Abstract
Background/Objectives: Crohn’s disease (CD) is a progressive condition, and early treatment with infliximab combined with an immunosuppressant within six months has been shown to improve clinical outcomes. However, the impact of early ustekinumab (UST) use in biologic-naïve CD patients remains unclear. This study aims to address this gap by evaluating the clinical outcomes of early UST intervention in such patients. Methods: In this retrospective cohort study, we included biologic-naïve CD patients treated with UST, with a clinical follow-up period of at least six months from October 2020 to January 2024 across four medical centers. Patients who received UST within six months of CD diagnosis were categorized into the Early-UST group, while those who were initially treated with conventional therapies and subsequently received UST after six months were assigned to the control group. The primary endpoint was the improvement of clinical outcomes at six months. Results: A total of 60 biologic-naïve CD patients were enrolled. Baseline characteristics were comparable between the two groups. At six months, the Early-UST group (n = 24) demonstrated significantly lower Crohn’s Disease Activity Index (CDAI) scores (73.03 vs. 112.42, p = 0.038), lower Harvey–Bradshaw Index (HBI) scores (1.46 ± 1.69 vs. 2.72 ± 2.17, p = 0.020), higher rates of clinical remission (91.7% vs. 63.9%, p = 0.017), and higher rates of steroid-free clinical remission (79.2% vs. 50.0%, p = 0.031) compared to the control group (n = 36). At one year, the early-UST group continued to exhibit lower CDAI scores (39.94 vs. 91.48, p = 0.005). Conclusions: Initiating ustekinumab within six months of CD diagnosis is associated with improved clinical outcomes and enhanced quality of life in biologic-naïve Crohn’s disease patients.
1. Introduction
Crohn’s disease (CD) is a chronic, progressive disorder involving the gastrointestinal tract that can result in complications such as fistulas, abscesses, and strictures [1]. Since the introduction of infliximab in CD in 1998, an increasing number of biologics with distinct mechanisms have become available for the treatment of CD. The advent of biologic therapies has significantly reduced surgical risk for CD patients [2]. However, despite these improvements, there are still some patients who require surgical intervention [3]. The timing of biologic therapies may also play a crucial role in modifying the disease course and improving the clinical outcomes. Studies have shown that early biologic treatment within one to three years of CD diagnosis is associated with lower surgery rates and higher remission rates compared to delayed treatment [4,5,6,7,8]. These studies primarily focused on the impact of anti-tumor necrosis factor alpha (anti-TNF-alpha), demonstrating that early use of biologics may lead to superior clinical outcomes for patients with CD.
Building on these findings, the ECCO guidelines discourage the use of 5-aminosalicylates (5-ASA) and thiopurine monotherapy as induction therapy for CD, as well as the use of 5-ASA and steroids for maintaining remission in CD [9]. The American Gastroenterological Association (AGA) similarly advocates for the early introduction of biologics, with or without an immunomodulator, rather than delaying their use until after the failure of 5-ASA and/or corticosteroids [10]. The Paris definition suggests that early biological treatment within 18 months of diagnosis can modify the natural course of CD, minimize bowel damage, prevent disability, and improve clinical outcomes [11]. Recently, the PRedicting Outcomes For Crohn’s disease using a moLecular biomarker (PROFILE) trial highlighted the advantages of early combination therapy with infliximab and immunosuppressants [12]. This trial enrolled biologic-naïve CD patients within 6 months of diagnosis, randomizing them to either a top-down therapy group or an accelerated step-up therapy group. After one year of follow-up, the top-down group demonstrated a significantly higher rate of sustained steroid-free and surgical-free remission (79% vs. 15%). The findings of PROFILE suggest that top-down therapy with infliximab and immunosuppressants may be considered a standard approach for newly diagnosed CD patients.
While infliximab was the first biologic approved for CD, the availability of advanced therapies for treating CD continues to expand. It remains unclear whether early top-down treatment using biologics other than infliximab provides similar benefits. Ustekinumab (UST), a monoclonal antibody targeting the p40 subunit of interleukin-12 and interleukin-23, is recommended by the ECCO guidelines for inducing and maintaining remission in moderate-to-severe CD [9]. Additionally, real-world studies have shown that UST, when used as a first-line biologic, is as effective as other biologic agents in achieving clinical remission [13,14,15]. However, the impact of early UST treatment in biologic-naïve CD within six months of diagnosis remains unknown [16]. To date, no study has specifically evaluated whether early UST therapy improves clinical outcomes in CD. Therefore, this study aimed to address this critical gap.
