Downstream Link of Vitamin D Pathway with Inflammation Irrespective of Plasma 25OHD3: Hints from Vitamin D-Binding Protein (DBP) and Receptor (VDR) Gene Polymorphisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Design, and Subjects
2.2. Ethical Issues
2.3. Blood Sample Collection
2.4. Measurements of Plasma Vitamin D and Inflammatory Biomarker Levels
2.4.1. Chemiluminescent Microparticle Immunoassay (CMIA)
2.4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Genotyping of Vitamin D Receptor (VDR) and Vitamin D-Binding Protein (GC) Genes
2.5.1. DNA Extraction
2.5.2. The Tested SNPs of DBP and VDR Genes
2.5.3. Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Description of Study Subjects
3.2. Correlation of Plasma Concentrations of Vitamin D with Inflammatory Biomarkers
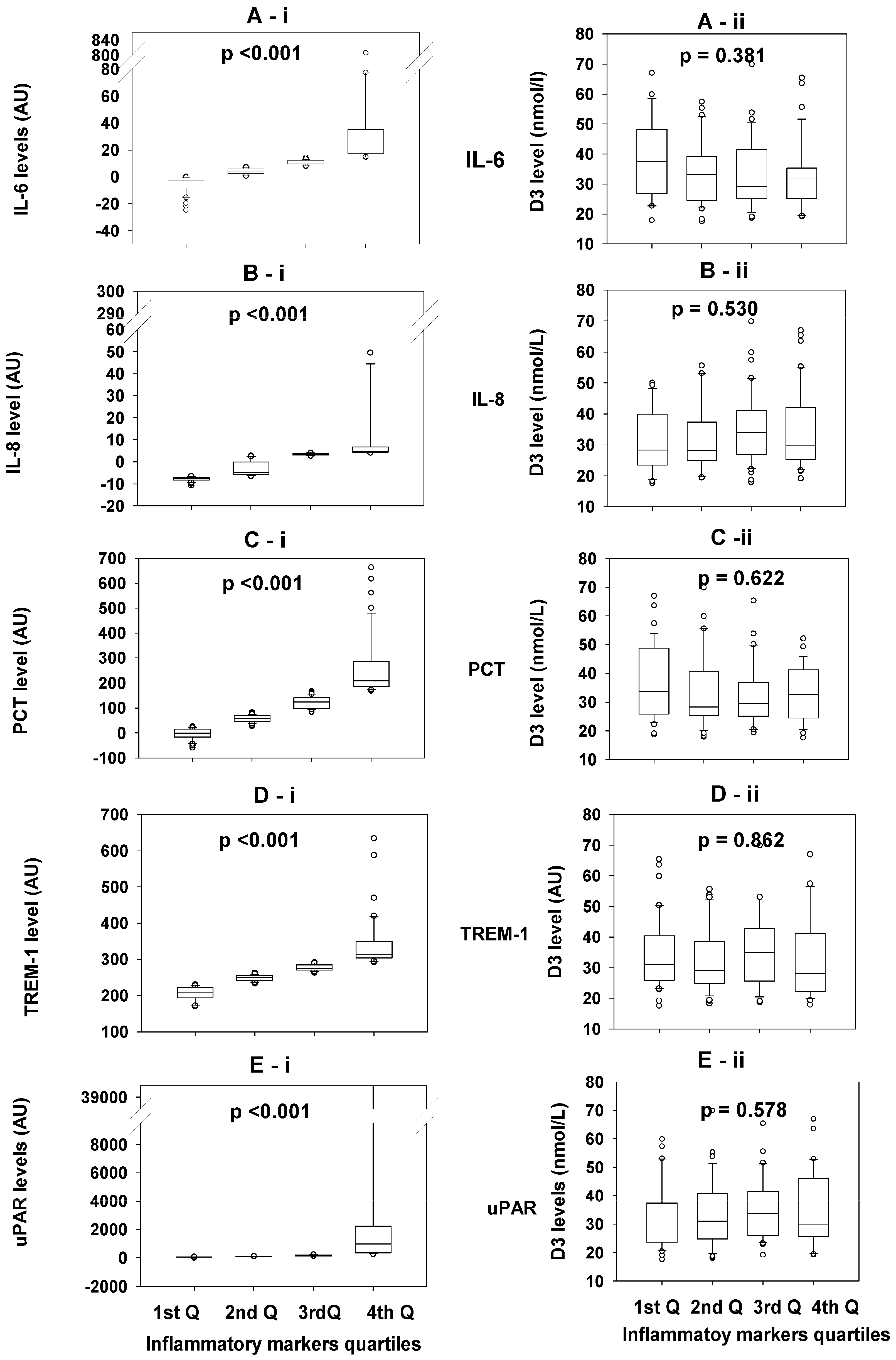
3.3. Comparisons of the 25OHD3 Concentrations Between Quartiles of Each Inflammatory Biomarker
3.4. Comparisons of the Concentrations of the Inflammatory Biomarkers in the Different Quartiles of 25OHD3 Concentrations
3.5. Association of Inflammatory Biomarker Levels with Vitamin D-Binding Protein (DBP) and Vitamin D Receptors (VDR) SNP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Larbi, A. Immunity and Inflammation: From Jekyll to Hyde. Exp. Gerontol. 2018, 107, 98–101. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, X.; He, F.; Huang, X. Inflammatory biomarkers of frailty: A review. Exp. Gerontol. 2023, 179, 112253. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 1992, 13, 719–764. [Google Scholar] [PubMed]
- Querfeld, U. Vitamin D and inflammation. Pediatr. Nephrol. 2013, 28, 605–610. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Nagy, L.; Szanto, A.; Szatmari, I.; Széles, L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiol. Rev. 2012, 92, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; van Geel, N.; Delanghe, J.R. Vitamin D binding protein: A multifunctional protein of clinical importance. Adv. Clin. Chem. 2014, 63, 1–57. [Google Scholar]
- Karlsson, T.; Osmancevic, A.; Jansson, N.; Hulthén, L.; Holmäng, A.; Larsson, I. Increased vitamin D-binding protein and decreased free 25(OH)D in obese women of reproductive age. Eur. J. Nutr. 2014, 53, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; van Baelen, H.; de Moor, P. The measurement of the vitamin D-binding protein in human serum. J. Clin. Endocrinol. Metab. 1977, 45, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norman, A.W. Receptors for 1alpha,25(OH)2D3: Past, present, and future. J. Bone Miner. Res. 1998, 13, 1360–1369. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Schwartz, Z.; Pedrozo, H.; Sylvia, V.L.; Dean, D.D.; Boyan, B.D. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J. Bone Miner. Res. 1998, 13, 1353–1359. [Google Scholar] [CrossRef]
