Gas Plasma-Induced Oxidative Transformation of Glucose
Abstract
1. Introduction
2. Materials and Methods
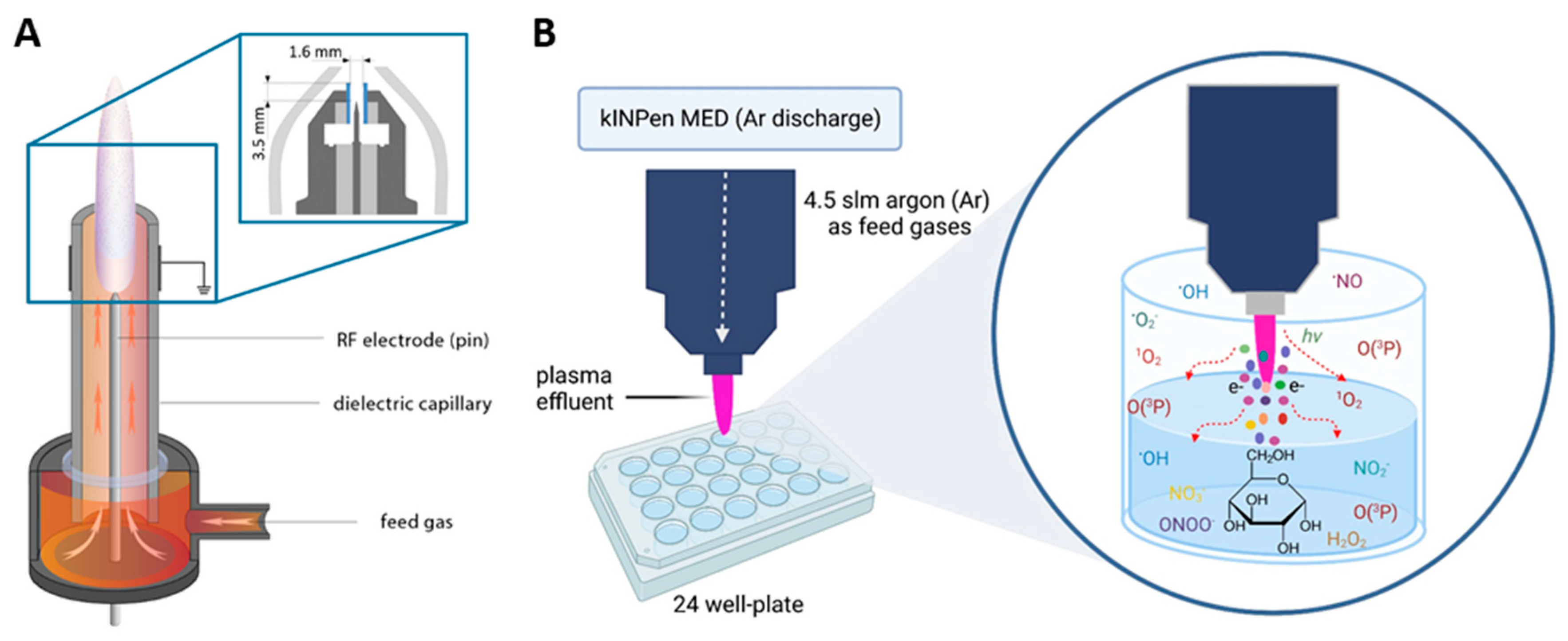
2.1. Sample Preparation
2.2. Gas Plasma Exposure
2.3. Mass Spectrometry (MS)
2.4. Data Analysis and Visualization
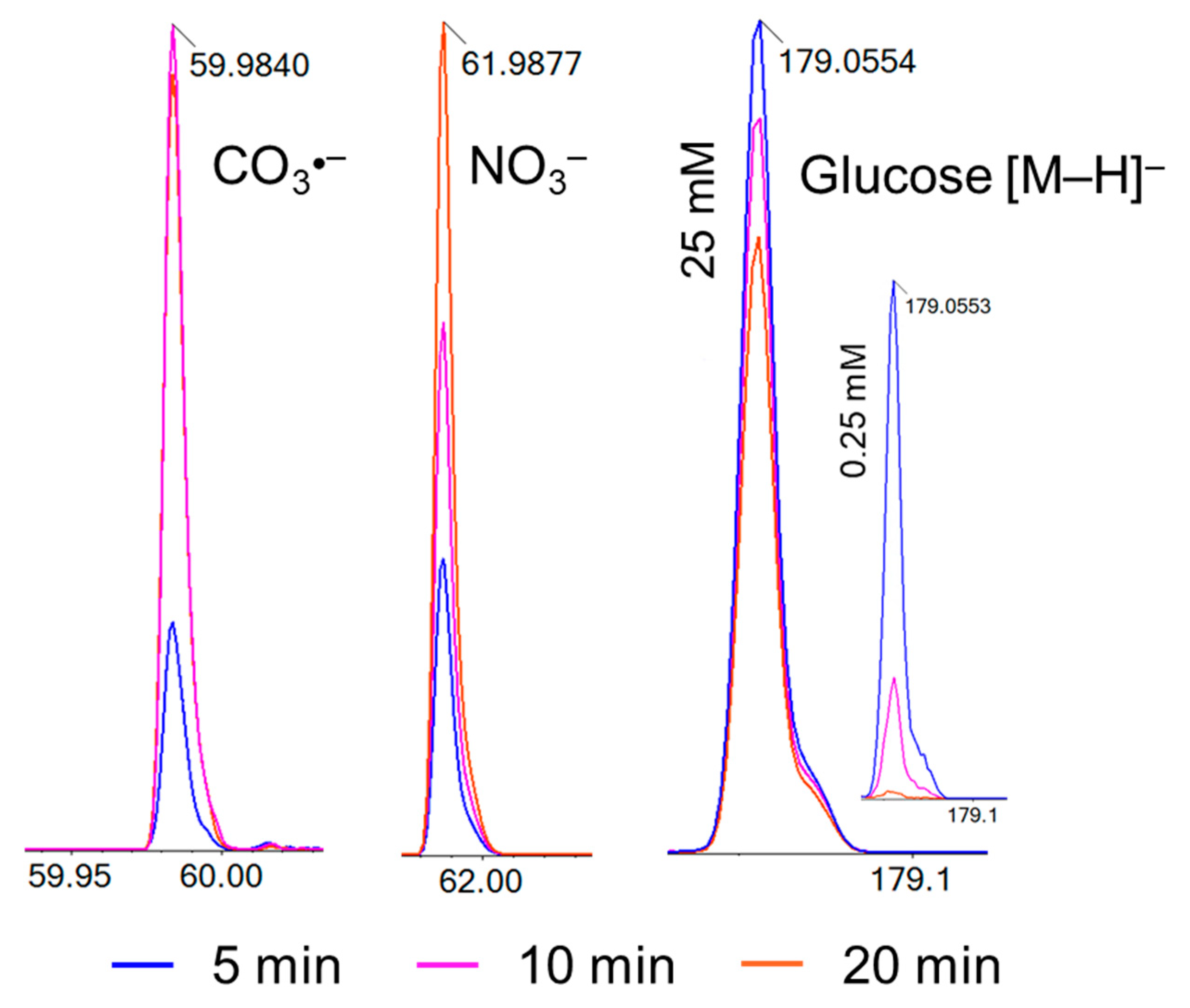
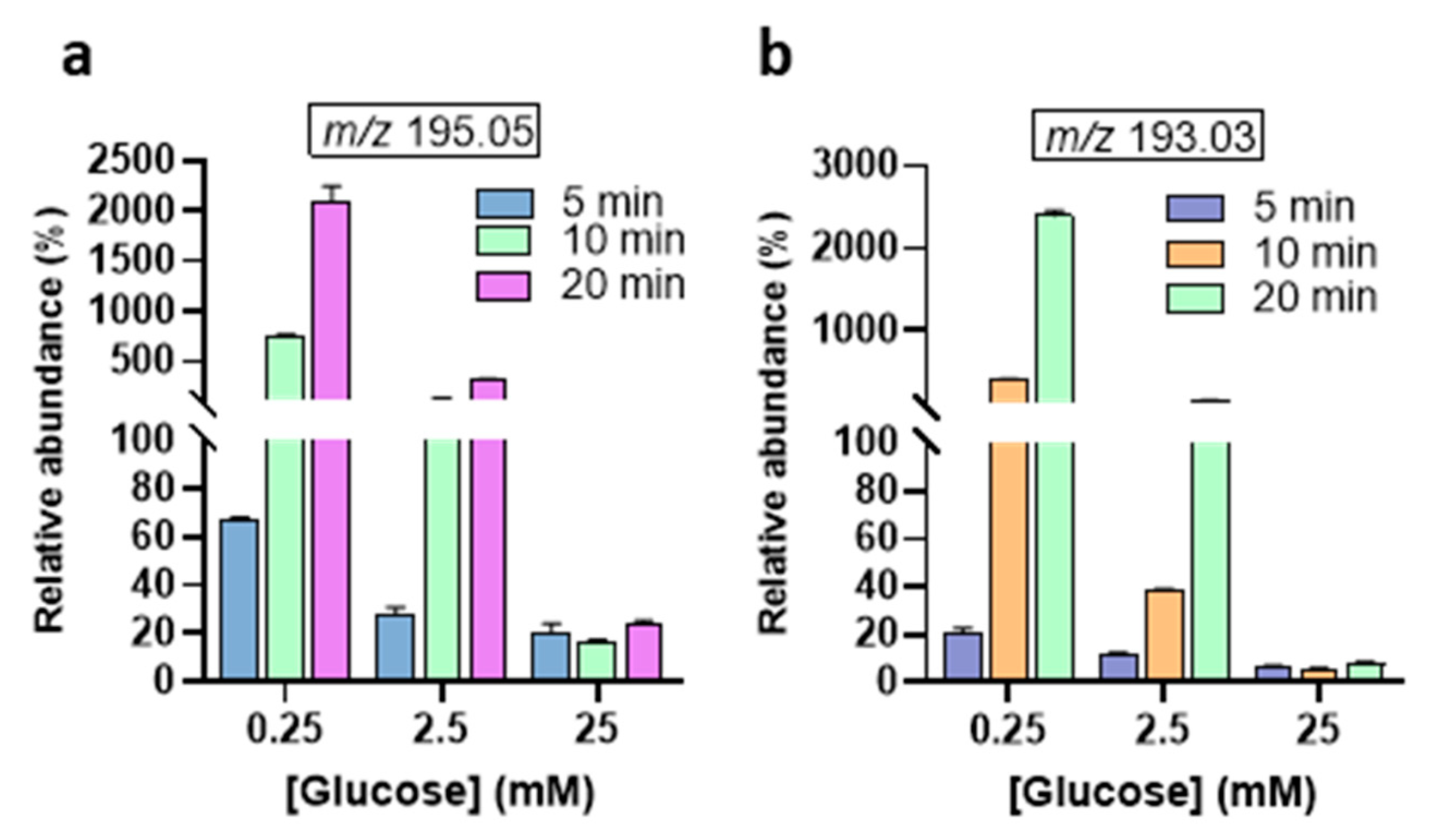
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Red. Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Bekeschus, S. Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef]
- Ahmadi, M.; Potlitz, F.; Link, A.; von Woedtke, T.; Nasri, Z.; Wende, K. Flucytosine-based prodrug activation by cold physical plasma. Arch. Pharm. 2022, 355, e2200061. [Google Scholar] [CrossRef]
- Nasri, Z.; Ahmadi, M.; Striesow, J.; Ravandeh, M.; von Woedtke, T.; Wende, K. Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. Int. J. Mol. Sci. 2022, 23, 5932. [Google Scholar] [CrossRef] [PubMed]
- Wenske, S.; Lackmann, J.W.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15, 061008. [Google Scholar] [CrossRef] [PubMed]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.W.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice. Adv. Sci. 2021, 8, 2003395. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.