Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies
Abstract
1. Introduction
AIM2 Inflammasome
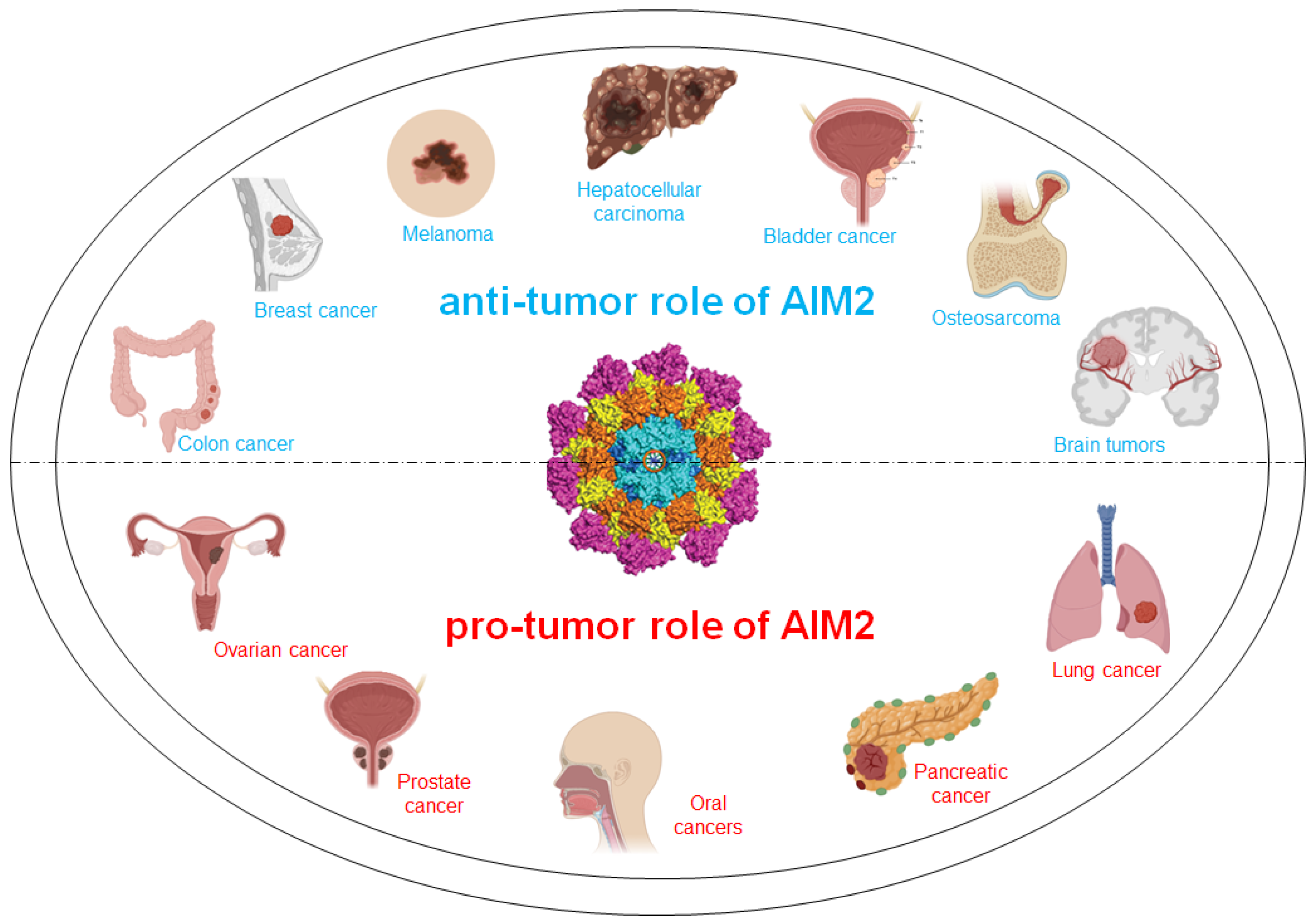
2. Anti-Tumor Role of AIM2 in Cancer
2.1. Colon and Colorectal Cancer
2.2. Breast Cancer
2.3. Melanoma
2.4. Hepatocellular Carcinoma
2.5. Bladder Cancer
2.6. Osteosarcoma
2.7. Brain Tumors
3. Pro-Tumor Role of AIM2 in Cancer
3.1. Ovarian Cancer
3.2. Prostate Cancer
3.3. Oral Cancers: Head and Neck Squamous Cell Carcinoma, Oral Squamous Cell Carcinoma and Hypopharyngeal Squamous Cell Carcinoma
3.4. Pancreatic Cancer
3.5. Lung Cancer
4. Targeting AIM2 Inflammasome as a Therapeutic Approach in Cancer
| Therapeutic Agent/s | Experimental Approach | Results | References |
|---|---|---|---|
| AIM2-deficient DC vaccine plus adoptive T cell therapy and anti–PD-1 immunotherapy | In vivo and ex vivo study | Silencing AIM2 in DC vaccination improves the efficacy of immunotherapy in melanoma | [4] |
| Docetaxel plus Thalidomide | Randomized phase II clinical trial | The addition of thalidomide to docetaxel resulted in an encouraging PSA decline rate and overall median survival rate in patients with metastatic AIPC | [106] |
| CAR-T and Thalidomide | In vitro study | CAR-T therapy combined with Thalidomide limits IL-1β-related toxic side effects of CAR-T treatment and improves the anti-tumor effect of CAR-T therapy. | [107] |
| Doxorubicin | In vivo and ex vivo study | AIM2 inflammasome signaling insufficiency (in AIM2 knock-out mice) limits Doxorubicin bone-damaging effects | [109] |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, M.M.; Shu, H.B. Innate Immune Response to Cytoplasmic DNA: Mechanisms and Diseases. Annu. Rev. Immunol. 2020, 38, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Shu, H.B. Cytoplasmic mechanisms of recognition and defense of microbial nucleic acids. Annu. Rev. Cell Dev. Biol. 2018, 34, 357–379. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Okamura, K.; Riding, R.L.; Fan, X.; Afshari, K.; Haddadi, N.S.; McCauley, S.M.; Guney, M.H.; Luban, J.; Funakoshi, T.; et al. AIM2 regulates anti-tumor immunity and is a viable therapeutic target for melanoma. J. Exp. Med. 2021, 218, e20200962. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; de Alba, E. Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. Int. J. Mol. Sci. 2021, 22, 872. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Kantono, M.; Guo, B. Inflammasomes and cancer: The dynamic role of the inflammasome in tumor development. Front. Immunol. 2017, 8, 1132. [Google Scholar] [CrossRef]