2. Materials and Methods
2.1. Patients and Data Collection
In this multicenter retrospective cohort study, we initially enrolled 140 adult patients with CD who received UST between October 2020 and January 2024 at four medical centers in Taiwan: Chang Gung Memorial Hospital, Cheng Kung University Hospital, Tri-Service General Hospital, and Far Eastern Memorial Hospital. Patients were excluded if they had prior exposure to biologics or a follow-up duration of less than 26 weeks. Eligible patients were categorized into two groups based on the duration of CD at the start of UST treatment. Patients who initiated UST therapy within six months of CD diagnosis were placed in the Early-UST group, while those who initially received conventional therapies such as 5-ASA, steroids, and immunomodulators during the first six months and started UST treatment at a later stage were assigned to the control group.
We extracted data from the electronic medical records of each institution, collecting information on age, gender, smoking status, body mass index (BMI), time from diagnosis to UST initiation, Crohn’s Disease Activity Index (CDAI), Harvey–Bradshaw Index (HBI), previous intestinal resections, disease location and behavior (according to Montreal classification), IBD medication, laboratory data, and extraintestinal manifestations (EIMs). Data were collected at the time of UST initiation, as well as at 26 weeks (six months) and 52 weeks (one year) after UST initiation.
2.2. Clinical Outcome Evaluation
The UST treatment regimen comprised an initial intravenous (IV) induction dose at week 0, administered based on patient weight: 260 mg for those weighing ≤55 kg, 390 mg for those weighing between 55 and 85 kg, and 520 mg for those weighing ≥85 kg. Following the induction, patients received subcutaneous (SC) maintenance doses of 90 mg at week 8, with subsequent doses administered every 12 weeks. Follow-up assessments were performed at 26 weeks (6 months) and 52 weeks (1 year) after UST initiation.
The primary endpoint was the clinical outcomes six months after the first dose of UST, while the secondary endpoint was the outcome at one year. Clinical outcomes included CDAI scores, HBI scores, clinical remission rate, steroid-free clinical remission rate, BMI, hemoglobin, albumin, and C-reactive protein (CRP) levels. Clinical remission was defined as a CDAI score of less than 150, and steroid-free remission was defined as achieving clinical remission without concurrent steroid use. Adverse events were recorded throughout the follow-up period, with assessments conducted at six months and one year after UST initiation.
2.3. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, while categorical variables were presented as percentages. Student’s t-test was used to analyze continuous data, while the chi-square test or Fisher’s exact test was applied for categorical data. A p-value of <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 29.0 (Armonk, NY, USA, IBM Corp).
