Effects of Teriparatide and Alendronate on Functional Recovery from Spinal Cord Injury and Postinjury Bone Loss
Abstract
1. Introduction
2. Materials and Methods
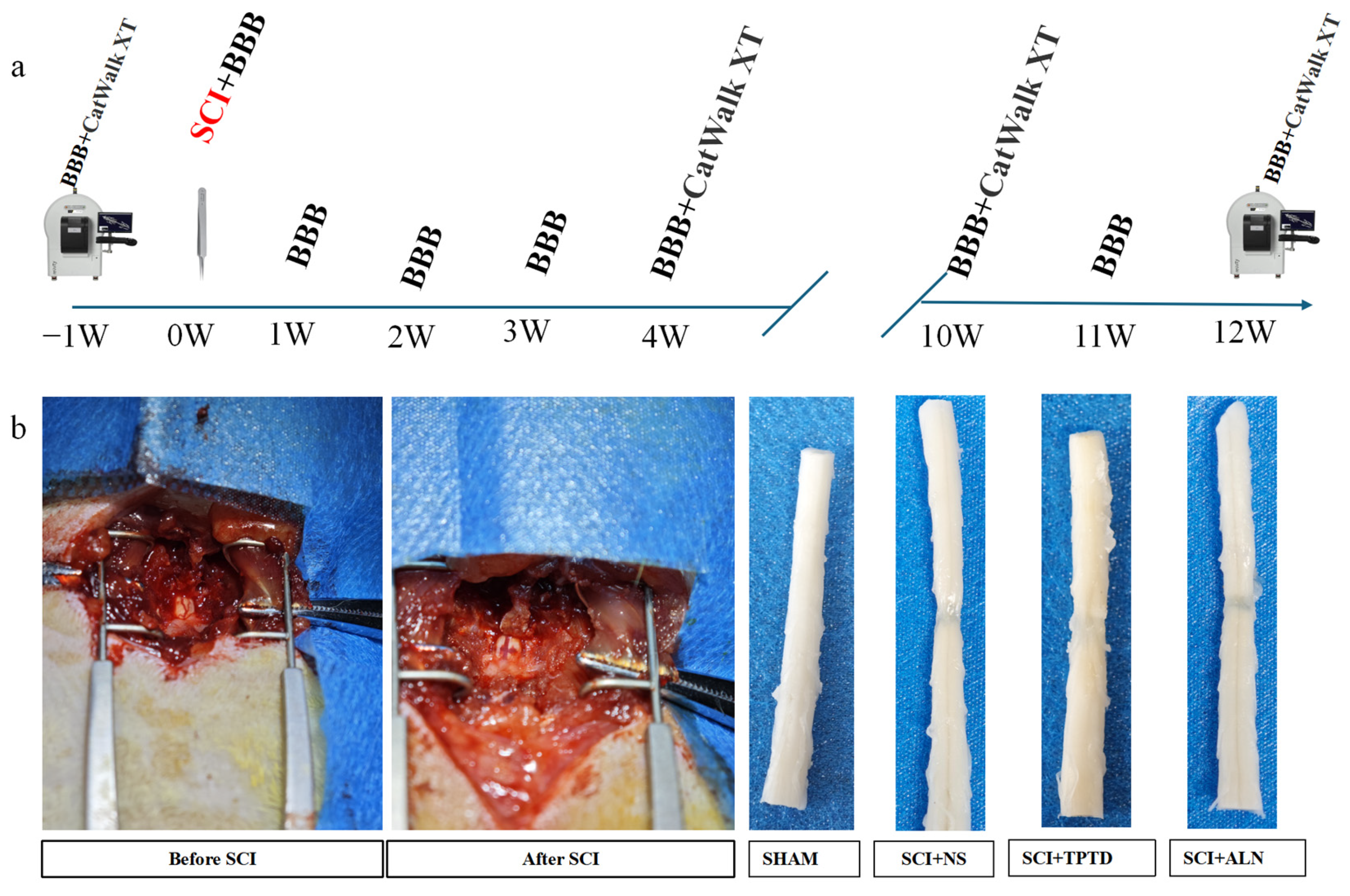
2.1. Animals and Experimental Design
2.2. SCI Modeling
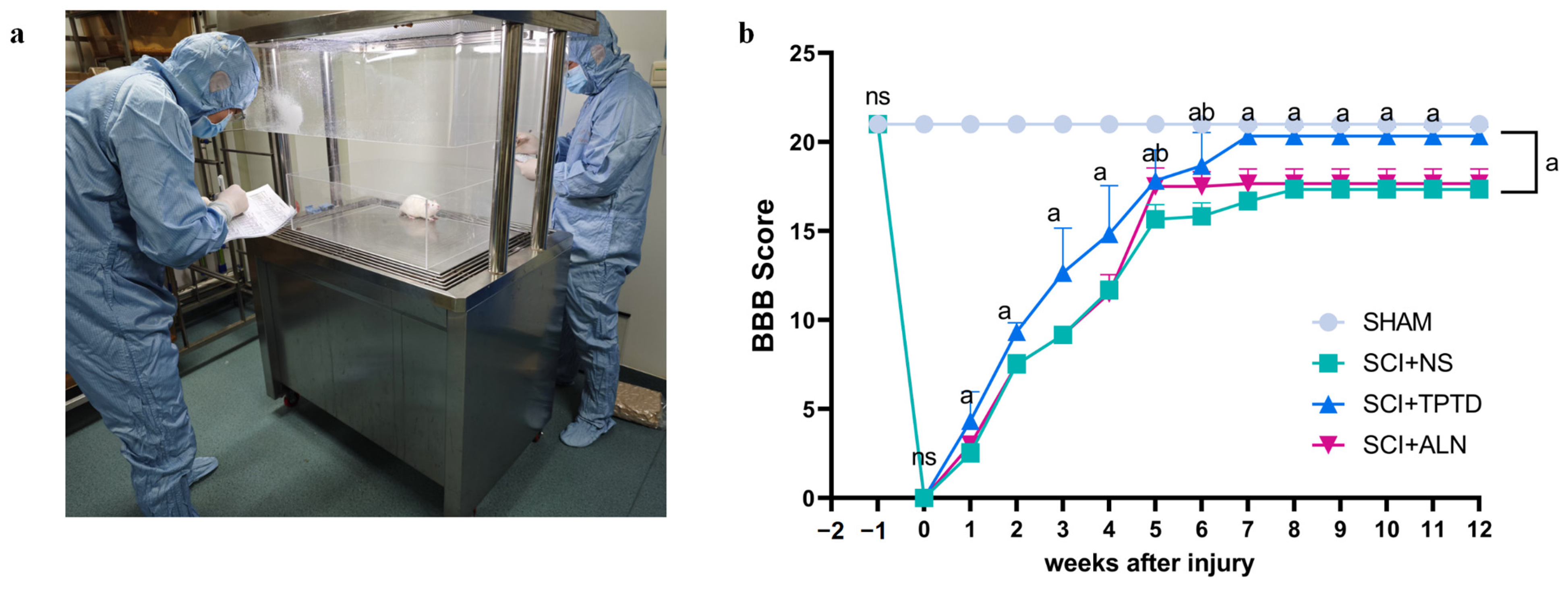
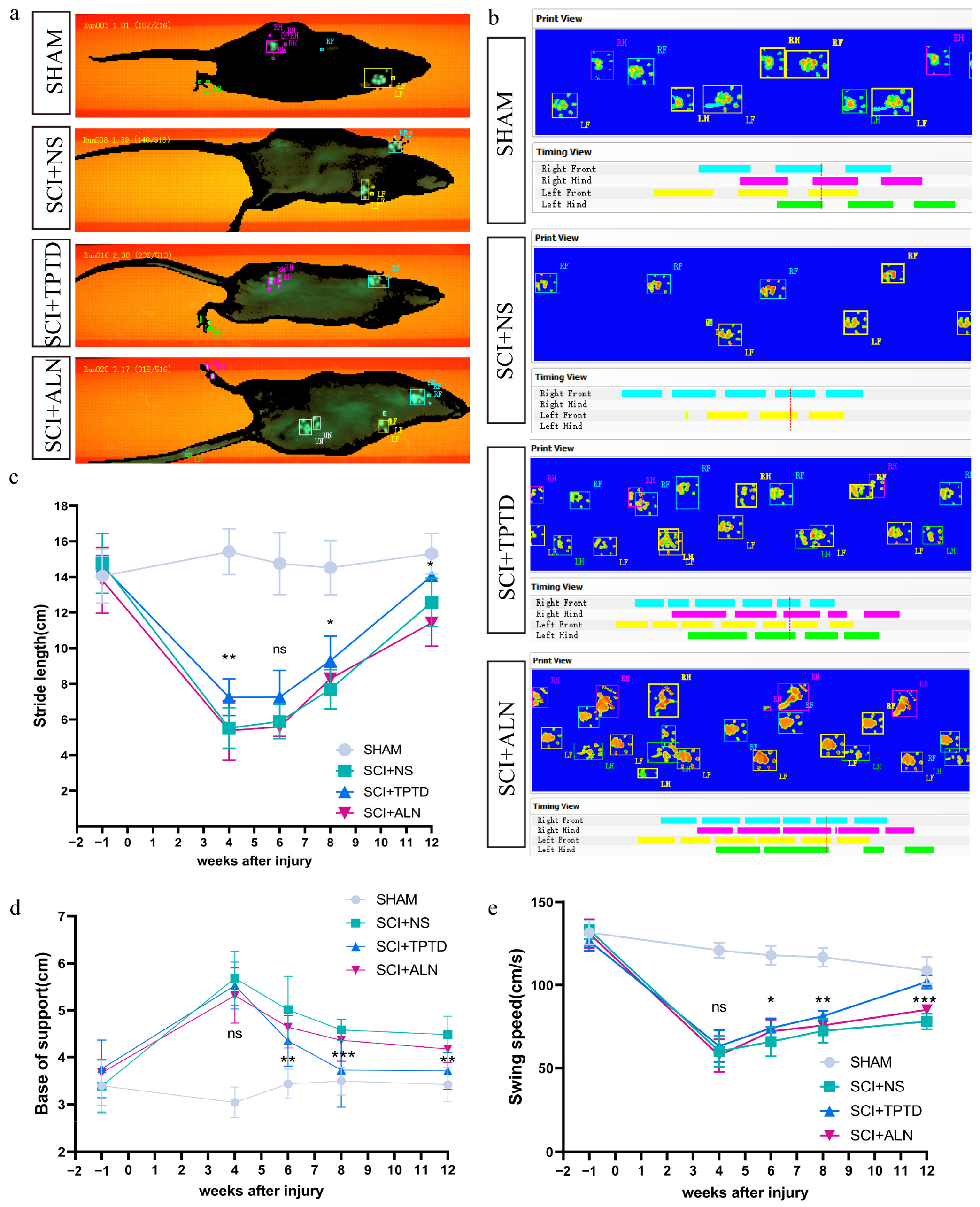
2.3. Behavioral Tests
2.4. Tissue Preparation and Histological Staining
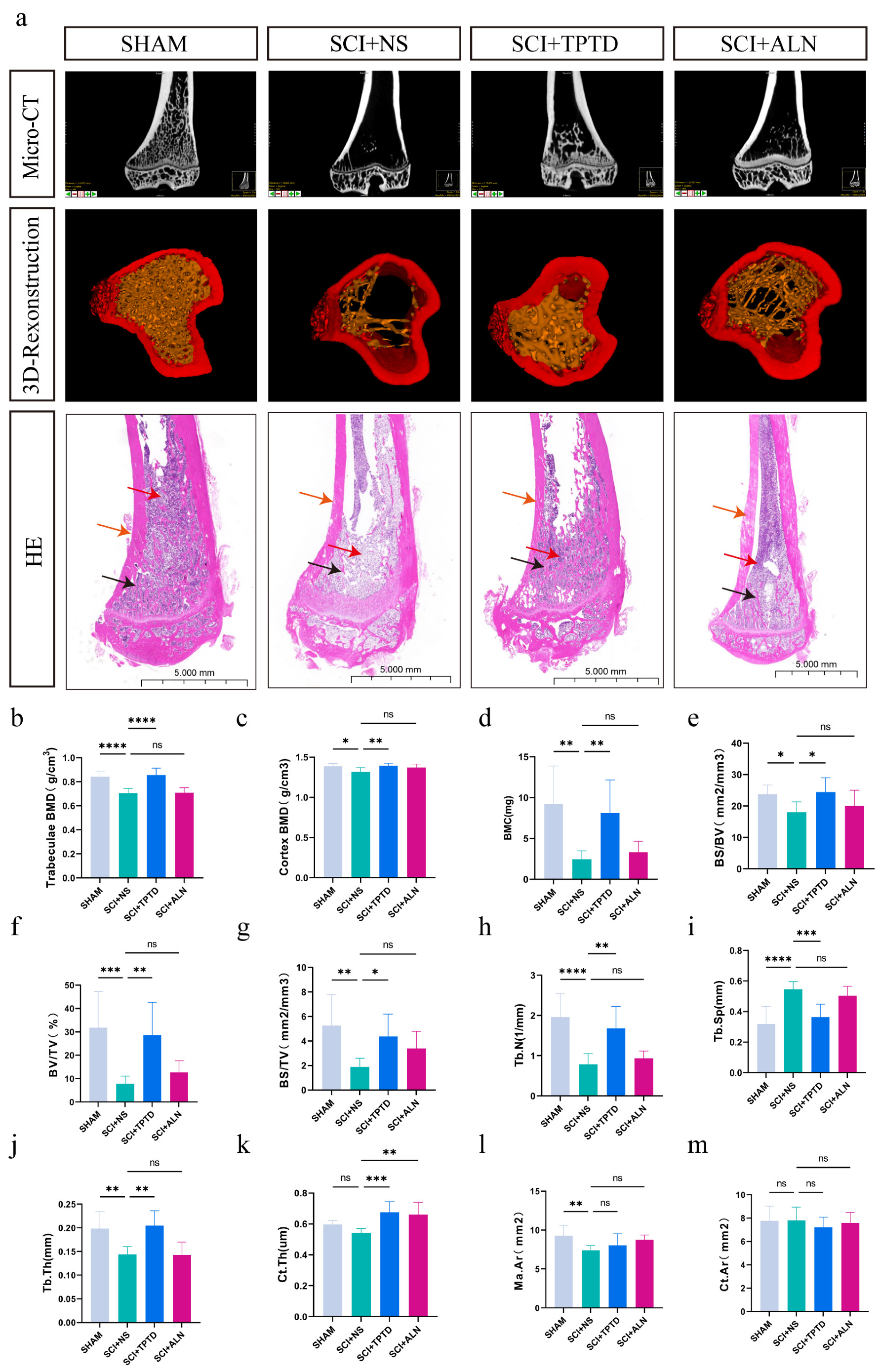
2.5. Micro-CT
2.6. Statistical Analysis
3. Results
3.1. TPTD Can Significantly Alleviate Bone Loss After SCI
3.2. TPTD Improves the Motor Function of Rats After SCI
3.3. TPTD Significantly Ameliorates Histopathological Changes After SCI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal Cord Injury: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent Update on Basic Mechanisms of Spinal Cord Injury. Neurosurg. Rev. 2020, 43, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, L.; Hong, B.; Xie, Y.; Li, T.; Feng, C.; Yang, F.; Wang, Y.; Zhang, J.; Yu, Y.; et al. Epidemiological Features of Traumatic Spinal Cord Injury in China: A Systematic Review and Meta-Analysis. Front. Neurol. 2023, 14, 1131791. [Google Scholar] [CrossRef]
- Williams, J.A.; Huesa, C.; Windmill, J.F.C.; Purcell, M.; Reid, S.; Coupaud, S.; Riddell, J.S. Spatiotemporal Responses of Trabecular and Cortical Bone to Complete Spinal Cord Injury in Skeletally Mature Rats. Bone Rep. 2022, 16, 101592. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cardozo, C.P. Osteoporosis in Individuals with Spinal Cord Injury. PMR 2015, 7, 188–201. [Google Scholar] [CrossRef]
- Dauty, M.; Perrouin Verbe, B.; Maugars, Y.; Dubois, C.; Mathe, J.F. Supralesional and Sublesional Bone Mineral Density in Spinal Cord-Injured Patients. Bone 2000, 27, 305–309. [Google Scholar] [CrossRef]