- Morrone, S.; Matyszewski, M.; Yu, X.; Delannov, M.; Egelman, E.H.; Sohn, J. Assembly-driven activation of the AIM2 foreign-dsDNA sensor provides a polymerization template for downstream ASC. Nat. Commun. 2015, 6, 7827. [Google Scholar] [CrossRef] [PubMed]
- Chew, Z.H.; Cui, J.; Sachaphibulkij, K.; Tan, I.; Kar, S.; Koh, K.K.; Singh, K.; Lim, H.M.; Lee, S.C.; Kumar, A.P.; et al. Macrophage IL-1b contributes to tumorigenesis through paracrine AIM2 inflammasome activation in the tumor microenvironment. Front. Immunol. 2023, 14, 1211730. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.; Casolaro, V.; Pinto, A.; Sorrentino, R. Inflammasome: Cancer’s friend or foe? Pharmacol. Ther. 2014, 143, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Imitazione, P.; Somma, P.; Rega, R.; Saccomanno, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. AIM2 inflammasome activation leads to IL-1α and TGF-β release from exacerbated chronic obstructive pulmonary disease-derived peripheral blood mononuclear cells. Front. Pharmacol. 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Hopfner, P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signaling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Zhu, Q.; Zhu, L.; Liu, Z.; Karki, R.; Malik, A.; Sharma, D.; Li, L.; Malireddi, R.K.; Gurung, P.; et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell 2015, 162, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Terlizzi, M.; Di Crescenzo, V.G.; Popolo, A.; Pecoraro, M.; Perillo, G.; Galderisi, A.; Pinto, A. Human lung cancer-derived immunosuppressive plasmacytoid dendritic cells release IL-1α in an AIM2 inflammasome-dependent manner. Am. J. Pathol. 2015, 185, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Lamort, A.; Cerqua, I.; Roviezzo, F.; Stathopoulos, G.; Pinto, A.; Sorrentino, R. Caspase-11 and AIM2 inflammasome are involved in smoking-induced COPD and lung adenocarcinoma. Oncotarget 2021, 12, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Falanga, A.; Stathopoulos, G.; De Lucia, L.; Hansbro, P.; Pinto, A.; Sorrentino, R. Absent in melanoma 2 (AIM2) positive profile identifies a poor prognosis of lung adenocarcinoma patients. Int. Immunopharmacol. 2023, 124 Pt B, 110990. [Google Scholar] [CrossRef]
- Colarusso, C.; Falanga, A.; Di Caprio, S.; Terlizzi, M.; Pinto, A.; Maiolino, P.; Sorrentino, R. The activation of the AIM2 inflammasome after cigarette smoke exposure leads to an immunosuppressive lung microenvironment. Int. Immunopharmacol. 2024, 131, 111832. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Colorectal Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 19 April 2024).
