Cardiac Biomarkers Predict Major Adverse Cardiac Events (MACE) in Incident Haemodialysis Patients: Results from a Global Federated Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
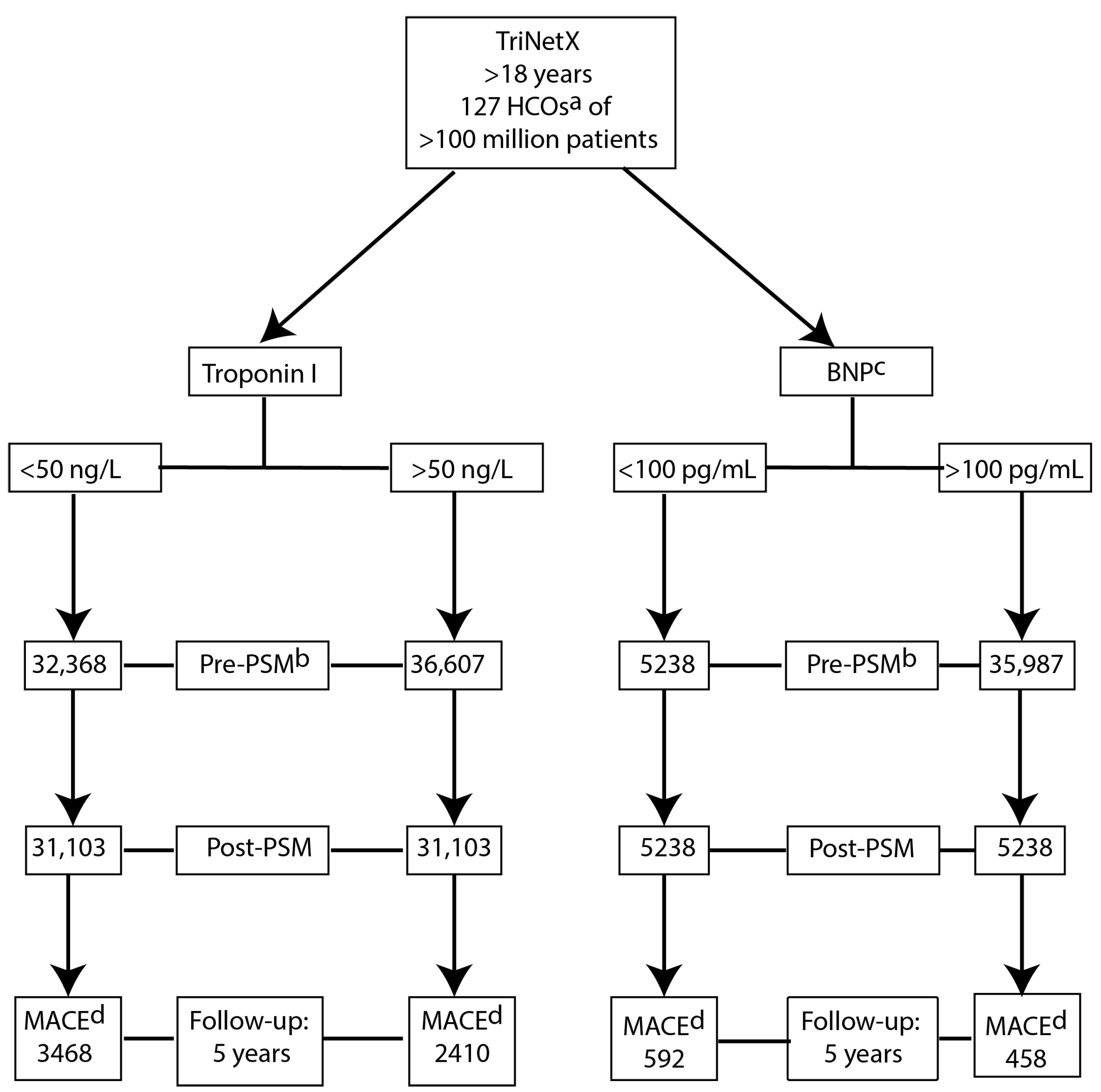
2.2. Data Source, Study Protocol and Patient Selection
2.3. Study Cohort
- Troponin I cohorts stratified as troponin I ≥ 50 ng/L or <50 ng/L.
- B-type natriuretic peptide (BNP) cohorts stratified as BNP ≥ 100 pg/mL or ˂100 pg/mL, respectively.
2.4. Index Event
2.5. Follow-Up and Clinical Outcome
2.6. Covariates
2.7. Statistical Analysis
- Socio-demographic characteristics (age at index, gender and race);
- Baseline CVD comorbidities (glomerular diseases (N08), hypertensive diseases (I10–I16), diabetes mellitus (E08–E13), chronic ischaemic heart disease (I25), cardiovascular procedures, smoking status (F17 nicotine dependence) and Body Mass Index (BMI kg/m2);
- Laboratory data (haemoglobin, serum albumin, alkaline phosphatase, potassium, sodium, calcium, phosphate, cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein-cholesterol and parathyroid hormone);
- Common cardiovascular medications (β-adrenergic receptor antagonists, antilipaemic agents, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, aspirin and clopidogrel);
- Left ventricular ejection fraction (%).