3. Results
3.1. Baseline Characteristics
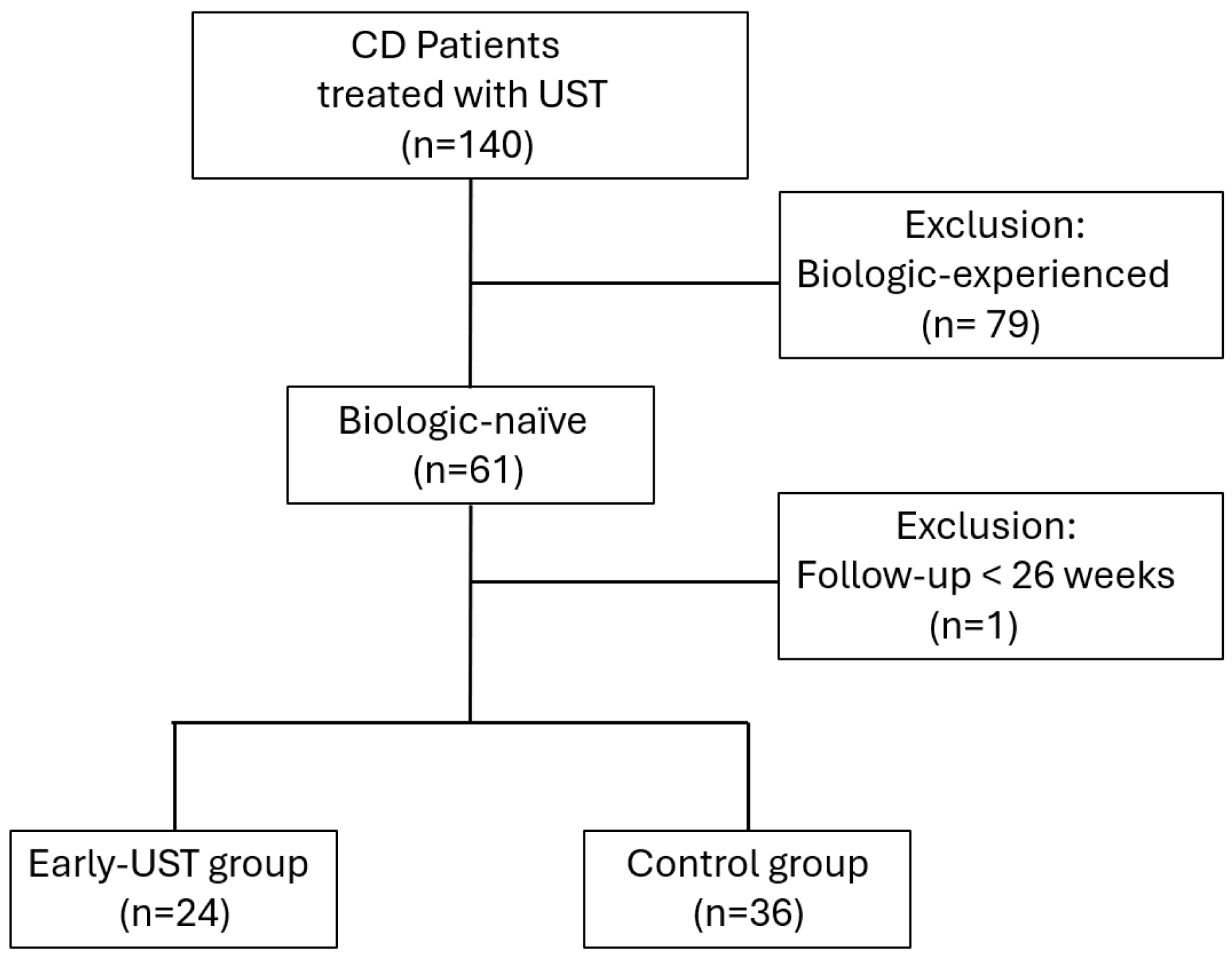
Initially, 140 patients with CD receiving UST treatment were assessed. Of these, 80 patients were excluded: 79 due to prior biologic exposure, and 1 due to a follow-up duration of less than six months. Ultimately, a total of 60 patients were included in the study. Among these, 24 patients (40%) initiated UST treatment within six months of diagnosis and were assigned to the Early-UST group, while the remaining 36 patients (60%) were classified as the control group. The patient inclusion process is illustrated in the flowchart (Figure 1).

Figure 1.
Flowchart of patient selection and grouping in this study. This flowchart illustrates the process of screening and categorizing patients with Crohn’s disease (CD) who received ustekinumab (UST) treatment. It includes the initial number of patients assessed, the exclusion criteria applied, and the final grouping into the Early-UST group and control group.
The mean time interval from diagnosis to the first UST dose was 13.2 weeks in the Early-UST group, compared to 314.2 weeks in the control group (p < 0.001). Baseline characteristics, including age, gender, and smoking status, were comparable between the Early-UST group and the control group. The detailed baseline characteristics of the two groups are presented in Table 1.

Table 1.
Baseline characteristics of biologic-naive Crohn’s disease patients receiving ustekinumab treatment in Early-UST and control groups.
3.2. Clinical Outcomes at Six Months
At the six-month follow-up, the Early-UST group demonstrated significantly lower CDAI scores (73.03 vs. 112.42, p = 0.038) and lower HBI scores (1.46 vs. 2.72, p = 0.020) compared to the control group. Additionally, a higher proportion of patients in the Early-UST group achieved clinical remission (91.7% vs. 63.9%, p = 0.017) and steroid-free remission (79.2% vs. 50.0%, p = 0.031). Laboratory parameters were comparable between the two groups.
Regarding adverse events, Clostridioides difficile infection was reported in one patient from the Early-UST group and two patients from the control group. Additionally, two patients in the Early-UST group and three patients in the control group required dose escalation at six months, which involved shortening the dosing interval from 12 weeks to 8 weeks. Hospitalizations and emergency department visits were similar between the two groups. No cases of malignancy or mortality were reported during the follow-up period. Additional details are provided in Table 2.

Table 2.
Clinical outcomes in biologic-naive Crohn’s disease patients receiving ustekinumab treatment in Early-UST and control groups.