- Janakiram, N.B.; Rao, C.V. The role of inflammation in colon cancer. Adv. Exp. Med. Biol. 2014, 816, 25–52. [Google Scholar] [PubMed]
- Dihlmann, S.; Tao, S.; Echterdiek, F.; Herpel, E.; Jansen, L.; Chang- Claude, J.; Brenner, H.; Hoffmeister, M.; Kloor, M. Lack of absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int. J. Cancer 2014, 135, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E.; Petrucelli, A.S.; Chen, L.; Koblansky, A.A.; Truax, A.D.; Oyama, Y.; Rogers, A.B.; Brickey, W.J.; Wang, Y.; Schneider, M.; et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat. Med. 2015, 21, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dong, X.; Yang, X.; Wan, D.; Sun, L.; Gu, M.; Li, M.; Zhu, Z.; Wang, J.; Shang, Z.; et al. Expression and clinical significance of absent in melanoma 2 in colorectal cancer. Biomed. Pharmacother. 2017, 94, 843–849. [Google Scholar] [CrossRef]
- Liu, R.; Truax, A.D.; Chen, L.; Hu, P.; Li, Z.; Chen, J.; Song, C.; Chen, L.; Ting, J.P. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: Correlation with cancer stages and inflammasome components. Oncotarget 2015, 6, 33456–33469. [Google Scholar] [CrossRef]
- Mori, Y.; Yin, J.; Rashid, A.; Leggett, B.A.; Young, J.; Simms, L.; Kuehl, P.M.; Langenberg, P.; Meltzer, S.J.; Stine, O.C. Instabilotyping: Comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res. 2001, 61, 6046–6049. [Google Scholar] [PubMed]
- Woerner, S.M.; Kloor, M.; Schwitalle, Y.; Youmans, H.; Doeberitz, M.; Gebert, J.; Dihlmann, S. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer 2007, 46, 1080–1089. [Google Scholar] [CrossRef]
- Schulmann, K.; Brasch, F.E.; Kunstmann, E.; Engel, C.; Pagenstecher, C.; Vogelsang, H.; Krüger, S.; Vogel, T.; Knaebel, H.P.; Rüschoff, J.; et al. HNPCC-associated small bowel cancer: Clinical and molecular characteristics. Gastroenterology 2005, 128, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Pan, L.; Qin, T.; Ruan, H.; Zhang, Y.; Zhang, Y.; Fu, Y.; Luo, Y.; Li, X.; Wei, X.; et al. Pan-cancer analysis of AIM2 inflammasomes with potential implications for immunotherapy in human cancer: A bulk omics research and single cell sequencing validation. Front. Immunol. 2022, 13, 998266. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Li, Y.; Fu, Y.; Yan, Y.; Tao, S.; Tang, S.; Gai, X.; Gai, X.; Ding, Z.; Gan, Z.; et al. Low-Dose Chemotherapy Preferentially Shapes the Ileal Microbiome and Augments the Response to Immune Checkpoint Blockade by Activating AIM2 Inflammasome in Ileal Epithelial Cells. Adv. Sci. 2024, 11, e2304781. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Breast Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 19 April 2024).