-J. Catalyst Preparation with Plasmas: How Does It Work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nasri, Z.; von Woedtke, T.; Wende, K. d-Glucose Oxidation by Cold Atmospheric Plasma-Induced Reactive Species. ACS Omega 2022, 7, 31983–31998. [Google Scholar] [CrossRef]
- Li, Y.; Friedman, G.; Fridman, A.; Ji, H.-F. Decomposition of sugars under non-thermal dielectric barrier discharge plasma. Clin. Plasma Med. 2014, 2, 56–63. [Google Scholar] [CrossRef]
- Mittelmaier, S.; Funfrocken, M.; Fenn, D.; Fichert, T.; Pischetsrieder, M. Identification and quantification of the glucose degradation product glucosone in peritoneal dialysis fluids by HPLC/DAD/MSMS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Linden, T.; Cohen, A.; Deppisch, R.; Kjellstrand, P.; Wieslander, A. 3,4-Dideoxyglucosone-3-ene (3,4-DGE): A cytotoxic glucose degradation product in fluids for peritoneal dialysis. Kidney Int. 2002, 62, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Auditore, A.; Gensberger-Reigl, S.; Pischetsrieder, M. In Vitro Reactivity of the Glucose Degradation Product 3,4-Dideoxyglucosone-3-ene (3,4-DGE) towards Abundant Components of the Human Blood Circulatory System. Int. J. Mol. Sci. 2022, 23, 4557. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Karam, A.; Trinh, Q.T.; Xu, K.; Hirao, H.; Jérôme, F.; Chatel, G. Selective and Catalyst-free Oxidation of D-Glucose to D-Glucuronic acid induced by High-Frequency Ultrasound. Sci. Rep. 2017, 7, 40650. [Google Scholar] [CrossRef]
- Rinsant, D.; Chatel, G.; Jérôme, F. Efficient and Selective Oxidation of D-Glucose into Gluconic acid under Low-Frequency Ultrasonic Irradiation. ChemCatChem 2014, 6, 3355–3359. [Google Scholar] [CrossRef]
- Helmke, A.; Hüsing, A.M.; Gaedcke, S.; Brauns, N.; Balzer, M.S.; Reinhardt, M.; Hiss, M.; Shushakova, N.; de Luca, D.; Prinz, I.; et al. Peritoneal dialysate-range hypertonic glucose promotes T-cell IL-17 production that induces mesothelial inflammation. Eur. J. Immunol. 2021, 51, 354–367. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef]
- Ahmadi, M.; Singer, D.; Potlitz, F.; Nasri, Z.; von Woedtke, T.; Link, A.; Bekeschus, S.; Wende, K. Cold Physical Plasma-Mediated Fenretinide Prodrug Activation Confers Additive Cytotoxicity in Epithelial Cells. Antioxidants 2023, 12, 1271. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef]
