Renal Abscess Associated with SGLT2 Inhibitors Administration in Heart Failure Without Other Previous Risk Factors: A Case Report
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
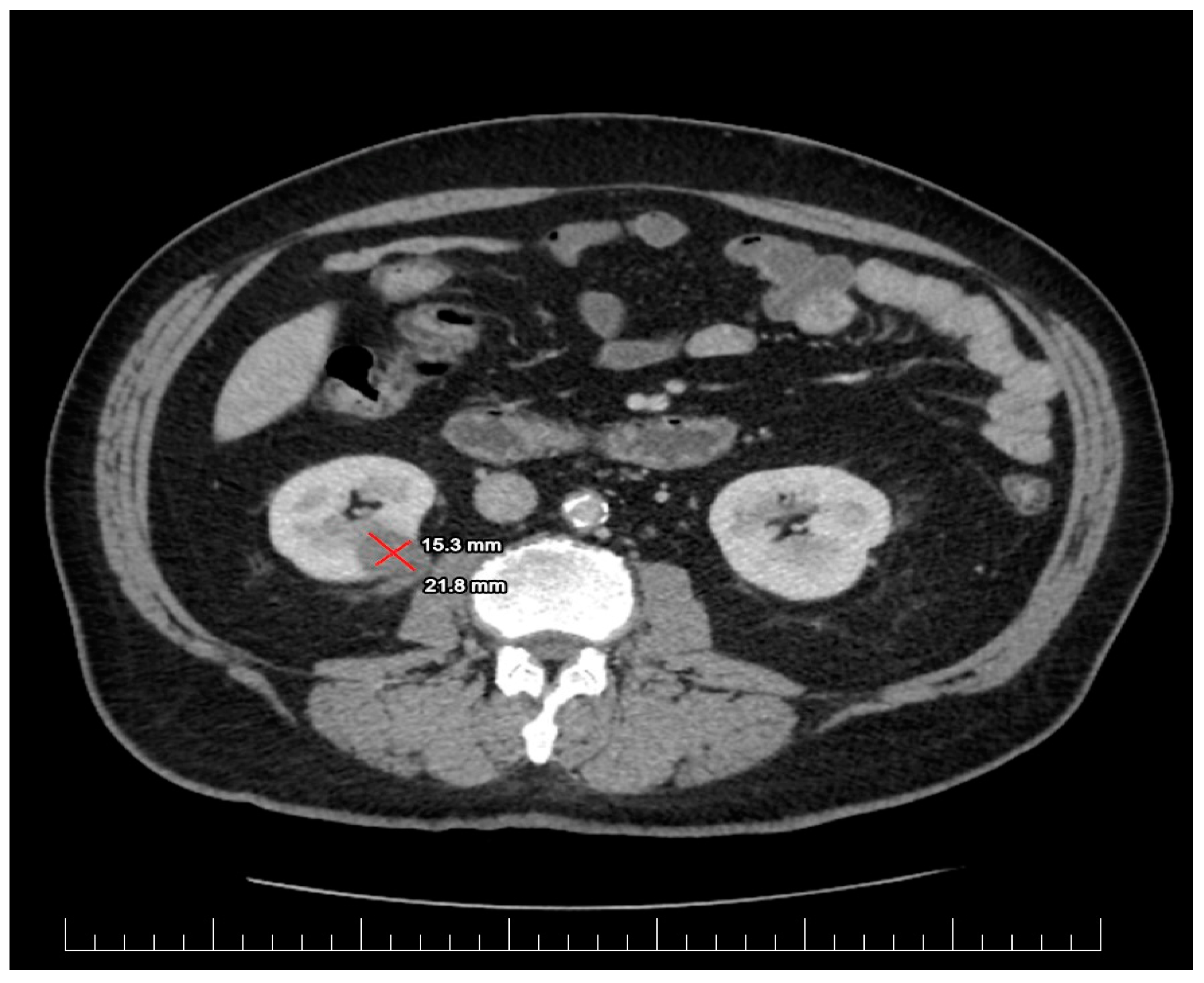
2.1. Diagnostic Assessment
2.2. Management
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Hirai, T.; Koya, D.; Kitada, M. Effects of SGLT2 Inhibitors on Atherosclerosis: Lessons from Cardiovascular Clinical Outcomes in Type 2 Diabetic Patients and Basic Researches. J. Clin. Med. 2021, 11, 137. [Google Scholar] [CrossRef]
- Krepostman, N.; Kramer, H. Lower Urinary Tract Symptoms Should Be Queried When Initiating Sodium Glucose Co-Transporter 2 Inhibitors. Kidney360 2021, 2, 751–754. [Google Scholar] [CrossRef]
- Heerspink, H.J.L. Sodium glucose co-transporter 2 inhibition: A new avenue to protect the kidney. Nephrol. Dial. Transplant. 2019, 34, 2015–2017. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Leslie, S.W.; Rad, J.; Foreman, J. Fournier Gangrene; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chowdhury, T.; Gousy, N.; Bellamkonda, A.; Dutta, J.; Zaman, C.F.; Zakia, U.B.; Tasha, T.; Dutta, P.; Roy, P.D.; Gomez, A.M.; et al. Fournier’s Gangrene: A Coexistence or Consanguinity of SGLT-2 Inhibitor Therapy. Cureus 2022, 14, e27773. [Google Scholar] [CrossRef]
- Coelho, R.F.; Schneider-Monteiro, E.D.; Mesquita, J.L.; Mazzucchi, E.; Marmo Lucon, A.; Srougi, M. Renal and perinephric abscesses: Analysis of 65 consecutive cases. World J. Surg. 2007, 31, 431–436. [Google Scholar] [CrossRef]
- Morgan, W.R.; Nyberg, L.M. Perinephric and intrarenal abscesses. Urology 1985, 1, 529–536. [Google Scholar] [CrossRef]
- Dembry, L.M.; Andriole, V.T. Renal and perirenal abscesses. Infect. Dis. Clin. N. Am. 1997, 11, 663–680. [Google Scholar] [CrossRef]
- Siegel, J.F.; Smith, A.; Moldwin, R. Minimally invasive treatment of renal abscess. J. Urol. 1996, 155, 52–55. [Google Scholar] [CrossRef]
- Rai, R.S.; Karan, S.C.; Kayastha, A. Renal and perinephric abscesses revisited. Med. J. Armed. Forces India 2007, 63, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.V.; Mario, L.A.; McAninch, J.W. Current treatment and outcomes of perinephric abscesses. J. Uro. 2002, 168, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Papamichail, A.; Briasoulis, A.; Loritis, K.; Bourazana, A.; Magouliotis, D.E.; Sarafidis, P.; Stefanidis, I.; Skoularigis, J.; Triposkiadis, F. Heart Failure in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 6105. [Google Scholar] [CrossRef]
- EAU Guidelines on Urological Infections Uroweb. Available online: https://uroweb.org/guidelines/urological-infections (accessed on 16 December 2024).