2.8. Exploratory Analysis
- The association of biomarkers and outcomes in patients ≥ 65 years of age.
- The association of biomarkers and outcomes in patients with BMI ≥ 25 kg/m2.
- The association of biomarkers and outcomes in female patients only.
- A combined biomarker approach combining both BNP AND troponin I using the thresholds above.
3. Results
3.1. Demographics
3.1.1. Troponin I
3.1.2. B-Type Natriuretic Peptide (BNP)
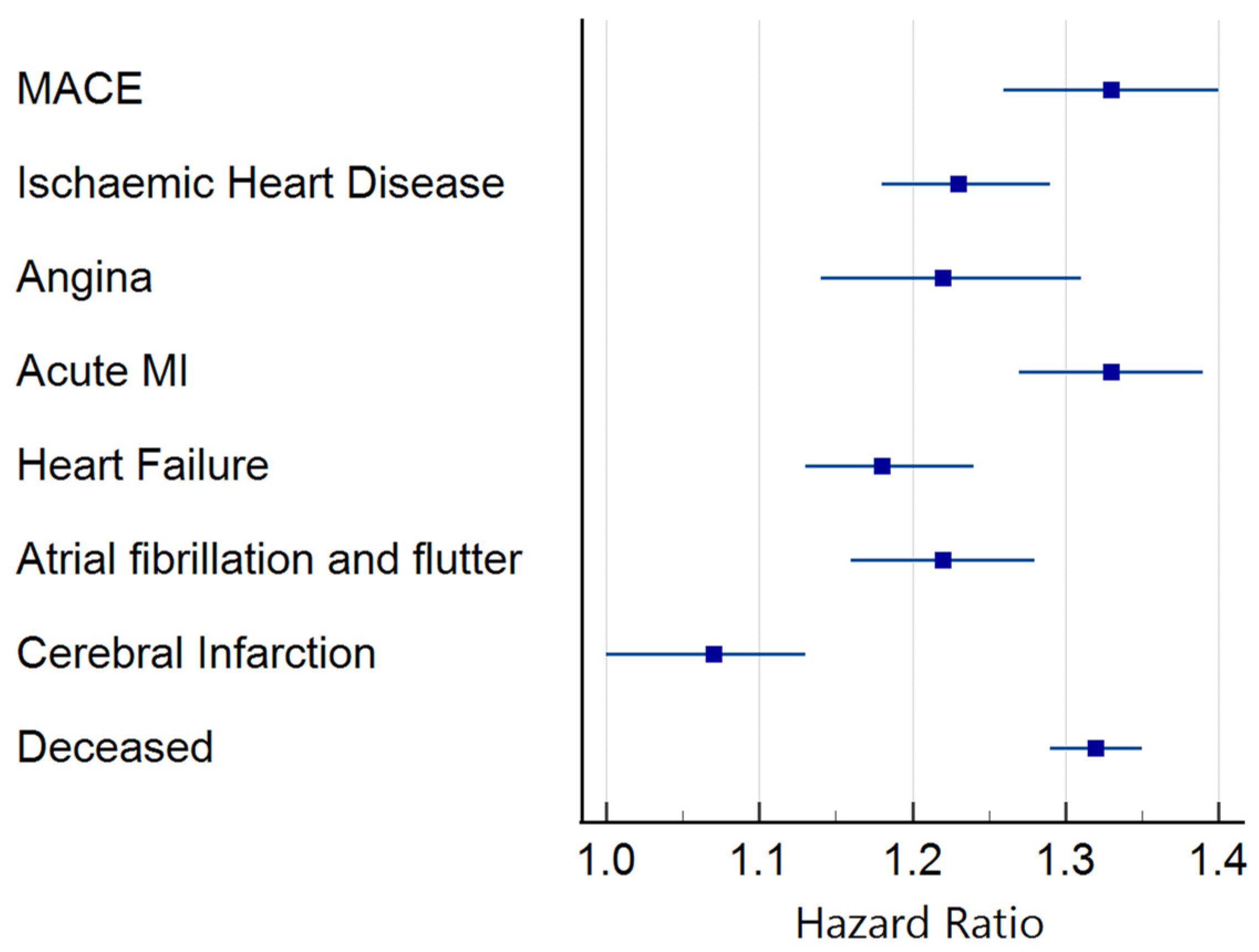
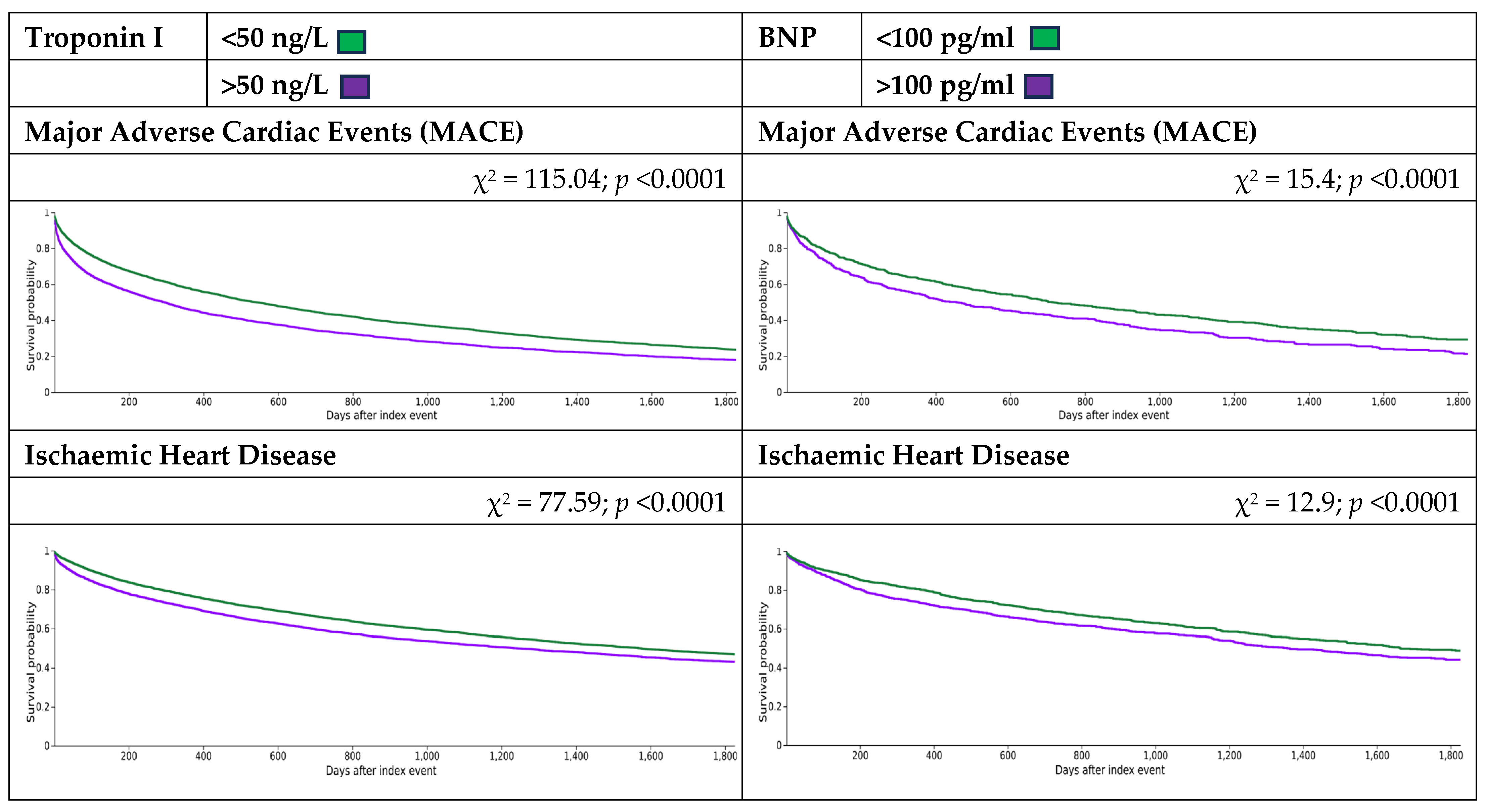
3.2. Clinical Outcomes
3.2.1. Troponin I
3.2.2. B-Type Natriuretic Peptide (BNP)
3.3. Exploratory Analysis
3.3.1. Troponin I: Patients ≥ 65 Years of Age
3.3.2. B-Type Natriuretic Peptide (BNP): Patients ≥ 65 Years of Age
3.3.3. Troponin I: BMI ≥ 25 kg/m2
3.3.4. B-Type Natriuretic Peptide (BNP): BMI ≥ 25 kg/m2
3.3.5. Troponin I: Female Only
3.3.6. B-Type Natriuretic Peptide (BNP): Female Only
3.3.7. Combined Biomarkers (Troponin I and BNP)
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujii, R.; Melotti, R.; Kottgen, A.; Teumer, A.; Giardiello, D.; Pattaro, C. Integrating multiple kidney function markers to predict all-cause and cardiovascular disease mortality: Prospective analysis of 366 758 UK Biobank participants. Clin. Kidney J. 2024, 17, sfae207. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.; Chalmers, J.; Heerspink, H.J.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Bolignano, D.; Mallamaci, F. Left ventricular hypertrophy in chronic kidney disease. In Oxford Textbook of Clinical Nephrology; Turner, N., Ed.; Oxford Academic: Oxford, UK, 2019; pp. 837–852. [Google Scholar]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Stirnadel-Farrant, H.A.; Karaboyas, A.; Cizman, B.; Bieber, B.A.; Kler, L.; Jones, D.; Cobitz, A.R.; Robinson, B.M. Cardiovascular Event Rates Among Hemodialysis Patients Across Geographical Regions-A Snapshot From The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. Rep. 2019, 4, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Lukowsky, L.R.; Mehrotra, R.; Kheifets, L.; Arah, O.A.; Nissenson, A.R.; Kalantar-Zadeh, K. Comparing mortality of peritoneal and hemodialysis patients in the first 2 years of dialysis therapy: A marginal structural model analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bos, W.J.; Bruin, S.; van Olden, R.W.; Keur, I.; Wesseling, K.H.; Westerhof, N.; Krediet, R.T.; Arisz, L.A. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am. J. Kidney Dis. 2000, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Ragosta, M.; Samady, H.; Isaacs, R.B.; Gimple, L.W.; Sarembock, I.J.; Powers, E.R. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am. Heart J. 2004, 147, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin. J. Am. Soc. Nephrol. 2009, 4, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Wayand, D.; Baum, H.; Schatzle, G.; Scharf, J.; Neumeier, D. Cardiac troponin T and I in end-stage renal failure. Clin. Chem. 2000, 46, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Resic, H.; Ajanovic, S.; Kukavica, N.; Masnic, F.; Coric, A. Plasma levels of brain natriuretic peptides and cardiac troponin in hemodialysis patients. Bosn. J. Basic. Med. Sci. 2009, 9, 137–141. [Google Scholar] [CrossRef]