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, K.; et al. If we build it they will come: Targeting the immune response to breast cancer. NPJ Breast Cancer 2019, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lu, L.; Li, B.; Shi, X.; Jin, H.; Hu, W. The roles of inflammasomes in cancer. Front. Immunol. 2023, 14, 1195572. [Google Scholar] [CrossRef]
- Chen, I.F.; Ou-Yang, F.; Hung, J.Y.; Liu, J.C.; Wang, H.; Wang, S.C.; Hou, M.F.; Hortobagyi, G.N.; Hung, M.C. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol. Cancer Ther. 2006, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Zhao, J.; Xing, Y.; Zhang, X.; Liu, J.; Ouyang, Q.; Chen, J.; Su, F.; Liu, Q.; Song, E. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell 2018, 175, 442–457.e23. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, J.; Zhang, Z.; Peng, Q.; Hao, L.; Guo, Y.; Zhou, W.; Cui, Q.; Shi, X. Cisplatin promotes the expression level of PD-L1 in the microenvironment of hepatocellular carcinoma through YAP1. Mol. Cell. Biochem. 2020, 475, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, K.L.; Ray, M.E.; Su, Y.A.; Anzick, S.L.; Johnstone, R.W.; Trapani, J.A.; Meltzer, P.S.; Trent, J.M. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 1997, 15, 453–457. [Google Scholar] [CrossRef]
- Choubey, D.; Walter, S.; Geng, Y.; Xin, H. Cytoplasmic localization of the interferon-inducible protein that is encoded by the AIM2 (absent in melanoma) gene from the 200-gene family. FEBS Lett. 2000, 474, 38–42. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.D.; Bergboer, J.G.; van den Bogaard, E.H.; van Vlijmen-Willems, I.M.; Rodijk-Olthuis, D.; Simon, A.; Zeeuwen, P.L.; Schalkwijk, J. Strong induction of AIM2 expression in human epidermis in acute and chronic inflammatory skin conditions. Exp. Dermatol. 2012, 21, 961–964. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.D.; van Vlijmen-Willems, I.M.; Zeeuwen, P.L.; Blokx, W.A.; Schalkwijk, J. Absent in melanoma 2 is predominantly present in primary melanoma and primary squamous cell carcinoma, but largely absent in metastases of both tumors. J. Am. Acad. Dermatol. 2014, 71, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Gluud, L.L.; Fialla, A.D.; Dahl, E.K.; Krag, A. Large variations in risk of hepatocellular carcinoma and mortality in treatment naïve hepatitis B patients: Systematic review with meta-analyses. PLoS ONE 2014, 9, e107177. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Hou, R.; Yan, D.; Liu, C.; Chen, S.; Li, X.; Du, W. Expression of AIM2 is correlated with increased inflammation in chronic hepatitis B patients. Virol. J. 2015, 12, 129. [Google Scholar] [CrossRef]
- Ma, X.; Guo, P.; Qiu, Y.; Mu, K.; Zhu, L.; Zhao, W.; Li, T.; Han, L. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget 2016, 7, 36185–36197. [Google Scholar] [CrossRef]
- Xie, S.; Wang, C.; Liu, X.; Li, C.; Yu, J.; Ma, S.; Li, Q.; Du, W. Hepatocellular carcinoma and AIM2: Therapeutic potential through regulation of autophagy and macrophage polarization. Immun. Inflamm. Dis. 2024, 12, e70002. [Google Scholar] [CrossRef]
- Sonohara, F.; Inokawa, Y.; Kanda, M.; Nishikawa, Y.; Yamada, S.; Fujii, T.; Sugimoto, H.; Kodera, Y.; Nomoto, S. Association of Inflammasome Components in Background Liver with Poor Prognosis After Curatively-resected Hepatocellular Carcinoma. Anticancer. Res. 2017, 37, 293–300. [Google Scholar] [CrossRef]
- Martínez-Cardona, C.; Lozano-Ruiz, B.; Bachiller, V.; Peiró, G.; Algaba-Chueca, F.; Gómez-Hurtado, I.; Such, J.; Zapater, P.; Francés, R.; González-Navajas, J.M. AIM2 deficiency reduces the development of hepatocellular carcinoma in mice. Int. J. Cancer 2018, 143, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Bladder Cancer. 2024. Available online: https://www.mayoclinic.org/diseases-conditions/bladder-cancer/symptoms-causes/syc-20356104 (accessed on 19 April 2024).
