Electroacupuncture Modulates Programmed Cell Death 1 Ligand 1 on Peripheral and Central Nervous Systems in a Mouse Fibromyalgia Pain Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and FM Pain Initiation
2.2. Electroacupuncture
2.3. Nociceptive Behavior Tests
2.4. Western Blot
2.5. Immunofluorescence
2.6. Intracerebroventricular Injection
2.7. Statistical Analysis
3. Results
3.1. EA, PD-L1 Injection, or Trpv1 Deletion Care for FM Pain in a Mouse Model
3.2. EA Controlled PD1-TRPV1 Pain Signaling in the Peripheral DRG of FM Mice
3.3. EA at ST36 Diminished Cold Stress-Induced FM Pain Through TRPV1-CB1 in the SCDH
3.4. EA at ST36 Altered FM Pain and Regulated PD-1 Signaling Pathway in the Thalamus
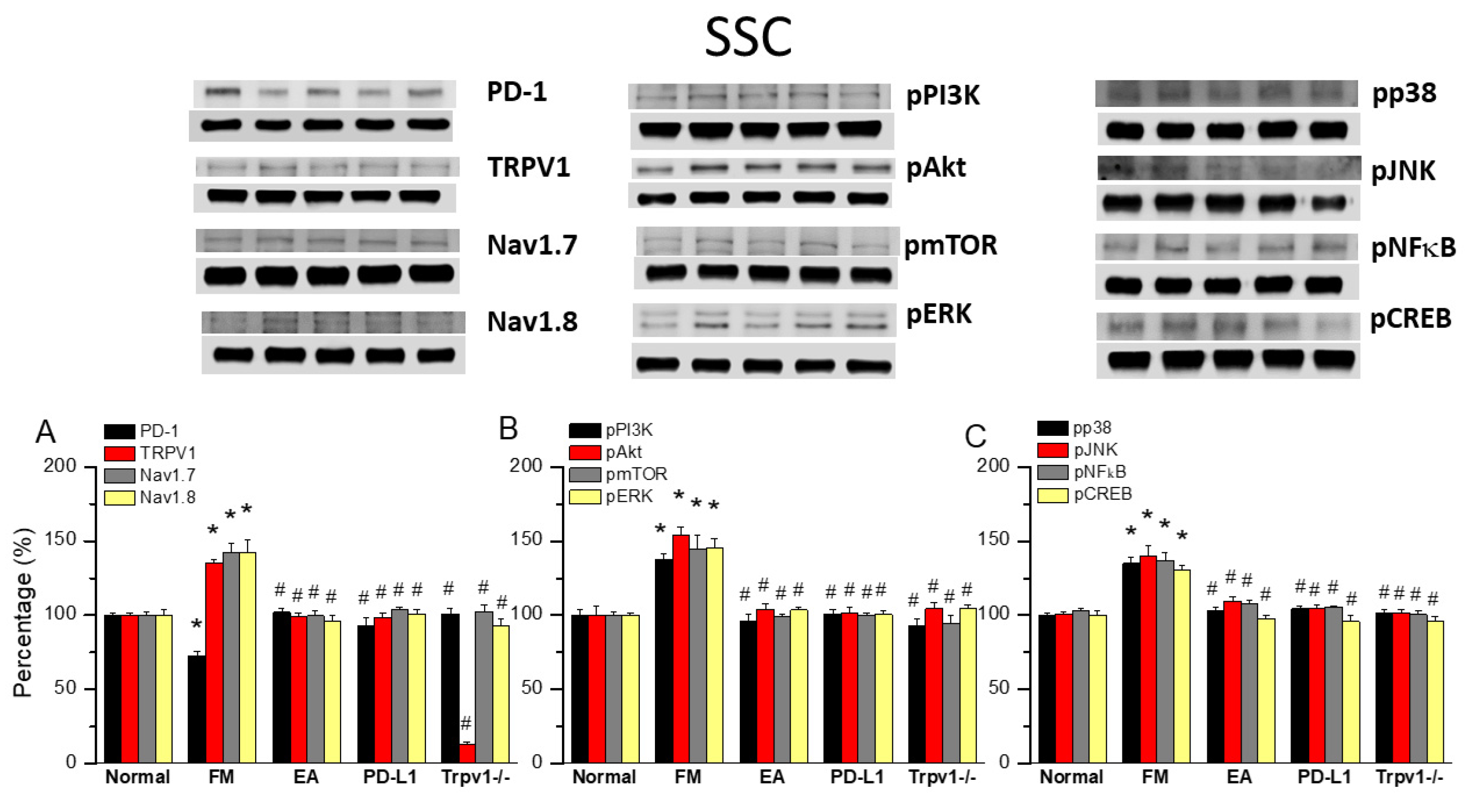
3.5. TRPV1 and Related Factors Were Inhibited in the Somatosensory Cortex After ICS and Recovered by 2 Hz EA and Trpv1 Deletion
3.6. EA, Intracerebral PD-L1 Injection, or TRPV1 Loss Mitigated FM in the DRG or SSC
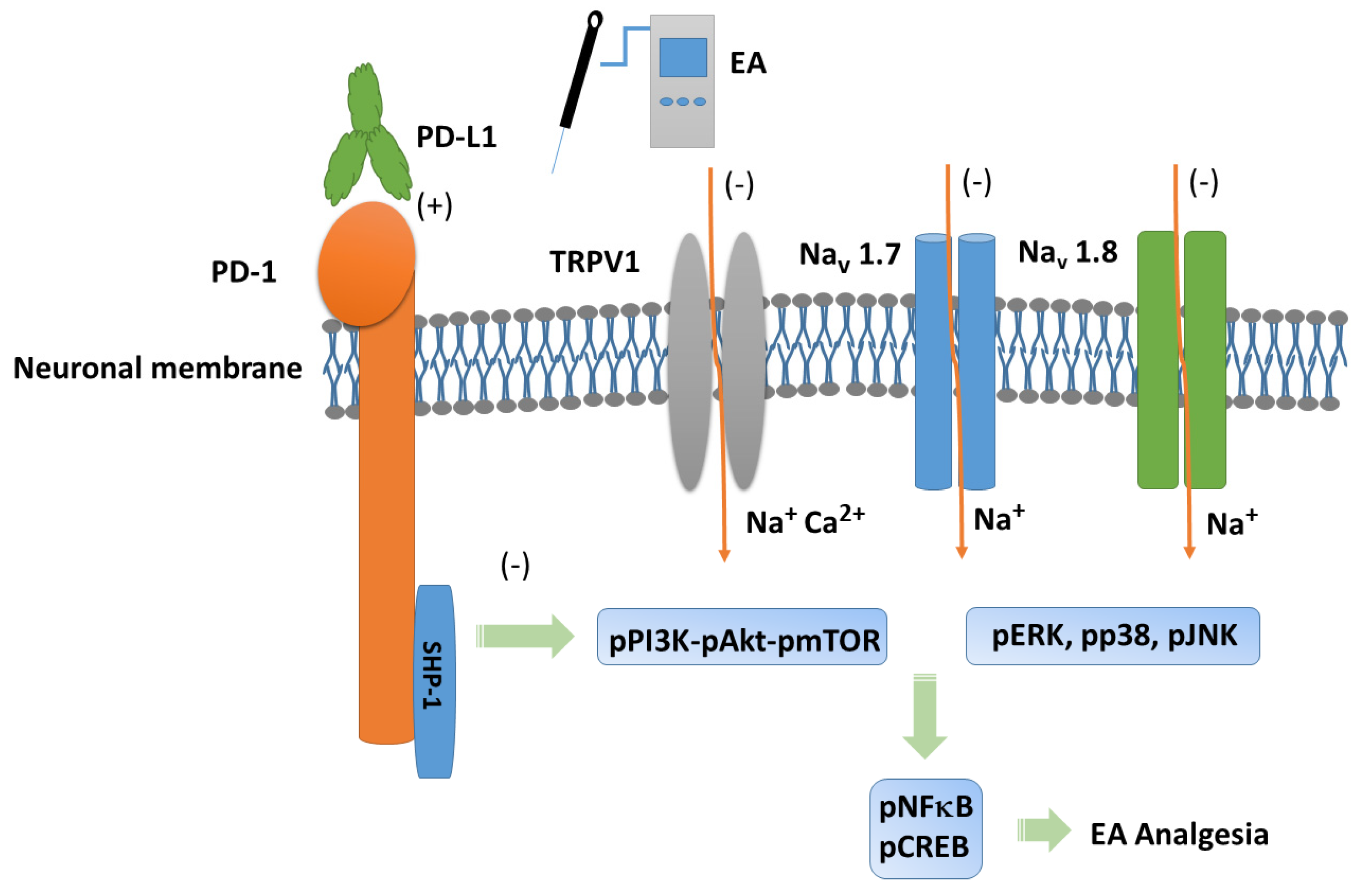
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Kang, X.; Chen, K.S.; Jehng, T.; Jones, L.; Chen, J.; Huang, X.F.; Chen, S.Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kim, Y.H.; Li, H.; Luo, H.; Liu, D.L.; Zhang, Z.J.; Lay, M.; Chang, W.; Zhang, Y.Q.; Ji, R.R. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci. 2017, 20, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal General Anesthesia: Theory and Practice. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Angeles, M.; Morales-Lazaro, S.L.; Juarez-Gonzalez, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Voets, T.; Iadarola, M.J.; Szallasi, A. Targeting TRP channels for pain relief: A review of current evidence from bench to bedside. Curr. Opin. Pharmacol. 2024, 75, 102447. [Google Scholar] [CrossRef]
- Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Ferrer-Montiel, A. TRPV1 in chronic pruritus and pain: Soft modulation as a therapeutic strategy. Front. Mol. Neurosci. 2022, 15, 930964. [Google Scholar] [CrossRef]
- Fischer, S.P.M.; Brusco, I.; Brum, E.S.; Fialho, M.F.P.; Camponogara, C.; Scussel, R.; Machado-de-Avila, R.A.; Trevisan, G.; Oliveira, S.M. Involvement of TRPV1 and the efficacy of α-spinasterol on experimental fibromyalgia symptoms in mice. Neurochem. Int. 2020, 134, 104673. [Google Scholar] [CrossRef]