- Snaedal, S.; Barany, P.; Lund, S.H.; Qureshi, A.R.; Heimburger, O.; Stenvinkel, P.; Lowbeer, C.; Szummer, K. High-sensitivity troponins in dialysis patients: Variation and prognostic value. Clin. Kidney J. 2021, 14, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.C.; Whitbourn, R.; MacIsaac, A.; Wilson, A.; Burns, A.; Palmer, S.; Layland, J. High-Sensitivity C-Reactive Protein Is a Predictor of Coronary Microvascular Dysfunction in Patients with Ischemic Heart Disease. Front. Cardiovasc. Med. 2017, 4, 81. [Google Scholar] [CrossRef]
- Palchuk, M.B.; London, J.W.; Perez-Rey, D.; Drebert, Z.J.; Winer-Jones, J.P.; Thompson, C.N.; Esposito, J.; Claerhout, B. A global federated real-world data and analytics platform for research. JAMIA Open 2023, 6, ooad035. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, U.; Palchuk, M.B. Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations. JCO Clin. Cancer Inform. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- WHO. ICD-10: International Statistical Classification of Diseases and Related Health Problems, Tenth revision 2nd ed.; World Health Organisation: Geneva, Switzerland, 2004. [Google Scholar]
- SNOMED CT Stands Systematized Nomenclature of Medicine Clinical Terms. Available online: https://www.nlm.nih.gov/healthit/snomedct/index.html (accessed on 11 December 2023).
- LOINC (Logical Observation Identifiers Names and Codes). Available online: https://loinc.org/kb/release-notes-archive/2.75/#loinc-table (accessed on 15 December 2023).
- Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification#:~:text=In%20the%20Anatomical%20Therapeutic%20Chemical,therapeutic%2C%20pharmacological%20and%20chemical%20properties. (accessed on 8 December 2023).
- Eriguchi, M.; Tsuruya, K.; Lopes, M.; Bieber, B.; McCullough, K.; Pecoits-Filho, R.; Robinson, B.; Pisoni, R.; Kanda, E.; Iseki, K.; et al. Routinely measured cardiac troponin I and N-terminal pro-B-type natriuretic peptide as predictors of mortality in haemodialysis patients. ESC Heart Fail 2022, 9, 1138–1151. [Google Scholar] [CrossRef]
- Kampmann, J.; Heaf, J.; Backer Mogensen, C.; Pedersen, A.K.; Granhoj, J.; Mickley, H.; Brandt, F. Troponin Cut-Offs for Acute Myocardial Infarction in Patients with Impaired Renal Function-A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 276. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, D.; Shen, D.; Zhang, L.; Wang, Y.; Sun, H.; Ma, Y. Brain Natriuretic Peptide as the long-term cause of mortality in patients with cardiovascular disease: A retrospective cohort study. Int. J. Clin. Exp. Med. 2015, 8, 16364–16368. [Google Scholar]
- Guo, S.; Fraser, M.W. Propensity Score Analysis: Statistical Methods and Applications; SAGE: London, UK, 2014. [Google Scholar]