- Shadpour, P.; Zamani, M.; Aghaalikhani, N.; Rashtchizadeh, N. Inflammatory cytokines in bladder cancer. J. Cell. Physiol. 2019, 234, 14489–14499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, L.; Luo, W.; Hong, H.; Tang, D.; Zhou, D.; Zhou, L.; Li, Y. AIM2 inflammasome activation benefits the therapeutic effect of BCG in bladder carcinoma. Front. Pharmacol. 2022, 13, 1050774. [Google Scholar] [CrossRef]
- Prater, S.; McKeon, B. Osteosarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK549868/ (accessed on 19 April 2024).
- Hu, K.; Dai, H.B.; Qiu, Z.L. mTOR signaling in osteosarcoma: Oncogenesis and therapeutic aspects. Oncol. Rep. 2016, 36, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, C.; Shi, J.; Wen, K.; Wang, X. AIM2 inhibits the proliferation, invasion and migration, and promotes the apoptosis of osteosarcoma cells by inactivating the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2022, 25, 53. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Sim, J.; Park, J.; Moon, J.; Lim, J. Dysregulation of inflammasome activation in glioma. Cell Commun. Signal. 2023, 21, 239. [Google Scholar] [CrossRef]
- Sharma, N.; Saxena, S.; Agrawal, I.; Singh, S.; Srinivasan, V.; Arvind, S.; Epari, S.; Paul, S.; Jha, S. Differential Expression Profile of NLRs and AIM2 in Glioma and Implications for NLRP12 in Glioblastoma. Sci. Rep. 2019, 9, 8480. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.A.; Shrivastava, G.; Balcom, E.F.; McKenzie, B.A.; Fernandes, J.; Branton, W.G.; Wheatley, B.M.; Petruk, K.; van Landeghem, F.K.H.; Power, C. Absent in melanoma 2 regulates tumor cell proliferation in glioblastoma multiforme. J. Neurooncol. 2019, 144, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Le, S.B.; Hutchinson, T.E.; Calinescu, A.A.; Sebastian, M.; Jin, D.; Liu, T.; Ghiaseddin, A.; Rahman, M.; Tran, D.D. Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Investig. 2022, 132, e149258. [Google Scholar] [CrossRef]
- National Cancer Institute. Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®)–Health Professional Version. 2023. Available online: https://www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdq (accessed on 19 April 2024).
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune interactions in endometriosis. Expert. Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Wang, M.L.; Lu, K.H.; Yang, Y.P.; Juang, C.M.; Wang, P.H.; Hsu, R.J.; Yu, M.H.; Chang, C.C. Integrating the dysregulated inflammasome-based molecular functionome in the malignant transformation of endometriosis-associated ovarian carcinoma. Oncotarget 2017, 9, 3704–3726. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Chao, T.K.; Chou, Y.C.; Yu, M.H.; Wang, Y.C.; Lin, Y.H.; Lee, Y.L.; Liu, L.C.; Chang, C.C. AIM2 Inflammasome in Tumor Cells as a Biomarker to Predicting the Treatment Response to Antiangiogenic Therapy in Epithelial Ovarian Cancer Patients. J. Clin. Med. 2021, 10, 4529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.S.; Zhou, L.; Yu, L.; Zhang, L.M. A network-pathway based module identification for predicting the prognosis of ovarian cancer patients. J. Ovarian Res. 2016, 9, 73. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, P.H.; Hoekx, L.; Wyndaele, J.J.; Jeuris, W.; Van Marck, E. Inflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: Correlation with total serum PSA and PSA density. Eur. Urol. 2000, 37, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Lucia, M.S.; Thompson, I.M., Jr.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar] [CrossRef]