- Wa, P. Consensus Development Conference: Diagnosis, Prophylaxis, and Treatment of Osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Dharnipragada, R.; Ahiarakwe, U.; Gupta, R.; Abdilahi, A.; Butterfield, J.; Naik, A.; Parr, A.; Morse, L.R. Pharmacologic and Nonpharmacologic Treatment Modalities for Bone Loss in SCI—Proposal for Combined Approach. J. Clin. Densitom. 2023, 26, 101359. [Google Scholar] [CrossRef]
- Walker, M.D.; Shane, E. Postmenopausal Osteoporosis. N. Engl. J. Med. 2023, 389, 1979–1991. [Google Scholar] [CrossRef]
- Cotts, K.G.; Cifu, A.S. Treatment of Osteoporosis. JAMA 2018, 319, 1040. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Jiang, L.; Dai, L. Mechanisms of Osteoporosis in Spinal Cord Injury. Clin. Endocrinol. 2006, 65, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, N.L.; Frampton, C.M.; Acland, R.H.; Nicholls, M.G.; March, R.L.; Maguire, P.; Heard, A.; Reilly, P.; Marshall, K. Alendronate Prevents Bone Loss in Patients with Acute Spinal Cord Injury: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2007, 92, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Bryson, J.E.; Gourlay, M.L. Bisphosphonate Use in Acute and Chronic Spinal Cord Injury: A Systematic Review. J. Spinal Cord. Med. 2009, 32, 215–225. [Google Scholar] [CrossRef]
- Bubbear, J.S.; Gall, A.; Middleton, F.R.I.; Ferguson-Pell, M.; Swaminathan, R.; Keen, R.W. Early Treatment with Zoledronic Acid Prevents Bone Loss at the Hip Following Acute Spinal Cord Injury. Osteoporos. Int. 2011, 22, 271–279. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cirnigliaro, C.M.; La Fountaine, M.F.; Martinez, L.; Kirshblum, S.C.; Spungen, A.M. Zoledronic Acid Administration Failed to Prevent Bone Loss at the Knee in Persons with Acute Spinal Cord Injury: An Observational Cohort Study. J. Bone Min. Metab. 2015, 33, 410–421. [Google Scholar] [CrossRef]