- Wood, G.N.; Keevil, B.; Gupta, J.; Foley, R.; Bubtana, A.; McDowell, G.; Ackrill, P. Serum troponin T measurement in patients with chronic renal impairment predicts survival and vascular disease: A 2 year prospective study. Nephrol. Dial. Transplant. 2003, 18, 1610–1615. [Google Scholar] [CrossRef][Green Version]
- Hayashi, T.; Kimura, T.; Yasuda, K.; Sasaki, K.; Obi, Y.; Rakugi, H.; Isaka, Y. Cardiac troponin T elevation at dialysis initiation is associated with all-cause and cardiovascular mortality on dialysis in patients without diabetic nephropathy. Clin. Exp. Nephrol. 2017, 21, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Adams-Huet, B.; Li, X.; Colbert, G.; Jain, N.; de Lemos, J.A.; Hedayati, S.S. Effect Modification of Chronic Kidney Disease on the Association of Circulating and Imaging Cardiac Biomarkers With Outcomes. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Kruzan, R.M.; Herzog, C.A.; Wu, A.; Sang, Y.; Parekh, R.S.; Matsushita, K.; Hwang, S.; Cheng, A.; Coresh, J.; Powe, N.R.; et al. Association of NTproBNP and cTnI with outpatient sudden cardiac death in hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study. BMC Nephrol. 2016, 17, 18. [Google Scholar] [CrossRef]
- Otsuka, K.; Nakanishi, K.; Shimada, K.; Nakamura, H.; Inanami, H.; Nishioka, H.; Fujimoto, K.; Kasayuki, N.; Yoshiyama, M. Ankle-brachial index, arterial stiffness, and biomarkers in the prediction of mortality and outcomes in patients with end-stage kidney disease. Clin. Cardiol. 2019, 42, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Zhang, N.; Xie, X.; Ding, M.; Guo, H.; Li, F.; Wang, X. High-Sensitive Cardiac Troponin T for Prediction of Cardiovascular Outcomes in Stable Maintenance Hemodialysis Patients: A 3-Year Prospective Study. Kidney Blood Press. Res. 2021, 46, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Zager, P.G.; Sozio, S.M.; Grams, M.E.; Jaar, B.G.; Christenson, R.H.; Boulware, L.E.; Parekh, R.S.; Powe, N.R.; Coresh, J. Troponin I and NT-proBNP and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 443–451. [Google Scholar] [CrossRef]
- Kanderian, A.S.; Francis, G.S. Cardiac troponins and chronic kidney disease. Kidney Int. 2006, 69, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Unlu, S.; Sahinarslan, A.; Sezenoz, B.; Uludag, O.M.; Gokalp, G.; Seckin, O.; Arinsoy, S.T.; Gulbahar, O.; Boyaci, N.B. High-sensitive troponin T increase after hemodialysis is associated with left ventricular global longitudinal strain and ultrafiltration rate. Cardiol. J. 2020, 27, 376–383. [Google Scholar] [CrossRef]
- Quiroga, B.; Villaverde, M.; Abad, S.; Vega, A.; Reque, J.; Lopez-Gomez, J.M. Diastolic dysfunction and high levels of new cardiac biomarkers as risk factors for cardiovascular events and mortality in hemodialysis patients. Blood Purif. 2013, 36, 98–106. [Google Scholar] [CrossRef]