- Yuksel, E.; Naziroglu, M.; Sahin, M.; Cig, B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci. Rep. 2017, 7, 17543. [Google Scholar] [CrossRef]
- Hsiao, I.H.; Lin, Y.W. Electroacupuncture Reduces Fibromyalgia Pain by Attenuating the HMGB1, S100B, and TRPV1 Signalling Pathways in the Mouse Brain. Evid.-Based Complement. Altern. Med. 2022, 2022, 2242074. [Google Scholar] [CrossRef]
- Liao, H.Y.; Lin, Y.W. Electroacupuncture reduces cold stress-induced pain through microglial inactivation and transient receptor potential V1 in mice. Chin. Med. 2021, 16, 43. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.C.; Lu, K.Q.; Xiao, R.; Tang, W.C.; Wang, F. The Role of p38 Mitogen-Activated Protein Kinase-Mediated F-Actin in the Acupuncture-Induced Mitigation of Inflammatory Pain in Arthritic Rats. Brain Sci. 2024, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.H.; Zhang, Y.Y.; Lin, J.; Liu, Y.J.; Li, Y.L.; Fang, Z.H.; Liao, H.L.; Wang, H.; Shen, J.F. Phosphorylation of the AMPARs regulated by protein kinase C (PKC) and protein interacting with C-kinase 1 (PICK1) contribute to orofacial neuropathic pain. Brain Res. 2023, 1820, 148578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, W.; Xu, X.; Ge, X.; Wang, F.; Zhang, G.Q.; Miao, L.; Deng, X. Aprepitant Inhibits JNK and p38/MAPK to Attenuate Inflammation and Suppresses Inflammatory Pain. Front. Pharmacol. 2021, 12, 811584. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, S.; Zhang, C.; Sun, J.; Zhang, D.; Chang, S.; Lin, Y.; Zhao, G. Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-kB Signaling Pathway. Front. Pharmacol. 2021, 12, 716684. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, L.; Singh, N.; Bhatti, M.S.; Bhatti, R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: Role of NMDA/NFkB mediated downstream signaling. Biochem. Pharmacol. 2019, 166, 56–69. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chou, A.I.W.; Su, H.; Su, K.P. Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids. Brain Behav. Immun. 2020, 89, 604–614. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Janssen, L.P.; Medeiros, L.F.; Souza, A.; Silva, J.D. Fibromyalgia: A Review of Related Polymorphisms and Clinical Relevance. An. Acad. Bras. Cienc. 2021, 93 (Suppl. S4), e20210618. [Google Scholar] [CrossRef]