- Ponomareva, L.; Liu, H.; Duan, X.; Dickerson, E.; Shen, H.; Panchanathan, R.; Choubey, D. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol. Cancer Res. 2013, 11, 1193–1202. [Google Scholar] [CrossRef]
- Panchanathan, R.; Liu, H.; Choubey, D. Hypoxia primes human normal prostate epithelial cells and cancer cell lines for the NLRP3 and AIM2 inflammasome activation. Oncotarget 2016, 7, 28183–28194. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Feng, S.H.; Zhu, J.; Zhu, G.P.; Li, D.S.; Wang, Y.; Zhu, Y.X.; Sun, G.H.; Ji, Q.H. Impact of lymph node ratio on the survival of patients with hypopharyngeal squamous cell carcinoma: A population-based analysis. PLoS ONE 2013, 8, e566132013. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Pecorari, G.; Biolatti, M.; Pautasso, S.; Lo Cigno, I.; Garzaro, M.; Dell’Oste, V.; Landolfo, S. PYHIN genes as potential biomarkers for prognosis of human papillomavirus-positive or -negative head and neck squamous cell carcinomas. Mol. Biol. Rep. 2019, 46, 3333–3347. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, H.; Zhang, L.; Zhang, G.; Wei, Y.; Zhang, W.; Yang, X.; Li, M.; Yin, G.; Guo, W.; et al. AIM2 promotes the progression of HNSCC via STAT1 mediated transcription and IL-17/MAPK signaling. Cell Signal 2024, 127, 111545. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Nagai, K.; Nakahata, S.; Saito, Y.; Ichikawa, T.; Suekane, A.; Taki, T.; Iwakawa, R.; Enari, M.; Taniwaki, M.; et al. Overexpression of the DNA sensor proteins, absent in melanoma 2 and interferon-inducible 16, contributes to tumorigenesis of oral squamous cell carcinoma with p53 inactivation. Cancer Sci. 2012, 103, 782–790. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakahata, S.; Kondo, Y.; Izumi, A.; Yamamoto, K.; Ichikawa, T.; Tamura, T.; Noumi, K.; Yamashita, Y.; Morishita, K. Overexpression of absent in melanoma 2 in oral squamous cell carcinoma contributes to tumor progression. Biochem. Biophys. Res. Commun. 2019, 509, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, X.; Li, H.; Wang, W.; Li, X. Low expression of AIM2 combined with high expression of p-STAT3 is associated with poor prognosis in hypopharyngeal squamous cell carcinoma. Oncol. Rep. 2019, 41, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Laversanne, M.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Piñeros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Antoni, S.; et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 2015, 137, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Kandikattu, H.K.; Venkateshaiah, S.U.; Mishra, A. Chronic Pancreatitis and the Development of Pancreatic Cancer. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1182–1210. [Google Scholar] [CrossRef]
- Algaba-Chueca, F.; de-Madaria, E.; Lozano-Ruiz, B.; Martínez-Cardona, C.; Quesada-Vázquez, N.; Bachiller, V.; Tarín, F.; Such, J.; Francés, R.; Zapater, P.; et al. The expression and activation of the AIM2 inflammasome correlates with inflammation and disease severity in patients with acute pancreatitis. Pancreatology 2017, 17, 364–371. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Cheng, X.; Yuan, H.; Zhu, S.; Liu, J.; Wen, Q.; Xie, Y.; Liu, J.; Kroemer, G.; et al. PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev. Cell 2018, 46, 441–455. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Lung Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 19 April 2024).