- Boeckel, J.N.; Palapies, L.; Zeller, T.; Reis, S.M.; Von Jeinsen, B.; Tzikas, S.; Bickel, C.; Baldus, S.; Blankenberg, S.; Munzel, T.; et al. Estimation of values below the limit of detection of a contemporary sensitive troponin I assay improves diagnosis of acute myocardial infarction. Clinical Chemistry 2015, 61, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Wanner, C.; Krane, V.; Kraus, D.; Genser, B.; Scharnagl, H.; Marz, W.; Drechsler, C. Prognostic Value of High-Sensitivity Versus Conventional Cardiac Troponin T Assays Among Patients With Type 2 Diabetes Mellitus Undergoing Maintenance Hemodialysis. Am. J. Kidney Dis. 2018, 71, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Hessel, M.H.; Atsma, D.E.; van der Valk, E.J.; Bax, W.H.; Schalij, M.J.; van der Laarse, A. Release of cardiac troponin I from viable cardiomyocytes is mediated by integrin stimulation. Pflugers Arch. 2008, 455, 979–986. [Google Scholar] [CrossRef]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002, 106, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Sharkey, S.W.; Hoeft, P.; Skeate, R.; Voss, E.; Dahlmeier, B.A.; Preese, L.M. Prognostic value of serum cardiac troponin I and T in chronic dialysis patients: A 1-year outcomes analysis. Am. J. Kidney Dis. 1997, 29, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Reyes, M.J.; Mon, C.; Heras, M.; Guevara, P.; Garcia, M.C.; Sanchez, R.; Velasco, S.; Alvarez-Ude, F. Predictive value of troponin T levels for ischemic heart disease and mortality in patients on hemodialysis. J. Nephrol. 2004, 17, 721–727. [Google Scholar]
- Taylor, C.J.; Lay-Flurrie, S.L.; Ordonez-Mena, J.M.; Goyder, C.R.; Jones, N.R.; Roalfe, A.K.; Hobbs, F.R. Natriuretic peptide level at heart failure diagnosis and risk of hospitalisation and death in England 2004-2018. Heart 2022, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- McDowell, G.; Shaw, C.; Buchanan, K.D.; Nicholls, D.P. The natriuretic peptide family. Eur. J. Clin. Investig. 1995, 25, 291–298. [Google Scholar] [CrossRef]
- Kawagoe, C.; Sato, Y.; Toida, T.; Nakagawa, H.; Yamashita, Y.; Fukuda, A.; Iwatsubo, S.; Fujimoto, S. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren. Fail. 2018, 40, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schwermer, K.; Hoppe, K.; Radziszewska, D.; Kłysz, P.; Sawatiuk, P.; Nealis, J.; Kałużna, M.; Kaczmarek, J.; Baum, E.; Lind-holm, B.; et al. N-terminal pro-B-type natriuretic peptide as a marker of hypervolemia and predictor of increased mortality in patients on hemodialysis. Pol. Arch. Med. Wewn. 2015, 125, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Voroneanu, L.; Siriopol, D.; Apetrii, M.; Hogas, S.; Onofriescu, M.; Nistor, I.; Kanbay, M.; Dumea, R.; Cusai, S.; Cianga, P.; et al. Prospective Validation of a Screening Biomarker Approach Combining Amino-Terminal Pro-Brain Natriuretic Peptide With Galectin-3 Predicts Death and Cardiovascular Events in Asymptomatic Hemodialysis Patients. Angiology 2018, 69, 449–455. [Google Scholar] [CrossRef]