3.3. Clinical Outcomes at One Year
For the one-year outcome analysis, we excluded patients without complete one-year follow-up data. Specifically, seven patients from the Early-UST group and six from the control group were excluded due to a follow-up period of less than one year. Additionally, one patient in the control group was excluded due to mortality at 10 months. This left 17 patients in the Early-UST group and 29 patients in the control group available for the one-year clinical outcome assessment.
At one year, the Early-UST group maintained significantly lower CDAI scores (39.94 vs. 91.48, p = 0.005) compared to the control group (Table 2). Disease recurrence occurred in one patient from the Early-UST group and three from the control group. Among those who achieved clinical remission at six months, sustained remission was maintained in 93.8% of the Early-UST group and 87.5% of the control group. However, there were no significant differences between the two groups in terms of HBI score, remission rates, laboratory data.
As for adverse events, Clostridioides difficile infection occurred in two patients from the Early-UST group by one year, while Clostridium innocuum infection was observed in two patients from the control group. The single mortality case in the control group resulted from pneumonia complicated by septic shock and was not considered related to a CD-related opportunistic infection. By one year, dose escalation was required in three patients from the Early-UST group and six from the control group. The overall incidence of adverse events, including emergency room visits, hospitalizations, and opportunistic infections, was comparable between the two groups. Further details are illustrated in Table 2. There were minimal missing data, primarily in a few baseline variables such as BMI. The primary outcome variables had no missing data.
4. Discussion
In this multicenter, retrospective cohort study conducted in Taiwan, we found that the Early-UST group had significantly lower CDAI scores (73.03 vs. 112.42, p = 0.038), lower HBI scores (1.46 vs. 2.72, p = 0.020), higher rates of clinical remission (91.7% vs. 63.9%, p = 0.017), and higher rates of steroid-free remission (79.2% vs. 50.0%, p = 0.031) at six months compared to the control group. At one year, the Early-UST group continued to exhibit significantly lower CDAI scores (39.94 vs. 91.48, p = 0.005). These findings suggest that early UST treatment within six months of CD diagnosis can lead to a lower disease burden and better clinical and patient-reported outcomes. This is consistent with the PROFILE trial, which highlighted the importance of early biologic intervention in optimizing clinical outcomes. While the PROFILE trial demonstrated significantly higher rates of sustained steroid-free and surgery-free remission at one year in the early infliximab with immunosuppressant group [12], our study also showed significantly higher rates of clinical remission in the Early-UST group. Notably, none of the patients in our study required surgery during the one-year follow-up period.
Although no prior studies have specifically examined early UST use, the benefits of early use of anti-TNF-alpha agents were observed in subgroup analyses of the CHARM and PRECiSE2 studies [7,17]. Additionally, a cohort study by Faleck et al. demonstrated significantly higher rates of steroid-free remission (43% vs. 14%) at six months in CD patients who received earlier vedolizumab treatment (≤2 years duration) compared to those with later intervention (>2 years) [18].
Recent randomized controlled trials and real-world studies on biologic-naïve CD patients have reported six-month clinical remission rates with UST ranging from 54% to 67.7% when using an eight-week dosing interval [19,20,21]. In comparison, our study demonstrated six-month clinical remission rates of 91.7% in the Early-UST group and 63.9% in the control group with a twelve-week dosing interval, highlighting the importance of therapeutic timing for UST treatment. To explore potential factors influencing treatment response, we compared patients who achieved clinical remission with those who did not. The two groups were similar in age, gender, and Montreal classification. Additionally, we did not identify any statistically significant predictors for dose escalation in this study.
In terms of adverse events, UST, an IgG1 monoclonal antibody that binds to the p40 subunit shared by the pro-inflammatory interleukins 12 and 23, demonstrated an outstanding safety profile [22,23,24]. Unlike in other countries, in Taiwan, the National Health Insurance regulations stipulate a 12-week dosing interval for UST in IBD patients. Consequently, dose escalation (shortening the dosing interval) was required in 8.3% of patients during the first six months and in 19.6% of patients over the one-year period. Notably, apart from one 88-year-old patient with multiple comorbidities in the control group who died from aspiration pneumonia-related septic shock at ten months, there were no other treatment discontinuations throughout the twelve-month follow-up period in this study. Moreover, the incidence of adverse events, including opportunistic infections, hospitalizations, and emergency room visits, was comparable between the Early-UST group and the control group. These findings suggest that early UST treatment does not increase the risk of adverse events and leads to better clinical outcomes in newly diagnosed CD.