- McLoed, A.G.; Sherrill, T.P.; Cheng, D.S.; Han, W.; Saxon, J.A.; Gleaves, L.A.; Wu, P.; Polosukhin, V.V.; Karin, M.; Yull, F.E.; et al. Neutrophil-Derived IL-1β Impairs the Efficacy of NF-κB Inhibitors against Lung Cancer. Cell Rep. 2016, 16, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Wang, Y.; Zeng, X.; Wang, Z.; Wang, H.; Xie, W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015, 36, 7501–7513. [Google Scholar] [CrossRef]
- Qi, M.; Dai, D.; Liu, J.; Li, Z.; Liang, P.; Wang, Y.; Cheng, L.; Zhan, Y.; An, Z.; Song, Y.; et al. AIM2 promotes the development of non-small cell lung cancer by modulating mitochondrial dynamics. Oncogene 2020, 39, 2707–2723. [Google Scholar] [CrossRef]
- Terlizzi, M.; Molino, A.; Colarusso, C.; Donovan, C.; Imitazione, P.; Somma, P.; Aquino, R.P.; Hansbro, P.M.; Pinto, A.; Sorrentino, R. Activation of the absent in melanoma 2 inflammasome in peripheral blood mononuclear cells from idiopathic pulmonary fibrosis patients leads to the release of pro-fibrotic mediators. Front. Immunol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Maglio, A.; Molino, A.; Candia, C.; Vitale, C.; Hansbro, P.M.; Vatrella, A.; Pinto, A.; Sorrentino, R. Activation of the AIM2 receptor in circulating cells of post-COVID-19 patients with signs of lung fibrosis is associated with the release of IL-1α, IFN-α and TGF-β. Front. Immunol. 2022, 13, 934264. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Molino, A.; Pinto, A.; Sorrentino, R. Role of the inflammasome in chronic obstructive pulmonary disease (COPD). Oncotarget 2017, 8, 81813–81824. [Google Scholar] [CrossRef]
- Durham, A.I.; Adcock, I.M. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef]
- Molino, A.; Terlizzi, M.; Colarusso, C.; Rossi, A.; Somma, P.; Saglia, A.; Pinto, A.; Sorrentino, R. AIM2/IL-1α/TGF-β Axis in PBMCs From Exacerbated Chronic Obstructive Pulmonary Disease (COPD) Patients Is Not Related to COX-2-Dependent Inflammatory Pathway. Front. Physiol. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Terlizzi, M.; Colarusso, C.; De Rosa, I.; De Rosa, N.; Somma, P.; Curcio, C.; Sanduzzi, A.; Micheli, P.; Molino, A.; Saccomanno, A.; et al. Circulating and tumor-associated caspase-4: A novel diagnostic and prognostic biomarker for Non-Small Cell Lung Cancer. Oncotarget 2018, 9, 19356–19367. [Google Scholar] [CrossRef]
- Terlizzi, M.; Molino, A.; Colarusso, C.; Somma, P.; De Rosa, I.; Troisi, J.; Scala, G.; Salvi, R.; Pinto, A.; Sorrentino, R. Altered lung tissue lipidomic profile in caspase-4 positive non-small cell lung cancer (NSCLC) patients. Oncotarget 2020, 11, 3515–3525. [Google Scholar] [PubMed]
- Terlizzi, M.; Colarusso, C.; De Rosa, I.; Somma, P.; Curcio, C.; Aquino, R.P.; Panico, L.; Salvi, R.; Zito Marino, F.; Botti, G.; et al. Identification of a novel subpopulation of Caspase-4 positive Non-Small Cell Lung Cancer patients. J. Exp. Clin. Cancer Res. 2020, 39, 242. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Lin, C.H.; Lee, H.H.; Chang, W.M.; Li, L.J.; Su, C.Y.; Lee, K.Y.; Chiu, H.W.; Lin, Y.F. AIM2 upregulation promotes metastatic progression and PD-L1 expression in lung adenocarcinoma. Cancer Sci. 2023, 114, 306–320. [Google Scholar] [CrossRef]
- Alanazi, M.; Weng, T.; McLeod, L.; Gearing, L.J.; Smith, J.A.; Kumar, B.; Saad, M.I.; Jenkins, B.J. Cytosolic DNA sensor AIM2 promotes KRAS-driven lung cancer independent of inflammasomes. Cancer Sci. 2024, 115, 1834–1850. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, M.; Ying, Q.; Xie, X.; Yue, S.; Tong, B.; Wei, Q.; Bai, Z.; Ma, L. Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Sollberger, G.; Beer, H.D. Thalidomide inhibits activation of caspase-1. J. Immunol. 2009, 183, 5593–5599. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Xu, J.; Yan, S.; Huang, M.; Ding, H.; Sun, X.; Bi, A.; Ding, J.; Sun, B.; Geng, M. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Dimopoulos, M.A. Thalidomide in the treatment of multiple myeloma. Best. Pract. Res. Clin. Haematol. 