- Assavarittirong, C.; Samborski, W.; Grygiel-Gorniak, B. Oxidative Stress in Fibromyalgia: From Pathology to Treatment. Oxid. Med. Cell. Longev. 2022, 2022, 1582432. [Google Scholar] [CrossRef]
- Farag, H.M.; Yunusa, I.; Goswami, H.; Sultan, I.; Doucette, J.A.; Eguale, T. Comparison of Amitriptyline and US Food and Drug Administration-Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2022, 5, e2212939. [Google Scholar] [CrossRef]
- Peck, M.M.; Maram, R.; Mohamed, A.; Ochoa Crespo, D.; Kaur, G.; Ashraf, I.; Malik, B.H. The Influence of Pro-inflammatory Cytokines and Genetic Variants in the Development of Fibromyalgia: A Traditional Review. Cureus 2020, 12, e10276. [Google Scholar] [CrossRef] [PubMed]
- Menzies, V.; Lyon, D.E. Integrated review of the association of cytokines with fibromyalgia and fibromyalgia core symptoms. Biol. Res. Nurs. 2010, 11, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ghowsi, M.; Qalekhani, F.; Farzaei, M.H.; Mahmudii, F.; Yousofvand, N.; Joshi, T. Inflammation, oxidative stress, insulin resistance, and hypertension as mediators for adverse effects of obesity on the brain: A review. Biomedicine 2021, 11, 13–22. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Su, Y.; Qi, L.; Yang, W.; Fu, M.; Jing, X.; Wang, Y.; Ma, Q. A neuroanatomical basis for electroacupuncture to drive the vagal–adrenal axis. Nature 2021, 598, 641–645. [Google Scholar] [CrossRef]
- Torres-Rosas, R.; Yehia, G.; Pena, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014, 20, 291–295. [Google Scholar] [CrossRef]
- Hsiao, I.H.; Liao, H.Y.; Cheng, C.M.; Yen, C.M.; Lin, Y.W. Paper-Based Detection Device for Microenvironment Examination: Measuring Neurotransmitters and Cytokines in the Mice Acupoint. Cells 2022, 11, 2869. [Google Scholar] [CrossRef]