- Akhigbe, T.; Chin, A.S.; Svircev, J.N.; Hoenig, H.; Burns, S.P.; Weaver, F.M.; Bailey, L.; Carbone, L. A Retrospective Review of Lower Extremity Fracture Care in Patients with Spinal Cord Injury. J. Spinal Cord. Med. 2015, 38, 2–9. [Google Scholar] [CrossRef]
- Hodsman, A.B.; Bauer, D.C.; Dempster, D.W.; Dian, L.; Hanley, D.A.; Harris, S.T.; Kendler, D.L.; McClung, M.R.; Miller, P.D.; Olszynski, W.P.; et al. Parathyroid Hormone and Teriparatide for the Treatment of Osteoporosis: A Review of the Evidence and Suggested Guidelines for Its Use. Endocr. Rev. 2005, 26, 688–703. [Google Scholar] [CrossRef]
- Blick, S.K.A.; Dhillon, S.; Keam, S.J. Teriparatide. Drugs 2008, 68, 2709–2737. [Google Scholar] [CrossRef]
- Yoshitake, S.; Mashiba, T.; Saito, M.; Fujihara, R.; Iwata, K.; Takao-Kawabata, R.; Yamamoto, T. Once-Weekly Teriparatide Treatment Prevents Microdamage Accumulation in the Lumbar Vertebral Trabecular Bone of Ovariectomized Cynomolgus Monkeys. Calcif. Tissue Int. 2019, 104, 402–410. [Google Scholar] [CrossRef]
- Xu, Q.; Li, D.; Chen, J.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Wang, X.; Cao, H. Crosstalk between the Gut Microbiota and Postmenopausal Osteoporosis: Mechanisms and Applications. Int. Immunopharmacol. 2022, 110, 108998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, Y.; Tian, J.; Kang, Y.; Xu, J.; Shao, H.; Zhou, J.; Xia, C.; Wang, Y.; Zhang, J. Unraveling the Relationship between Serum Parathyroid Hormone Levels and Trabecular Bone Score: A Cross-Sectional Study. Sci. Rep. 2024, 14, 13065. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Hao, Z.; Hu, Y.; Li, J. Parathyroid Hormone and Its Related Peptides in Bone Metabolism. Biochem. Pharmacol. 2021, 192, 114669. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Santora, A.C.; Black, D.M.; Russell, R.G.G. History of Alendronate. Bone 2020, 137, 115411. [Google Scholar] [CrossRef]