2007, 20, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Dahut, W.L.; Gulley, J.L.; Arlen, P.M.; Liu, Y.; Fedenko, K.M.; Steinberg, S.M.; Wright, J.J.; Parnes, H.; Chen, C.C.; Jones, E.; et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J. Clin. Oncol. 2004, 2, 2532–2539. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Dai, Y.; Zhao, X.; Bao, S.; Ma, W.; Zha, L.; Liu, S.; Liu, Y.; Zheng, Y.; et al. Blockade of AIM2 inflammasome or α1-AR ameliorates IL-1β release and macrophage-mediated immunosuppression induced by CAR-T treatment. J. Immunother. Cancer 2021, 9, e001466. [Google Scholar] [CrossRef] [PubMed]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Wang, C.; Kaur, K.; Xu, C.; Abu-Amer, Y.; Mbalaviele, G. Chemotherapy activates inflammasomes to cause inflammation-associated bone loss. Elife 2024, 13, RP92885. [Google Scholar] [CrossRef]
- Green, J.P.; El-Sharkawy, L.Y.; Roth, S.; Zhu, J.; Cao, J.; Leach, A.G.; Liesz, A.; Freeman, S.; Brough, D. Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome. iScience 2023, 26, 106758. [Google Scholar] [CrossRef]
- Lambrecht, G. Agonists and antagonists acting at P2X receptors: Selectivity profiles and functional implications. Naunyn-Schmiedebergs Arch. Pharmacol. 2000, 362, 340–350. [Google Scholar] [CrossRef]
- Yousaf, A.; Hamid, S.A.; Bunnori, N.M.; Ishola, A.A. Applications of calixarenes in cancer chemotherapy: Facts and perspectives. Drug Des. Devel Ther. 2015, 9, 2831–2838. [Google Scholar] [PubMed]
- Puri, S.; Mazza, M.; Roy, G.; England, R.M.; Zhou, L.; Nourian, S.; Subramony, J.A. Evolution of nanomedicine formulations for targeted delivery and controlled release. Adv. Drug Deliv. Rev. 2023, 200, 114962. [Google Scholar] [CrossRef]
- Chai, D.; Liu, N.; Li, H.; Wang, G.; Song, J.; Fang, L.; Lu, Z.; Yao, H.; Zheng, J. H1/pAIM2 nanoparticles exert anti-tumour effects that is associated with the inflammasome activation in renal carcinoma. J. Cell Mol. Med. 2018, 22, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, C.; Li, H.B.; Tong, J.; Ouyang, X.; Cetinbas, N.M.; Zhu, S.; Strowig, T.; Lam, F.C.; Zhao, C.; et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 2016, 354, 765–768. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Peng, S.; Shan, X.; Deng, G.; Shen, L.; Sun, J.; Jiang, C.; Yang, X.; Chang, Z.; Sun, X.; et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 2019, 10, 957. [Google Scholar] [CrossRef]
- Han, C.; Godfrey, V.; Liu, Z.; Han, Y.; Liu, L.; Peng, H.; Weichselbaum, R.R.; Zaki, H.; Fu, Y.X. The AIM2 and NLRP3 inflammasomes trigger IL-1–mediated antitumor effects during radiation. Sci. Immunol. 2021, 6, eabc6998. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.W.; Lee, H.L.; Lee, H.H.; Lu, H.W.; Lin, K.Y.; Lin, Y.F.; Lin, C.H. AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-κB activation in oral squamous cell carcinoma. J. Transl. Med. 2024, 22, 13. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colarusso, C.; Terlizzi, M.; Di Caprio, S.; Falanga, A.; D’Andria, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R. Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies. Biomedicines 2025, 13, 395. https://doi.org/10.3390/biomedicines13020395
Colarusso C, Terlizzi M, Di Caprio S, Falanga A, D’Andria E, d’Emmanuele di Villa Bianca R, Sorrentino R. Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies. Biomedicines. 2025; 13(2):395. https://doi.org/10.3390/biomedicines13020395
Chicago/Turabian StyleColarusso, Chiara, Michela Terlizzi, Simone Di Caprio, Anna Falanga, Emmanuel D’Andria, Roberta d’Emmanuele di Villa Bianca, and Rosalinda Sorrentino. 2025. "Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies" Biomedicines 13, no. 2: 395. https://doi.org/10.3390/biomedicines13020395
APA StyleColarusso, C., Terlizzi, M., Di Caprio, S., Falanga, A., D’Andria, E., d’Emmanuele di Villa Bianca, R., & Sorrentino, R. (2025). Role of the AIM2 Inflammasome in Cancer: Potential Therapeutic Strategies. Biomedicines, 13(2), 395. https://doi.org/10.3390/biomedicines13020395