- Andreea, M.M.; Surabhi, S.; Razvan-Ionut, P.; Lucia, C.; Camelia, N.; Emil, T.; Tiberiu, N.I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Harms or Unexpected Benefits? Medicina 2023, 59, 742. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Furtado, R.H.M.; et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019, 393, 31–39. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Häring, H.U.; Merker, L.; Weimer, M.; Meinicke, T.; Broedl, U.C.; Woerle, H.J.; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014, 37, 1650–1659. [Google Scholar] [CrossRef]
- Häring, H.U.; Merker, L.; Seewaldt-Becker, E.; Weimer, M.; Meinicke, T.; Broedl, U.C.; Woerle, H.J.; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013, 36, 3396–3404. [Google Scholar] [CrossRef] [PubMed]
- Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium-glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Matthews, D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 2099. [Google Scholar] [CrossRef] [PubMed]
- Puckrin, R.; Saltiel, M.P.; Reynier, P.; Azoulay, L.; Yu, O.H.Y.; Filion, K.B. SGLT-2 inhibitors and the risk of infections: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 503–514. [Google Scholar] [CrossRef]
- Echeverria, P.; Saa, J.; Miño, L.D.P.Y. Emphysematous Kidney Related to the Use of Empagliflozin in a Diabetic Woman. AACE Clin. Case Rep. 2023, 9, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Simhadri, P.K.; Vaitla, P.; Sriperumbuduri, S.; Chandramohan, D.; Singh, P.; Murari, U. Sodium-glucose Co-transporter-2 Inhibitors Causing Candida tropicalis Fungemia and Renal Abscess. JCEM Case Rep. 2024, 2, luae010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanagida, K.; Watanabe, D.; Yoshida, T.; Nakagawa, T.; Mizushima, A.; Miura, K.; Ishihara, T. Successful management of renal abscess secondary to diabetes mellitus with surgical treatment and hyperbaric oxygen therapy. Undersea Hyperb. Med. 2024, 51, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Drobac, M.; Vidović, B.; Pavlović, D.; Krajnović, D.; Tadić, I. Herbal Products versus Antibiotics for Urinary Tract Infections-Analysis of Patient Attitudes. J. Herb. Med. 2024, 46, 100892. [Google Scholar] [CrossRef]
- Gorgojo-Martínez, J.J.; Górriz, J.L.; Cebrián-Cuenca, A.; Conde, A.C.; Arribas, M.V. Clinical Recommendations for Managing Genitourinary Adverse Effects in Patients Treated with SGLT-2 Inhibitors: A Multidisciplinary Expert Consensus. J. Clin. Med. 2024, 13, 6509. [Google Scholar] [CrossRef]

| LV-EDD (mm) | 60 |
| LV-ESD (mm) | 50 |
| LV-EDV (mL) | 151 |
| LV-ESV (mL) | 109 |
| LV-EF (%) | 39 |
| LV mass index (g/m2) | 142 |
| LA volume index (mL/m2) | 60 |
| Mitral annular e′ velocity (cm/s) | 4.7 |
| Mitral annular s′ velocity (cm/s) | 4.7 |
| Mitral annular E/e′ ratio | 21 |
| MAPSE (mm) | 8 |
| PASP (mmHg) | 43 |
| Global longitudinal strain (%) | 7.5 |
| Lab | Results | Normal Range |