- Yang, D.; Tan, J.; Long, Y.; Huang, K.; Han, W.; Wang, M.; Zhu, S.; Zeng, S.; Yi, W. Sequential Treatment of Teriparatide and Alendronate versus Alendronate Alone for Elevation of Bone Mineral Density and Prevention of Refracture after Percutaneous Vertebroplasty in Osteoporosis: A Prospective Study. Aging Clin. Exp. Res. 2023, 35, 531–539. [Google Scholar] [CrossRef]
- Ananchenko, G.; Novakovic, J.; Tikhomirova, A. Chapter One—Alendronate Sodium. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Profiles of Drug Substances, Excipients, and Related Methodology; Academic Press: Cambridge, MA, USA, 2013; Volume 38, pp. 1–33. [Google Scholar]
- Choi, Y.; Shin, T. Alendronate Enhances Functional Recovery after Spinal Cord Injury. Exp. Neurobiol. 2022, 31, 54–64. [Google Scholar] [CrossRef]
- Xiong, M.; Feng, Y.; Luo, C.; Guo, J.; Zeng, J.; Deng, L.; Xiao, Q. Teriparatide: An Innovative and Promising Strategy for Protecting the Blood-Spinal Cord Barrier Following Spinal Cord Injury. Front. Pharmacol. 2024, 15, 1386565. [Google Scholar] [CrossRef]
- Ridlen, R.; McGrath, K.; Gorrie, C.A. Animal Models of Compression Spinal Cord Injury. J. Neurosci. Res. 2022, 100, 2201–2212. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Garrick, J.M.; Costa, L.G.; Cole, T.B.; Marsillach, J. Evaluating Gait and Locomotion in Rodents with the CatWalk. Curr. Protoc. 2021, 1, e220. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the Glial Scar for Spinal Cord Repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, M.; Kwaśniewska, A.; Miazga, K.; Sławińska, U. Perspectives in the Cell-Based Therapies of Various Aspects of the Spinal Cord Injury-Associated Pathologies: Lessons from the Animal Models. Cells 2021, 10, 2995. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Miron, V.E. Microglia Regulation of Central Nervous System Myelin Health and Regeneration. Nat. Rev. Immunol. 2024, 24, 49–63. [Google Scholar] [CrossRef]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability From the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Shen, C.; Jiang, L.-S.; Dai, L.-Y. Differences of Bone Mass and Bone Structure in Osteopenic Rat Models Caused by Spinal Cord Injury and Ovariectomy. Osteoporos. Int. 2007, 18, 743–750. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Jiang, L.-S.; Dai, L.-Y. Spinal Cord Injury Causes More Damage to Bone Mass, Bone Structure, Biomechanical Properties and Bone Metabolism than Sciatic Neurectomy in Young Rats. Osteoporos. Int. 2006, 17, 1552–1561. [Google Scholar] [CrossRef]
- Leone, G.E.; Shields, D.C.; Haque, A.; Banik, N.L. Rehabilitation: Neurogenic Bone Loss after Spinal Cord Injury. Biomedicines 2023, 11, 2581. [Google Scholar] [CrossRef]
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. The Pathophysiology of Osteoporosis after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 3057. [Google Scholar] [CrossRef]
- Abdelrahman, S.; Ireland, A.; Winter, E.M.; Purcell, M.; Coupaud, S. Osteoporosis after Spinal Cord Injury: Aetiology, Effects and Therapeutic Approaches. J. Musculoskelet. Neuronal Interact. 2021, 21, 26. [Google Scholar]
- Lin, T.; Tong, W.; Chandra, A.; Hsu, S.-Y.; Jia, H.; Zhu, J.; Tseng, W.-J.; Levine, M.A.; Zhang, Y.; Yan, S.