- Ferrer-Suay, S.; Alonso-Iglesias, E.; Tortajada-Girbés, M.; Carrasco-Luna, J.; Codoñer-Franch, P. Vitamin D receptor gene ApaI and FokI polymorphisms and its association with inflammation and oxidative stress in vitamin D sufficient Caucasian Spanish children. Transl. Pediatr. 2021, 10, 103–111. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar]
- Filgueiras, M.S.; Rocha, N.P.; Novaes, J.F.; Bressan, J. Vitamin D status, oxidative stress, and inflammation in children and adolescents: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Almesri, N.; Das, N.S.; Ali, M.E.; Gumaa, K.; Giha, H.A. Gender-Dependent Association of Vitamin D Deficiency with Obesity and Hypercholesterolemia (LDLC) in Adults. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Sater, M.S.; AlDehaini, D.M.B.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Plasma IL-6, TREM1, uPAR, and IL6/IL8 biomarkers increment further witnessing the chronic inflammation in type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2023, 44, 259–269. [Google Scholar] [CrossRef]
- Olliver, M.; Spelmink, L.; Hiew, J.; Meyer-Hoffert, U.; Henriques-Normark, B.; Bergman, P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J. Infect. Dis. 2013, 208, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Wildbaum, G.; Gepstein, V.; Verbitsky, O.; Weisman, Y.; Karin, N.; Eztioni, A. The role of vitamin D receptor in innate and adaptive immunity: A study in hereditary vitamin D-resistant rickets patients. J. Clin. Endocrinol. Metab. 2013, 98, 1685–1693. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice-Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef]
- Graham, C.; Chooniedass, R.; Stefura, W.P.; Lotoski, L.; Lopez, P.; Befus, A.D.; Becker, A.B.; HayGlass, K.T. Stability of pro- and anti-inflammatory immune biomarkers for human cohort studies. J. Transl. Med. 2017, 15, 53. [Google Scholar] [CrossRef]