- Obokata, M.; Sunaga, H.; Ishida, H.; Ito, K.; Ogawa, T.; Ando, Y.; Kurabayashi, M.; Negishi, K. Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients. Am. Heart J. 2016, 179, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Campbell, R.T.; Mooney, L.; Kimenai, D.M.; Hayward, C.; Campbell, A.; Porteous, D.; Mills, N.L.; Lang, N.N.; Petrie, M.C.; et al. Reference Ranges for NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and Risk Factors for Higher NT-proBNP Concentrations in a Large General Population Cohort. Circ. Heart Fail. 2022, 15, e009427. [Google Scholar] [CrossRef] [PubMed]
- Lowry, M.T.H.; Doudesis, D.; Wereski, R.; Kimenai, D.M.; Tuck, C.; Ferry, A.V.; Bularga, A.; Taggart, C.; Lee, K.K.; Chapman, A.R.; et al. Influence of Age on the Diagnosis of Myocardial Infarction. Circulation 2022, 146, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Ha Manh, T.; Do Anh, D.; Le Viet, T. Effect of body mass index on N-terminal pro-brain natriuretic peptide values in patients with heart failure. Egypt. Heart J. 2023, 75, 75. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Szummer, K.; Barany, P.; Heimburger, O.; Magin, H.; Almquist, T.; Uhlin, F.; Dekker, F.W.; Wanner, C.; Jager, K.J.; et al. Association Between Renal Function and Troponin T Over Time in Stable Chronic Kidney Disease Patients. J. Am. Heart Assoc. 2019, 8, e013091. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Herzog, C.A.; Love, S.A.; Cao, J.; Hu, Y.; Wu, A.H.; Gilbertson, D.; Brunelli, S.M.; Young, A.; Ler, R.; et al. Prognostic Value of Serial Changes in High-Sensitivity Cardiac Troponin I and T over 3 Months Using Reference Change Values in Hemodialysis Patients. Clin. Chem. 2016, 62, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.L.; Grebel, L.E.; Butler, C.C.; Hood, K.; Verheij, T.J.M.; Little, P. Comparison between treatment effects in a randomised controlled trial and an observational study using propensity scores in primary care. Br. J. Gen. Pract. 2017, 67, e643–e649. [Google Scholar] [CrossRef]
- Chazot, C.; Rozes, M.; Vo-Van, C.; Deleaval, P.; Hurot, J.M.; Lorriaux, C.; Mayor, B.; Zaoui, E.; Jean, G. Brain Natriuretic Peptide Is a Marker of Fluid Overload in Incident Hemodialysis Patients. Cardiorenal Med. 2017, 7, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Ventura, M.D.; Avila-Diaz, M.; Hinojosa-Heredia, H.; Mendez-Duran, A.; Cueto-Manzano, A.; Cisneros, A.; Ramos, A.; Madonia-Juseino, C.; Belio-Caro, F.; et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol. Dial. Transplant. 2010, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]


| (a) | ||||
|---|---|---|---|---|
| Troponin I | ||||
| ≥50 ng/L | <50 ng/L | p-Value | Std Diff. | |
| Sample size | 31,103 | 31,103 | ||
| Demographics | ||||
| Age at index Mean ± SD | 61.4 ± 14.1 | 61.3 ± 14.2 | 0.453 | 0.006 |
| Male N (%) | 17,034 (54.8%) | 17,103 (55.0%) | 0.578 | 0.004 |
| White N (%) | 13,722 (44.1%) | 13,561 43.6% | 0.193 | 0.010 |
| American Indian or Alaska Native N (%) | 156 (0.5%) | 170 (0.5%) | 0.437 | 0.006 |