-G.; et al. A Comprehensive Study of Long-Term Skeletal Changes after Spinal Cord Injury in Adult Rats. Bone Res. 2015, 3, 15028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haider, I.T.; Simonian, N.; Saini, A.S.; Leung, F.M.; Edwards, W.B.; Schnitzer, T.J. Open-Label Clinical Trial of Alendronate after Teriparatide Therapy in People with Spinal Cord Injury and Low Bone Mineral Density. Spinal Cord. 2019, 57, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Edwards, W.B.; Simonian, N.; Haider, I.T.; Anschel, A.S.; Chen, D.; Gordon, K.E.; Gregory, E.K.; Kim, K.H.; Parachuri, R.; Troy, K.L.; et al. Effects of Teriparatide and Vibration on Bone Mass and Bone Strength in People with Bone Loss and Spinal Cord Injury: A Randomized, Controlled Trial. J. Bone Miner. Res. 2018, 33, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Fernández Dorado, M.T.; Díaz Merino, M.D.S.; García Marco, D.; Cuena Boy, R.; Blanco Samper, B.; Martínez Dhier, L.; Labarta Bertol, C. Preventive Treatment with Alendronate of Loss of Bone Mineral Density in Acute Traumatic Spinal Cord Injury. Randomized Controlled Clinical Trial. Spinal Cord. 2022, 60, 687–693. [Google Scholar] [CrossRef]
- Wang, L.; Yao, X.; Xiao, L.; Tang, X.; Ding, H.; Zhang, H.; Yuan, J. The Effects of Spinal Cord Injury on Bone Healing in Patients with Femoral Fractures. J. Spinal Cord. Med. 2014, 37, 414–419. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Jiang, L.-S.; Dai, L.-Y. Changes in Bone Mass, Bone Structure, Bone Biomechanical Properties, and Bone Metabolism after Spinal Cord Injury: A 6-Month Longitudinal Study in Growing Rats. Calcif. Tissue Int. 2007, 80, 167–175. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Badhiwala, J.H.; Fehlings, M.G. “Time Is Spine”: The Importance of Early Intervention for Traumatic Spinal Cord Injury. Spinal Cord. 2020, 58, 1037–1039. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Chen, J.Y.; Safarians, G.; Ezubeik, S.; Pedroncelli, N.; Duquette, R.D.; Prasse, T.; Seidlits, S.K. Clinical Trials Targeting Secondary Damage after Traumatic Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 3824. [Google Scholar] [CrossRef]