- Almesri, N.; Das, N.S.; Ali, M.E.; Gumaa, K.; Giha, H.A. Independent associations of polymorphisms in vitamin D binding protein (GC) and vitamin D receptor (VDR) genes with obesity and plasma 25OHD3 levels demonstrate sex dimorphism. Appl. Physiol. Nutr. Metab. 2016, 41, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; O’Halloran, A.M.; Molloy, A.M.; Healy, M.; Bourke, N.; Kenny, R.A. Vitamin D status & associations with inflammation in older adults. PLoS ONE 2023, 18, e0287169. [Google Scholar]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Xiang, Y.; Zhou, Y.; Tang, M.; Cai, J.; Xu, X.; Cui, H.; Feng, Y.; Xu, R. The Association between Serum Level of Vitamin D and Inflammatory Biomarkers in Hospitalized Adult Patients: A Cross-Sectional Study Based on Real-World Data. Mediat. Inflamm. 2024, 2024, 8360538. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.J.; van Leeuwen, H.; Pols, H.A.P. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lee, Y.H. Vitamin D level and risk of systemic lupus erythematosus and rheumatoid arthritis: A Mendelian randomization. Clin. Rheumatol. 2018, 37, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Tangjittipokin, W.; Umjai, P.; Khemaprasit, K.; Charoentawornpanich, P.; Chanprasert, C.; Teerawattanapong, N.; Narkdontri, T.; Santiprabhob, J. Vitamin D pathway gene polymorphisms, vitamin D level, and cytokines in children with type 1 diabetes. Gene 2021, 791, 145691. [Google Scholar] [CrossRef] [PubMed]
- Ponasenko, A.; Sinitskaya, A.; Sinitsky, M.; Khutornaya, M.; Barbarash, O. The Role of Polymorphism in the Endothelial Homeostasis and Vitamin D Metabolism Genes in the Severity of Coronary Artery Disease. Biomedicines 2023, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, C.; Hooker, S.; Mason, T.; Bock, C.H.; Kittles, R.A. Prostate cancer susceptibility Loci identified on chromosome 12 in African Americans. PLoS ONE 2011, 6, e16044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.L.; Gozdzik, A.; Cole, D.E. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Guerini, F.R.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M.; Agliardi, C.; Costa, A.S.; Saulle, I.; Mohammed, A.K.; et al. Vitamin D receptor gene polymorphisms are associated with obesity and inflammosome activity. PLoS ONE 2014, 9, e102141. [Google Scholar] [CrossRef]
- Yousefzadeh, P.; Shapses, S.A.; Wang, X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int. J. Endocrinol. 2014, 2014, 981581. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Values |
|---|---|
| Number | 162 |
| Age (years) | 29.99 ± 5.65 |
| Sex (M/F) | 76/86 |
| BMI (kg/m2) | 30.46 ± 7.921 |
| Weight (kg) | 84.61 ± 23.65 |
| Obesity grades [kg/m2] | |
| Normal [18.5 to 24.9] | 43 |
| Overweight [25 to 29.9] | 41 |
| Obese [30 to 34.9] | 39 |
| Severely obese [≥35] | 39 |