| Native Hawaiian or other Pacific Islander N (%) | 501 (1.6%) | 548 (1.8%) | 0.143 | 0.012 |
| Black or African American N (%) | 12,167 (39.1%) | 12,136 (39.0%) | 0.799 | 0.002 |
| Asian N (%) | 1459 (4.7%) | 1477 (4.7%) | 0.734 | 0.003 |
| Other race N (%) | 1052 (3.4%) | 1082 (3.5%) | 0.509 | 0.005 |
| Co-morbidities | ||||
| Diabetes mellitus N (%) | 21,002 (67.5%) | 20,901 (67.2%) | 0.388 | 0.007 |
| Glomerular disorders N (%) | 3076 (9.9%) | 2924 (9.4%) | 0.039 | 0.017 |
| Hypertensive diseases N (%) | 27,136 (87.2%) | 27,067 (87.0%) | 0.409 | 0.007 |
| Smoker N (%) | 7282 (23.4%) | 7178 (23.1%) | 0.324 | 0.008 |
| Chronic ischemic heart disease N (%) | 17,251 (55.5%) | 15,448 (49.7%) | <0.001 | 0.116 |
| Cardiovascular procedures N (%) | 27,295 (87.8%) | 26,835 (86.3%) | <0.001 | 0.044 |
| Medications | ||||
| Beta-adrenergic receptor antagonists N (%) | 24,448 (78.6%) | 24,908 (80.1%) | <0.001 | 0.037 |
| Antilipaemic agents N (%) | 19,711 (63.4%) | 19,704 (63.4%) | 0.954 | <0.001 |
| ACE inhibitors N (%) | 12,995 (41.8%) | 13,268 (42.7%) | 0.027 | 0.018 |
| Angiotensin II receptor antagonists N (%) | 8957 (28.8%) | 9291 (29.9%) | 0.003 | 0.024 |
| Aspirin N (%) | 19,799 (63.7%) | 19,259 (61.9%) | <0.001 | 0.036 |
| Clopidogrel N (%) | 7738 (24.9%) | 6970 (22.4%) | <0.001 | 0.058 |
| Laboratory Results | ||||
| Haemoglobin (g/dL) | 9.7 ± 1.9 | 9.6 ± 1.8 | 0.094 | 0.014 |
| Albumin (g/dL) | 3.2 ± 0.7 | 3.2 ± 0.7 | 0.806 | 0.002 |
| Alkaline phosphatase (U/L) | 127.5 ± 121.6 | 124.5 ± 110.5 | 0.002 | 0.026 |
| Potassium (mmol/L) | 4.4 ± 0.7 | 4.4 ± 0.7 | 0.001 | 0.026 |
| Sodium (mmol/L) | 136.4 ± 4.1 | 136.5 ± 3.9 | 0.001 | 0.027 |
| Calcium (mg/dL) | 8.6 ± 0.9 | 8.6 ± 0.9 | 0.174 | 0.011 |
| Phosphate (mg/dL) | 4.8 ± 1.8 | 4.6 ± 1.7 | <0.001 | 0.107 |
| Cholesterol (mg/dL) | 149.3 ± 55.7 | 150.8 ± 55.8 | 0.009 | 0.027 |
| HDL-C (mg/dl) | 42.1 ± 17.3 | 42.6 ± 17.5 | 0.007 | 0.028 |
| LDL-C (mg/dL) | 80.1 ± 42.7 | 80.5 ± 43.2 | 0.427 | 0.008 |
| PTH (pg/mL) | 347.5 ± 371.76 | 334.6 ± 353.6 | 0.002 | 0.036 |
| BMI (kg/m2) | 28.7 ± 7.1 | 28.8 ± 7.2 | 0.546 | 0.008 |
| LVEF (%) | 51.1 ± 15.3 | 54.3 ± 14.0 | <0.001 | 0.217 |
| (b) | ||||
| BNP | ||||
| ≥100 pg/mL | <100 pg/mL | p-Value | Std diff. | |
| Sample size | 5238 | 5238 | ||
| Demographics | ||||
| Age at index Mean ± SD | 57.5 ± 14.5 | 57.2 ± 14.8 | 0.341 | 0.019 |
| Male N (%) | 2853 (54.5%) | 2854 (54.5%) | 0.984 | <0.001 |
| White N (%) | 1943 (37.1%) | 1935 (36.9%) | 0.871 | 0.003 |
| American Indian or Alaska Native N (%) | 11 (0.2%) | 15 (0.3%) | 0.432 | 0.015 |
| Native Hawaiian or other Pacific Islander N (%) | 73 (1.4%) | 77 (1.5%) | 0.742 | 0.006 |
| Black or African American N (%) | 2700 (51.5%) | 2674 (51.1%) | 0.611 | 0.010 |
| Asian N (%) | 164 (3.1%) | 170 (3.2%) | 0.739 | 0.007 |
| Other race N (%) | 105 (2.0%) | 112 (2.1%) | 0.631 | 0.009 |
| Co-morbidities | ||||
| Diabetes mellitus N (%) | 3551 (67.8%) | 3489 (66.6%) | 0.197 | 0.025 |