- Guo, L.; Hu, H.; Jiang, N.; Yang, H.; Sun, X.; Xia, H.; Ma, J.; Liu, H. Electroacupuncture blocked motor dysfunction and gut barrier damage by modulating intestinal NLRP3 inflammasome in MPTP-induced Parkinson’s disease mice. Heliyon 2024, 10, e30819. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Fan, M.; Tian, J.; Zhang, S.; Zhao, Z.; Liu, X.; Ma, H.; Yang, L.; Chen, Y. Electroacupuncture improves gastrointestinal motility through a central-cholinergic pathway-mediated GDNF releasing from intestinal glial cells to protect intestinal neurons in Parkinson’s disease rats. Neurotherapeutics 2024, 21, e00369. [Google Scholar] [CrossRef]
- Lu, S.F.; Tang, Y.X.; Zhang, T.; Fu, S.P.; Hong, H.; Cheng, Y.; Xu, H.X.; Jing, X.Y.; Yu, M.L.; Zhu, B.M. Electroacupuncture Reduces Body Weight by Regulating Fat Browning-Related Proteins of Adipose Tissue in HFD-Induced Obese Mice. Front. Psychiatry 2019, 10, 353. [Google Scholar] [CrossRef]
- Qin, Y.; He, J.; Xia, L.; Guo, H.; He, C. Effects of electro-acupuncture on oestrogen levels, body weight, articular cartilage histology and MMP-13 expression in ovariectomised rabbits. Acupunct. Med. 2013, 31, 214–221. [Google Scholar] [CrossRef]
- Yen, L.T.; Hsieh, C.L.; Hsu, H.C.; Lin, Y.W. Targeting ASIC3 for Relieving Mice Fibromyalgia Pain: Roles of Electroacupuncture, Opioid, and Adenosine. Sci. Rep. 2017, 7, 46663. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Y.; Zhang, N.; Li, C.; Sandhu, A.F.; Williams, G., 3rd; Shen, Y.; Li, H.; Wu, Q.; Yu, S. Electroacupuncture Improves M2 Microglia Polarization and Glia Anti-inflammation of Hippocampus in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 689629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Cao, Q.L.; Zhao, X.; Liu, H.Z.; Zhang, Y.Q. Inhibition of TRPV1 by SHP-1 in nociceptive primary sensory neurons is critical in PD-L1 analgesia. JCI Insight 2020, 5, e137386. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Sun, K.; Zhu, J.; Li, F.; Lin, F.; Sun, X.; Luo, X.; Ren, C.; Lu, L.; Zhao, S.; et al. PD-L1 Improves Motor Function and Alleviates Neuropathic Pain in Male Mice After Spinal Cord Injury by Inhibiting MAPK Pathway. Front. Immunol. 2021, 12, 670646. [Google Scholar] [CrossRef]
- Shi, S.; Han, Y.; Wang, D.; Guo, P.; Wang, J.; Ren, T.; Wang, W. PD-L1 and PD-1 expressed in trigeminal ganglia may inhibit pain in an acute migraine model. Cephalalgia 2020, 40, 288–298. [Google Scholar] [CrossRef]