| Lipid profile | |
| TC (mmol/L) | 4.94 ± 1.04 |
| LDL-c (mmol/L) | 3.02 ± 0.96 |
| HDL-c (mmol/L) | 1.19 ± 0.34 |
| TAG (mmol/L) | 1.43 ± 1.29 |
| Glycemic profile | |
| FBG (mmol/L) | 5.54 ± 1.39 |
| HbA1c (mmol/mol) | 36.75 ± 6.07 |
| Inflammatory Biomarker Concentrations (Arbitrary Units) | |||||
|---|---|---|---|---|---|
| D3 level (nmol/L) | 1st Q (n = 32) (17.6–25.2) | 2nd Q (n = 32) (25.3–31.7) | 3rd Q (n = 32) (31.8–41.1) | 4th Q (n = 31) (41.3–69.9) | p-value |
| IL-6 | 10.52, 2.84–14.56 | 10.72, 5.66–15.00 | 8.10, 3.64–18.79 | 6.83, 0.31–11.84 | 0.422 |
| IL-8 | 3.131, −5.670–4.756 | 3.399, −6.769–4.282 | 3.295, −3.119–4.093 | 3.363, −5.287–4.643 | 0.895 |
| PCT | 105.18, 30.44–172.66 | 65.10, 27.13–150.01 | 83.17, 16.82–178.61 | 58.96, 13.05–136.17 | 0.740 |
| TREM-1 | 262.76, 234.87–296.64 | 239.45, 214.21–270.04 | 254.97, 223.48–287.51 | 256.46, 222.00–281.89 | 0.236 |
| uPAR | 114.35, 81.92–209.13 | 116.09, 83.70–282.52 | 127.07, 89.42–185.52 | 143.17, 98.37–243.76 | 0.830 |
| Levels of | IL-6 (AU) | IL-8 (AU) | PCT (AU) | TREM-1 (AU) | uPAR (AU) |
|---|---|---|---|---|---|
| rs2282679 AC vitamin D-binding protein (DBP) | |||||
| AA [18] | 3.52, −3.48–17.13 | −7.07, −8.11–−5.92 | 137.10, 58.48–203.79 | 282.12, 267.43–347.81 | 155.68, 58.55–307.93 |
| AC [63] | 9.98, −0.95–15.00 | 2.69, −6.10–3.85 | 66.91, 16.50–168.71 | 262.47, 230.01–296.13 | 124.06, 87.73–218.78 |
| CC [77] | 7.95, 2.57–12.76 | 3.29, −5.79–4.40 | 82.76, 40.75–175.00 | 254.73, 225.17–281.48 | 128.15, 89.90–226.04 |
| p-values | 0.463 | <0.001 | 0.388 | 0.005 | 0.858 |
| rs4588 CA (DBP) | |||||
| CC [10] | 1.66, −5.42–7.76 | −1.95, −7.38–4.50 | 41.21, 19.40–209.91 | 274.06, 229.55–335.78 | 102.69, 60.61–223.03 |
| CA [56] | 9.07, −0.22–15.06 | 2.69, −5.64–3.78 | 76.13, 13.05–161.86 | 256.77, 230.23–287.62 | 118.66, 93.16–216.08 |
| AA [91] | 8.09, 0.80–14.99 | 2.87, −6.73–4.22 | 95.86, 43.48–170.73 | 263.06, 227.88–291.59 | 136.92, 84.74–227.58 |
| p-values | 0.089 | 0.925 | 0.443 | 0.708 | 0.506 |
| rs7041 GT (DBP) | |||||
| GG [35] | 3.54, −0.16–12.55 | 2.20, −6.77–4.33 | 70.26, 25.69–134.60 | 273.53, 224.16–294.99 | 116.09, 93.05–227.58 |
| GT [75] | 8.15, −0.24–15.00 | 2.69, −5.81–4.05 | 93.78, 18.31–178.10 | 260.76, 232.77–290.27 | 129.82, 89.15–251.30 |
| TT [46] | 8.191, 0.393–15.102 | 2.61, −6.98–4.40 | 96.29, 43.24–177.28 | 263.11, 225.60–289.82 | 114.74, 80.85–218.80 |
| p-values | 0.794 | 0.766 | 0.698 | 0.880 | 0.729 |
| rs2298849 TC (DBP) | |||||