|---|---|---|
| WBC | 12,100/mm3 (25.1 × 109/L) | 4500–10,000/mm3 (4.5–11.0 × 109/L) |
| Bands | 21% (21) | 0–3% (0–3) |
| Hemoglobin | 15.3 g/dL (6.95 mmol/L) | 13.5–17.5 g/dL (8.38–10.86 mmol/L) |
| Hematocrit | 38.3% (38) | 39–49% (39–49) |
| Platelet count | 144,000/mm3 (144 × 109/L) | 150,000–400,000/mm3 (150–400 × 109/L) |
| BUN | 34 mg/dL (10.7 mmol/L) | 8–25 mg/dL (2.9–8.9 mmol/L) |
| Creatinine | 1.21 mg/dL (314.71 µmol/L) | 0.5–1 mg/dL (74.3–107 µmol/L) |
| Sodium | 140 mmol/L | 135–145 mmol/L (135–145 mmol/L) |
| Potassium | 5.4 mmol/L | 3.4–5.0 mmol/L (3.4–5.0 mmol/L) |
| Bicarbonate | 21 mmol/L | 20–32 mmol/L (20–32 mmol/L) |
| Procalcitonin | 0.03 ng/mL (0.03 µg/L) | 0.05 ng/mL (0.05 µg/L) |
| Lactic acid | 2.1 mmol (2.1 mmol/L) | 0.5–2.2 mmol/L (0.5–2.2 mmol/L) |
| Hemoglobin A1C | 5.4% | <6% |
| Blood glucose C reactive protein | 110 mg/dL (16.04 mmol/L) 20 mg/L | 70–110 mg/dL (3.9–6.1 mmol/L) 0.10–5.00 mg/L |
| Nr | Study Details | Underlying Disease | Bacterial Agent | Renal Manifestation | Treatment | SGLT2 Inhibitor |
|---|---|---|---|---|---|---|
| 1. | (Pablo Echeveria et al., 2023) [27] | T2DM Hypertension Dyslipidemia | E. coli | Emphysematous Pyelonephritis | Nephrectomy | Empaglifozin |
| 2. | (Prathap Kumar Simhadri et al., 2024) [28] | T2DM Hypertension CKD BPH | Candida tropicalis | Renal Abscess | Percutaneous drainage | Canaglifozin |
| 3. | (Kazuki Yanagida et al., 2024) [29] | T2DM | Not Specified | Renal Abscess | Percutaneous drainage Open surgical drainage and HBO2 therapy | Not specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, M.A.; Nicolae, C.; Popescu, R.I.; Rusescu, A.; Paun, N.; Nanea, T.I. Renal Abscess Associated with SGLT2 Inhibitors Administration in Heart Failure Without Other Previous Risk Factors: A Case Report. Biomedicines 2025, 13, 389. https://doi.org/10.3390/biomedicines13020389
Munteanu MA, Nicolae C, Popescu RI, Rusescu A, Paun N, Nanea TI. Renal Abscess Associated with SGLT2 Inhibitors Administration in Heart Failure Without Other Previous Risk Factors: A Case Report. Biomedicines. 2025; 13(2):389. https://doi.org/10.3390/biomedicines13020389
Chicago/Turabian StyleMunteanu, Madalina Andreea, Camelia Nicolae, Razvan Ionut Popescu, Andreea Rusescu, Nicolae Paun, and Tiberiu Ioan Nanea. 2025. "Renal Abscess Associated with SGLT2 Inhibitors Administration in Heart Failure Without Other Previous Risk Factors: A Case Report" Biomedicines 13, no. 2: 389. https://doi.org/10.3390/biomedicines13020389
APA StyleMunteanu, M. A., Nicolae, C., Popescu, R. I., Rusescu, A., Paun, N., & Nanea, T. I. (2025). Renal Abscess Associated with SGLT2 Inhibitors Administration in Heart Failure Without Other Previous Risk Factors: A Case Report. Biomedicines, 13(2), 389. https://doi.org/10.3390/biomedicines13020389