- Tan, H.; Ding, Z.; Zhang, C.; Yan, J.; Yang, Y.; Li, P. The Programmed Cell Death Ligand-1/Programmed Cell Death-1 Pathway Mediates Pregnancy-Induced Analgesia via Regulating Spinal Inflammatory Cytokines. Anesth. Analg. 2021, 133, 1321–1330. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, H.; Ma, Y.; Zhu, S.; Wu, Y.; Lu, M.; Yao, X.; Liu, X.; Chen, G. An analgesic peptide H-20 attenuates chronic pain via the PD-1 pathway with few adverse effects. Proc. Natl. Acad. Sci. USA 2022, 119, e2204114119. [Google Scholar] [CrossRef]
- Stone, J.A.; Johnstone, P.A. Mechanisms of action for acupuncture in the oncology setting. Curr. Treat. Options Oncol. 2010, 11, 118–127. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Du, X.; Shi, J.; Yu, R.; Li, S.; Na, R.; Zhao, Y.; Zhou, M.; Guo, Y.; et al. Combination of Anti-PD-1 and Electroacupuncture Induces a Potent Antitumor Immune Response in Microsatellite-Stable Colorectal Cancer. Cancer Immunol. Res. 2024, 12, 26–35. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-C.; Hsu, H.-C.; Liao, H.-Y.; Chen, A.L.P.; Lin, Y.-W. Electroacupuncture Modulates Programmed Cell Death 1 Ligand 1 on Peripheral and Central Nervous Systems in a Mouse Fibromyalgia Pain Model. Biomedicines 2025, 13, 396. https://doi.org/10.3390/biomedicines13020396
Lin H-C, Hsu H-C, Liao H-Y, Chen ALP, Lin Y-W. Electroacupuncture Modulates Programmed Cell Death 1 Ligand 1 on Peripheral and Central Nervous Systems in a Mouse Fibromyalgia Pain Model. Biomedicines. 2025; 13(2):396. https://doi.org/10.3390/biomedicines13020396
Chicago/Turabian StyleLin, Huan-Chin, Hsin-Cheng Hsu, Hsien-Yin Liao, Arbee L.P. Chen, and Yi-Wen Lin. 2025. "Electroacupuncture Modulates Programmed Cell Death 1 Ligand 1 on Peripheral and Central Nervous Systems in a Mouse Fibromyalgia Pain Model" Biomedicines 13, no. 2: 396. https://doi.org/10.3390/biomedicines13020396
APA StyleLin, H.-C., Hsu, H.-C., Liao, H.-Y., Chen, A. L. P., & Lin, Y.-W. (2025). Electroacupuncture Modulates Programmed Cell Death 1 Ligand 1 on Peripheral and Central Nervous Systems in a Mouse Fibromyalgia Pain Model. Biomedicines, 13(2), 396. https://doi.org/10.3390/biomedicines13020396