| TT [26] | 3.55, −3.16–18.34 | −6.74, −7.95-−6.07 | 135.93, 70.24–173.61 | 288.71, 248.57–347.47 | 158.44, 84.39–332.99 |
| TC [64] | 7.30, −0.36–15.06 | 2.69, −5.78–4.11 | 93.65, 39.86–180.14 | 258.02, 228.14–283.01 | 130.18, 92.83–222.23 |
| CC [66] | 9.58, 3.56–12.72 | 3.15, −2.70–4.39 | 56.03, 13.36–130.50 | 256.59, 222.89–289.01 | 114.35, 80.25–182.27 |
| p-values | 0.699 | <0.001 | 0.011 | 0.012 | 0.350 |
| rs731236 TC vitamin D receptor (VDR) gene | |||||
| TT [14] | 3.76, −1.67–11.83 | −4.76, −7.92–4.18 | 106.73, 56.22–160.11 | 241.04, 206.19–268.44 | 107.67, 76.5–136.93 |
| TC [67] | 7.59, −0.86–12.89 | −0.25, −6.60–4.10 | 82.76, 28.21–163.70 | 259.48, 241.10–290.27 | 119.13, 84.74–218.78 |
| CC [72] | 9.58, 2.00–16.02 | 3.12, −5.66–4.13 | 79.82, 15.17–177.07 | 269.03, 226.66–302.32 | 129.82, 89.90–234.25 |
| p-values | 0.231 | 0.307 | 0.765 | 0.077 | 0.397 |
| rs12721377AG (VDR) | |||||
| AA [2] | Ex | ||||
| AG [20] | 6.01, −0.88–11.28 | 2.93, −6.42–4.26 | 76.47, 42.68–165.43 | 269.36, 250.62–293.71 | 80.06, 62.67–211.56 |
| GG [132] | 8.106, 0.54–14.56 | 2.64, −6.58–4.11 | 83.17, 23.22–170.23 | 258.23, 226.66–291.29 | 128.67, 92.89–229.57 |
| p-values | 0.389 | 0.601 | 0.965 | 0.191 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sater, M.S.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Downstream Link of Vitamin D Pathway with Inflammation Irrespective of Plasma 25OHD3: Hints from Vitamin D-Binding Protein (DBP) and Receptor (VDR) Gene Polymorphisms. Biomedicines 2025, 13, 385. https://doi.org/10.3390/biomedicines13020385
Sater MS, Malalla ZHA, Ali ME, Giha HA. Downstream Link of Vitamin D Pathway with Inflammation Irrespective of Plasma 25OHD3: Hints from Vitamin D-Binding Protein (DBP) and Receptor (VDR) Gene Polymorphisms. Biomedicines. 2025; 13(2):385. https://doi.org/10.3390/biomedicines13020385
Chicago/Turabian StyleSater, Mai S., Zainab H. A. Malalla, Muhalab E. Ali, and Hayder A. Giha. 2025. "Downstream Link of Vitamin D Pathway with Inflammation Irrespective of Plasma 25OHD3: Hints from Vitamin D-Binding Protein (DBP) and Receptor (VDR) Gene Polymorphisms" Biomedicines 13, no. 2: 385. https://doi.org/10.3390/biomedicines13020385
APA StyleSater, M. S., Malalla, Z. H. A., Ali, M. E., & Giha, H. A. (2025). Downstream Link of Vitamin D Pathway with Inflammation Irrespective of Plasma 25OHD3: Hints from Vitamin D-Binding Protein (DBP) and Receptor (VDR) Gene Polymorphisms. Biomedicines, 13(2), 385. https://doi.org/10.3390/biomedicines13020385