| Glomerular disorders N (%) | 446 (8.5%) | 466 (8.9%) | 0.488 | 0.014 |
| Hypertensive diseases N (%) | 4748 (90.6%) | 4693 (89.6%) | 0.072 | 0.035 |
| Smoker N (%) | 1301 (24.8%) | 1301 (24.8%) | 1 | <0.001 |
| Chronic ischemic heart disease N (%) | 2622 (50.1%) | 2347 (44.8%) | <0.001 | 0.105 |
| Cardiovascular procedures N (%) | 4716 (90.0%) | 4700 (89.7%) | 0.604 | 0.010 |
| Medications | ||||
| Beta-adrenergic receptor antagonists N (%) | 4380 (83.6%) | 4281 (81.7%) | 0.011 | 0.050 |
| Antilipaemic agents N (%) | 3294 (62.9%) | 3282 (62.7%) | 0.808 | 0.005 |
| ACE inhibitors N (%) | 2242 (42.8%) | 2218 (42.3%) | 0.635 | 0.009 |
| Angiotensin II receptor antagonists N (%) | 1611 (30.8%) | 1653 (31.6%) | 0.376 | 0.017 |
| Aspirin N (%) | 3344 (63.8%) | 3274 (62.5%) | 0.156 | 0.028 |
| Clopidogrel N (%) | 1142 (21.8%) | 1052 (20.1%) | 0.031 | 0.042 |
| Laboratory Results | ||||
| Haemoglobin (g/dL) | 9.5 ± 1.8 | 9.9 ± 1.9 | <0.001 | 0.242 |
| Albumin (g/dL) | 3.1 ± 0.7 | 3.2 ± 0.7 | <0.001 | 0.150 |
| Alkaline phosphatase (U/L) | 129.0 ± 126.9 | 120.2 ± 101.3 | <0.001 | 0.077 |
| Potassium (mmol/L) | 4.4 ± 0.7 | 4.4 ± 0.7 | 0.474 | 0.014 |
| Sodium (mmol/L) | 136.7 ± 4.0 | 136.8 ± 3.9 | 0.034 | 0.042 |
| Calcium (mg/dL) | 8.7 ± 0.9 | 8.8 ± 0.9 | <0.001 | 0.118 |
| Phosphate (mg/dL) | 4.7 ± 1.8 | 4.7 ± 1.8 | 0.144 | 0.030 |
| Cholesterol (mg/dL) | 150.0 ± 55.3 | 152.6 ± 55.4 | 0.050 | 0.047 |
| HDL-C (mg/dl) | 42.8 ± 17.7 | 41.8 ± 17.2 | 0.026 | 0.053 |
| LDL-C (mg/dL) | 80.9 ± 44.7 | 81.4 ± 44.0 | 0.663 | 0.010 |
| PTH (pg/mL) | 367.4 ± 391.8 | 341.6 ± 360.6 | 0.011 | 0.069 |
| BMI (kg/m2) | 29.1 ± 7.4 | 30.1 ± 7.9 | <0.001 | 0.130 |
| LVEF (%) | 51.6 ± 15.5 | 56.6 ± 13.6 | <0.001 | 0.339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, E.M.; Buckley, B.J.R.; Austin, P.; Lip, G.Y.H.; Rao, A.; McDowell, G. Cardiac Biomarkers Predict Major Adverse Cardiac Events (MACE) in Incident Haemodialysis Patients: Results from a Global Federated Database. Biomedicines 2025, 13, 367. https://doi.org/10.3390/biomedicines13020367
Davies EM, Buckley BJR, Austin P, Lip GYH, Rao A, McDowell G. Cardiac Biomarkers Predict Major Adverse Cardiac Events (MACE) in Incident Haemodialysis Patients: Results from a Global Federated Database. Biomedicines. 2025; 13(2):367. https://doi.org/10.3390/biomedicines13020367
Chicago/Turabian StyleDavies, Elin Mitford, Benjamin J. R. Buckley, Philip Austin, Gregory Y. H. Lip, Anirudh Rao, and Garry McDowell. 2025. "Cardiac Biomarkers Predict Major Adverse Cardiac Events (MACE) in Incident Haemodialysis Patients: Results from a Global Federated Database" Biomedicines 13, no. 2: 367. https://doi.org/10.3390/biomedicines13020367
APA StyleDavies, E. M., Buckley, B. J. R., Austin, P., Lip, G. Y. H., Rao, A., & McDowell, G. (2025). Cardiac Biomarkers Predict Major Adverse Cardiac Events (MACE) in Incident Haemodialysis Patients: Results from a Global Federated Database. Biomedicines, 13(2), 367. https://doi.org/10.3390/biomedicines13020367