- Kim, H.N.; McCrea, M.R.; Li, S. Advances in Molecular Therapies for Targeting Pathophysiology in Spinal Cord Injury. Expert Opin. Ther. Targets 2023, 27, 171–187. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Guerra-Araiza, C.; Orozco-Suárez, S.; Salgado-Ceballos, H.; Feria-Romero, I.A.; Gallardo, J.M.; Orozco-Barrios, C.E. The Importance of Natural Antioxidants in the Treatment of Spinal Cord Injury in Animal Models: An Overview. Oxidative Med. Cell. Longev. 2019, 2019, 3642491. [Google Scholar] [CrossRef]
- De Almeida, F.; Marques, S.; Dos Santos, A.R.; Prins, C.; Dos Santos Cardoso, F.; Dos Santos Heringer, L.; Mendonça, H.; Martinez, A.B. Molecular Approaches for Spinal Cord Injury Treatment. Neural Regen. Res. 2023, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wei, W.; Yang, D.; Zhang, H.; Yang, W.; Zhang, Y.; Nie, Y.; Hao, M.; Wang, P.; Ruan, H.; et al. Dual Electrical Stimulation at Spinal-Muscular Interface Reconstructs Spinal Sensorimotor Circuits after Spinal Cord Injury. Nat. Commun. 2024, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S.; et al. Walking Naturally after Spinal Cord Injury Using a Brain–Spine Interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zipser, C.M.; Cragg, J.J.; Guest, J.D.; Fehlings, M.G.; Jutzeler, C.R.; Anderson, A.J.; Curt, A. Cell-Based and Stem-Cell-Based Treatments for Spinal Cord Injury: Evidence from Clinical Trials. Lancet Neurol. 2022, 21, 659–670. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The Leading Edge: Emerging Neuroprotective and Neuroregenerative Cell-Based Therapies for Spinal Cord Injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Cai, M.; Chen, L.; Wang, T.; Liang, Y.; Zhao, J.; Zhang, X.; Li, Z.; Wu, H. Hydrogel Scaffolds in the Treatment of Spinal Cord Injury: A Review. Front. Neurosci. 2023, 17, 1211066. [Google Scholar] [CrossRef]