This study has several limitations. First, 40% of patients were using immunosuppressants at baseline, 11.7% at six months, and 21.7% at one year. Although immunosuppressants can be effective for induction and maintenance therapy [25], the proportion of patients receiving combination therapy did not differ significantly between the two groups at baseline or during outcome assessments. Additionally, previous studies have shown that concurrent use of immunosuppressants does not affect the rates of clinical remission and steroid-free remission in UST treatment for CD [26,27]. Second, the study is limited by its small sample size, relatively short follow-up period, and lack of data on fecal calprotectin, endoscopic remission, and transmural healing. These limitations are largely attributed to the reimbursement policies of Taiwan’s National Health Insurance, which restrict certain diagnostic tests, and the inherent constraints associated with a retrospective study design. Despite these limitations, this study is, to our knowledge, the first to evaluate the benefit of early UST treatment within six months of diagnosis in biologic-naïve CD patients. Future large-scale, long-term, prospective randomized controlled trials are warranted to confirm these findings.
In conclusion, initiating UST treatment within six months of diagnosis improves clinical and patient-reported outcomes in biologic-naïve CD patients. Therefore, it can be considered a superior therapeutic strategy in newly diagnosed CD patients.
Author Contributions
Conceptualization, P.-H.L.; data acquisition, Y.-C.C., C.-H.C., T.-Y.H., C.-S.C., C.-J.K. and P.-H.L.; data analysis and interpretation, Y.-C.C., P.-H.L. and C.-S.C.; writing—original draft preparation, Y.-C.C.; writing—review and editing, P.-H.L., C.-H.C. and T.-Y.H.; statistical analysis, Y.-B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by Chang Gung Memorial Hospital, Linkou Branch, Taiwan (CPRPG3L0052).
Institutional Review Board Statement
The study protocol was approved by the Institutional Review Board (IRB) of the Chang Gung Medical Foundation (approval No. 202302078B0, “Epidemiological Study of Small Intestinal Diseases: A Multi-Center Retrospective Study in Taiwan”), the Research Ethics Review Committee of Far Eastern Memorial Hospital (approval No. 112192-E), the IRB of Tri-Service General Hospital (approval No. C202405111), and the IRB of National Cheng Kung University Hospital (approval No. A-ER-107-319). Written informed consent from patients was waived due to the retrospective design of the study.
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
The datasets analyzed in the study are available from the corresponding author upon reasonable request due to privacy and ethical reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.J.; Roth, E.M.; Feuerstein, J.D.; Poylin, V.Y. Surgery in the age of biologics. Gastroenterol. Rep. 2019, 7, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Beilman, C.L.; Huang, V.W.; Fedorak, D.K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Anti-TNF Therapy Within 2 Years of Crohn’s Disease Diagnosis Improves Patient Outcomes: A Retrospective Cohort Study. Inflamm. Bowel Dis. 2016, 22, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Law, C.C.Y.; Tkachuk, B.; Lieto, S.; Narula, N.; Walsh, S.; Colombel, J.-F.; Ungaro, R.C. Early Biologic Treatment Decreases Risk of Surgery in Crohn’s Disease but not in Ulcerative Colitis: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2023, 30, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Han, M.; Park, S.; Cheon, J.H. Impact of early anti-TNF use on clinical outcomes in Crohn’s disease: A nationwide population-based study. Korean J. Intern. Med. 2020, 35, 1104–1113. [Google Scholar] [CrossRef]
- Schreiber, S.; Reinisch, W.; Colombel, J.F.; Sandborn, W.J.; Hommes, D.W.; Robinson, A.M.; Huang, B.; Lomax, K.G.; Pollack, P.F. Subgroup analysis of the placebo-controlled CHARM trial: Increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J. Crohns Colitis 2013, 7, 213–221. [Google Scholar] [CrossRef]
- Colombel, J.F.; Rutgeerts, P.J.; Sandborn, W.J.; Yang, M.; Camez, A.; Pollack, P.F.; Thakkar, R.B.; Robinson, A.M.; Chen, N.; Mulani, P.M.; et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2014, 12, 414–422.e415. [Google Scholar] [CrossRef]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Billioud, V.; D’Haens, G.; Panaccione, R.; Feagan, B.; Panés, J.; Danese, S.; Schreiber, S.; Ogata, H.; Hibi, T.; et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: Results of an international expert opinion process. Am. J. Gastroenterol. 2012, 107, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.M.; Lee, J.C.; Bond, S.; Dowling, F.; Brezina, B.; Patel, K.V.; Ahmad, T.; Banim, P.J.; Berrill, J.W.; Cooney, R.; et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): A multicentre, open-label randomised controlled trial. Lancet Gastroenterol. Hepatol. 2024, 9, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.; Alsoud, D.; Verstockt, B.; Sabino, J.; Ferrante, M.; Vermeire, S. Adalimumab versus ustekinumab as first-line biological in moderate-to-severe Crohn’s disease: Real-life cohort from a tertiary referral center. Eur. J. Gastroenterol. Hepatol. 2022, 34, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Teresa, V.D.; Rául, O.M.; Marisa, I.; Claudia, H.G.; Esteban, F.V.; Luigi, M.; MR, M.M.; Lilyan, K.C.; Luisa, C.P.; Ángel, P.D.; et al. Effectiveness and safety of ustekinumab in bio-naïve Crohn’s disease patients: A multicentre observational retrospective study. Therap Adv. Gastroenterol. 2023, 16, 17562848231153560. [Google Scholar] [CrossRef]
- Bokemeyer, B.; Plachta-Danielzik, S.; di Giuseppe, R.; Mohl, W.; Teich, N.; Hoffstadt, M.; Schweitzer, A.; von der Ohe, M.; Gauss, A.; Atreya, R.; et al. Real-world Comparative Effectiveness of Ustekinumab vs Anti-TNF in Crohn’s Disease with Propensity Score Adjustment: Induction Phase Results from the Prospective, Observational RUN-CD Study. Inflamm. Bowel Dis. 2023, 29, 1741–1750. [Google Scholar] [CrossRef]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Schreiber, S.; Colombel, J.F.; Bloomfield, R.; Nikolaus, S.; Schölmerich, J.; Panés, J.; Sandborn, W.J. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: An analysis of PRECiSE 2 randomized maintenance trial data. Am. J. Gastroenterol. 2010, 105, 1574–1582. [Google Scholar] [CrossRef]
- Faleck, D.M.; Winters, A.; Chablaney, S.; Shashi, P.; Meserve, J.; Weiss, A.; Aniwan, S.; Koliani-Pace, J.L.; Kochhar, G.; Boland, B.S.; et al. Shorter Disease Duration Is Associated with Higher Rates of Response to Vedolizumab in Patients with Crohn’s Disease But Not Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2497–2505.e2491. [Google Scholar] [CrossRef]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: A multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef]
- Parra, R.S.; Chebli, J.M.F.; Queiroz, N.S.F.; Damião, A.; de Azevedo, M.F.C.; Chebli, L.A.; Bertges, E.R.; Alves Junior, A.J.T.; Ambrogini Junior, O.; da Silva, B.; et al. Long-term effectiveness and safety of ustekinumab in bio-naïve and bio-experienced anti-tumor necrosis factor patients with Crohn’s disease: A real-world multicenter Brazilian study. BMC Gastroenterol. 2022, 22, 199. [Google Scholar] [CrossRef]
- Bessissow, T.; Narula, N.; Ma, C.; In, T.S.H.; Eberg, M.; Karra, K.; Jairath, V. Real-world effectiveness and safety of ustekinumab in bio-naive patients with moderate-to-severe Crohn’s disease: A Canadian multi-center study. Dig. Liver Dis. 2024, 56, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Maida, M.; Ventimiglia, M.; Cottone, M.; Orlando, A. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: A meta-analysis of observational studies. Expert. Opin. Biol. Ther. 2020, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Yung, D.E.; Ma, C.; Pariente, B.; Wils, P.; Eliakim, R.; Ungar, B.; Ben-Horin, S.; Kopylov, U. Effectiveness and safety of Ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig. Liver Dis. 2019, 51, 1232–1240. [Google Scholar] [CrossRef]
- Honap, S.; Meade, S.; Ibraheim, H.; Irving, P.M.; Jones, M.P.; Samaan, M.A. Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- De Boer, N.K.H.; Peyrin-Biroulet, L.; Jharap, B.; Sanderson, J.D.; Meijer, B.; Atreya, I.; Barclay, M.L.; Colombel, J.-F.; Lopez, A.; Beaugerie, L.; et al. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J. Crohn’s Colitis 2017, 12, 610–620. [Google Scholar] [CrossRef]
- Battat, R.; Kopylov, U.; Bessissow, T.; Bitton, A.; Cohen, A.; Jain, A.; Martel, M.; Seidman, E.; Afif, W. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1427–1434.e1422. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohns Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).