- Ai, G.; Xiong, M.; Deng, L.; Zeng, J.; Xiao, Q. Research Progress on the Inhibition of Oxidative Stress by Teriparatide in Spinal Cord Injury. Front. Neurol. 2024, 15, 1358414. [Google Scholar] [CrossRef]
- Xiong, M.; Feng, Y.; Huang, S.; Lv, S.; Deng, Y.; Li, M.; Wang, P.; Luo, M.; Wen, H.; Zhang, W. Teriparatide Induces Angiogenesis in Ischemic Cerebral Infarction Zones of Rats through AC/PKA Signaling and Reduces Ischemia-Reperfusion Injury. Biomed. Pharmacother. 2022, 148, 112728. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhu, J.; Feng, Y.; Hua, Y.; You, G.; Su, J.; Shi, B. Effects of Teriparatide and Alendronate on Functional Recovery from Spinal Cord Injury and Postinjury Bone Loss. Biomedicines 2025, 13, 342. https://doi.org/10.3390/biomedicines13020342
Wang S, Zhu J, Feng Y, Hua Y, You G, Su J, Shi B. Effects of Teriparatide and Alendronate on Functional Recovery from Spinal Cord Injury and Postinjury Bone Loss. Biomedicines. 2025; 13(2):342. https://doi.org/10.3390/biomedicines13020342
Chicago/Turabian StyleWang, Shuai, Jingliang Zhu, Yuping Feng, Yuchen Hua, Gangjun You, Jahui Su, and Benchao Shi. 2025. "Effects of Teriparatide and Alendronate on Functional Recovery from Spinal Cord Injury and Postinjury Bone Loss" Biomedicines 13, no. 2: 342. https://doi.org/10.3390/biomedicines13020342
APA StyleWang, S., Zhu, J., Feng, Y., Hua, Y., You, G., Su, J., & Shi, B. (2025). Effects of Teriparatide and Alendronate on Functional Recovery from Spinal Cord Injury and Postinjury Bone Loss. Biomedicines, 13(2), 342. https://doi.org/10.3390/biomedicines13020342