- Omri, M.; Sauvage, F.; Golonu, S.; Wadouachi, A.; Pourceau, G. Photocatalyzed Transformation of Free Carbohydrates. Catalysts 2018, 8, 672. [Google Scholar] [CrossRef]
- Marcq, O.; Barbe, J.-M.; Trichet, A.; Guilard, R. Origin and significance of the production of carbon dioxide during the ozonization of 13C-labeled d-glucose at different pH values. Carbohydr. Res. 2001, 333, 233–240. [Google Scholar] [CrossRef]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2010, 21, 401–409. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Highly selective oxidation of glucose to gluconic acid and glucaric acid in water catalyzed by an efficient synergistic photocatalytic system. Catal. Sci. Technol. 2020, 10, 2231–2241. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Hirayama, J.; Knight, D.W.; Hutchings, G.J. Quantitative Determination of Pt- Catalyzed d-Glucose Oxidation Products Using 2D NMR. ACS Catal. 2019, 9, 325–335. [Google Scholar] [CrossRef]
- Hellwig, M.; Degen, J.; Henle, T. 3-deoxygalactosone, a “new” 1,2-dicarbonyl compound in milk products. J. Agric. Food Chem. 2010, 58, 10752–10760. [Google Scholar] [CrossRef]
- Zimmeck, T.; Tauer, A.; Fuenfrocken, M.; Pischetsrieder, M. How to Reduce 3-Deoxyglucosone and Acetaldehyde in Peritoneal Dialysis Fluids. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2020, 22, 350–356. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Boulos, S.; Nyström, L. Oxidized polysaccharides: A review on structural insights using MS-based approaches. Carbohydr. Polym. 2025, 367, 123946. [Google Scholar] [CrossRef]
- Zhou, X.; Nolte, M.W.; Mayes, H.B.; Shanks, B.H.; Broadbelt, L.J. Experimental and Mechanistic Modeling of Fast Pyrolysis of Neat Glucose-Based Carbohydrates. 1. Experiments and Development of a Detailed Mechanistic Model. Ind. Eng. Chem. Res. 2014, 53, 13274–13289. [Google Scholar] [CrossRef]
- Gensberger-Reigl, S.; Weigel, I.; Stutzer, J.; Auditore, A.; Nikolaus, T.; Pischetsrieder, M. Degradation and de novo formation of nine major glucose degradation products during storage of peritoneal dialysis fluids. Sci. Rep. 2022, 12, 4268. [Google Scholar] [CrossRef]
- Bae, J.H.; Lee, H.; Huh, S.-C.; Park, S. Nitric and nitrous acid formation in plasma-treated water: Decisive role of nitrogen oxides (NOx=1–3). Chemosphere 2024, 364, 143105. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Tresp, H.; Hammer, M.U.; Weltmann, K.-D.; Reuter, S. Effects of Atmosphere Composition and Liquid Type on Plasma-Generated Reactive Species in Biologically Relevant Solutions. Plasma Med. 2013, 3, 45–55. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Tóth, T.; Takács, E. Rate constants of carbonate radical anion reactions with molecules of environmental interest in aqueous solution: A review. Sci. Total Environ. 2020, 717, 137219. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; de Oliveira, M.G.; Mónica, F.Z.; Antunes, E. Methylglyoxal and Advanced Glycation End Products (AGEs): Targets for the Prevention and Treatment of Diabetes-Associated Bladder Dysfunction? Biomedicines 2024, 12, 939. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef]
- Erixon, M.; Lindén, T.; Kjellstrand, P.; Carlsson, O.; Ernebrant, M.; Forsbäck, G.; Wieslander, A.; Jönsson, J.Å. PD Fluids Contain High Concentrations of Cytotoxic GDPs Directly after Sterilization. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2020, 24, 392–398. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tomo, T.; Okabe, E.; Namoto, S.; Suzuki, K.; Hirao, Y. Glutathione depletion as a mechanism of 3,4-dideoxyglucosone-3-ene-induced cytotoxicity in human peritoneal mesothelial cells: Role in biocompatibility of peritoneal dialysis fluids. Nephrol. Dial. Transplant. 2008, 24, 1436–1442. [Google Scholar] [CrossRef]
- Distler, L.; Georgieva, A.; Kenkel, I.; Huppert, J.; Pischetsrieder, M. Structure- and Concentration-Specific Assessment of the Physiological Reactivity of α-Dicarbonyl Glucose Degradation Products in Peritoneal Dialysis Fluids. Chem. Res. Toxicol. 2014, 27, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Ahmad, S.; Rabbani, G.; Hasan, Q.; Jan, A.T.; Lee, E.J.; Khan, R.H.; Alam, K.; Choi, I. 3-Deoxyglucosone: A potential glycating agent accountable for structural alteration in H3 histone protein through generation of different AGEs. PLoS ONE 2015, 10, e0116804. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Haybrard, J.; Simon, N.; Danel, C.; Pincon, C.; Barthelemy, C.; Tessier, F.J.; Decaudin, B.; Boulanger, E.; Odou, P. Factors Generating Glucose Degradation Products in Sterile Glucose Solutions for Infusion: Statistical Relevance Determination of Their Impacts. Sci. Rep. 2017, 7, 11932. [Google Scholar] [CrossRef] [PubMed]
- Justo, P.; Sanz, A.B.; Egido, J.; Ortiz, A. 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005, 54, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
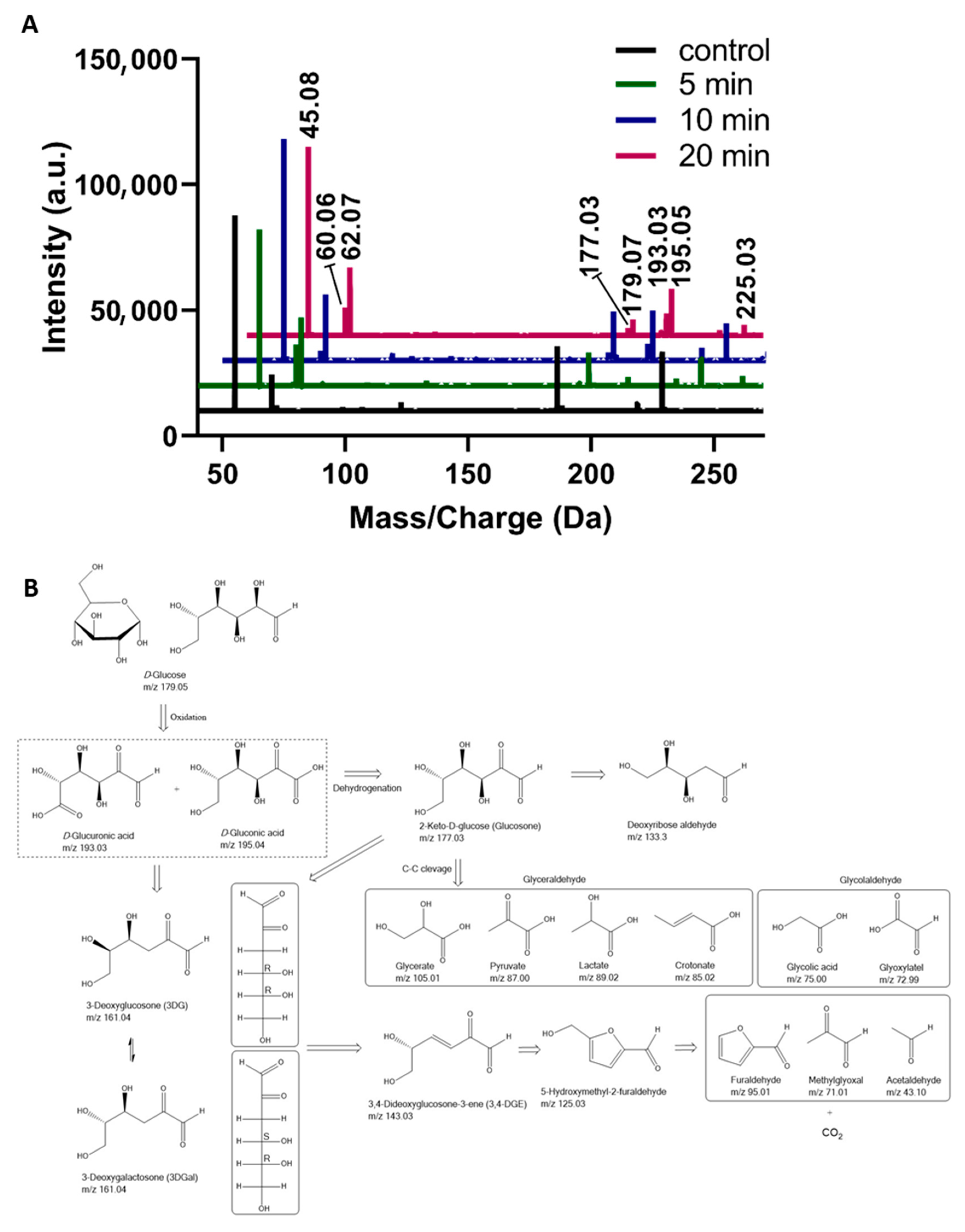
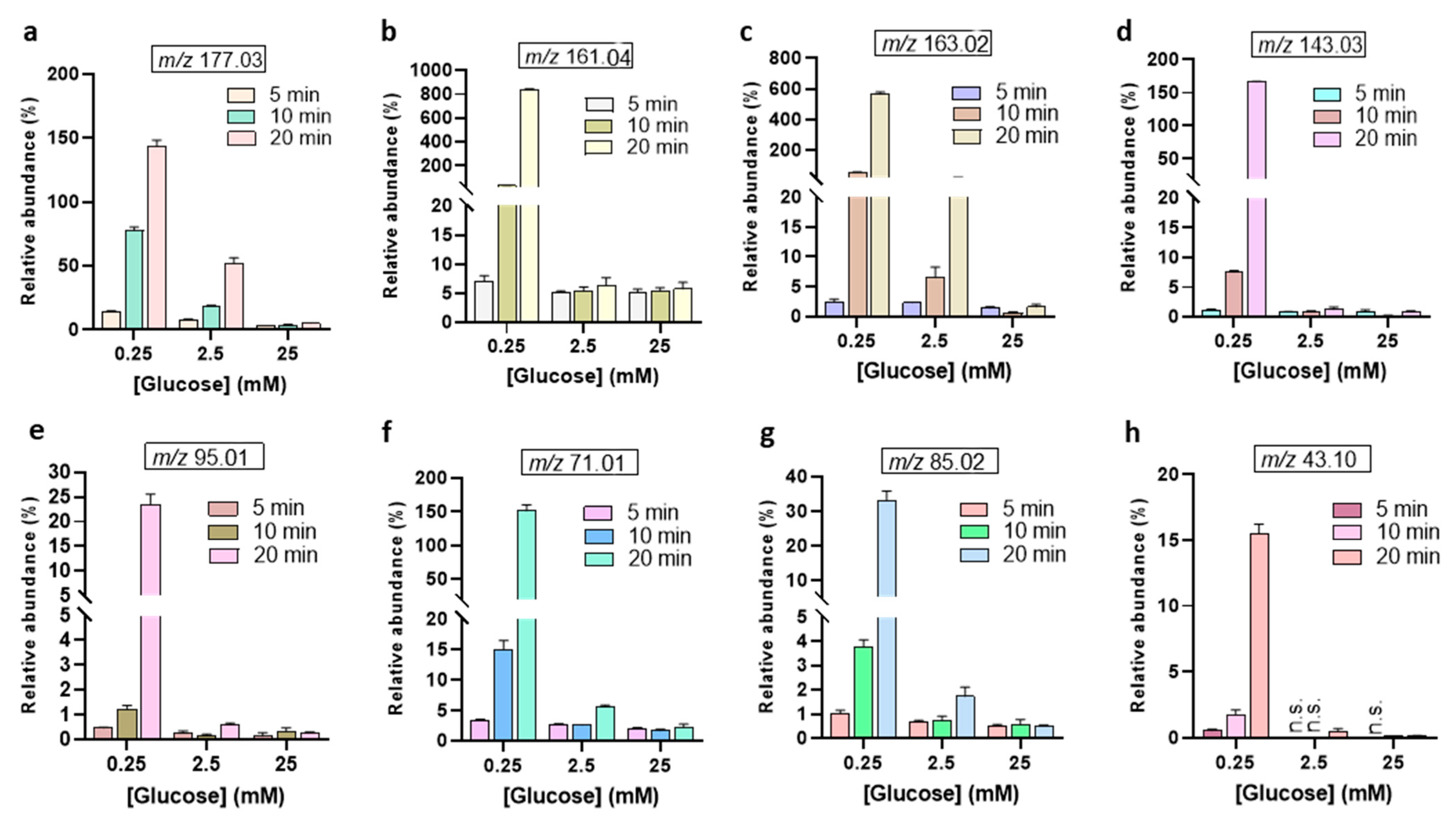
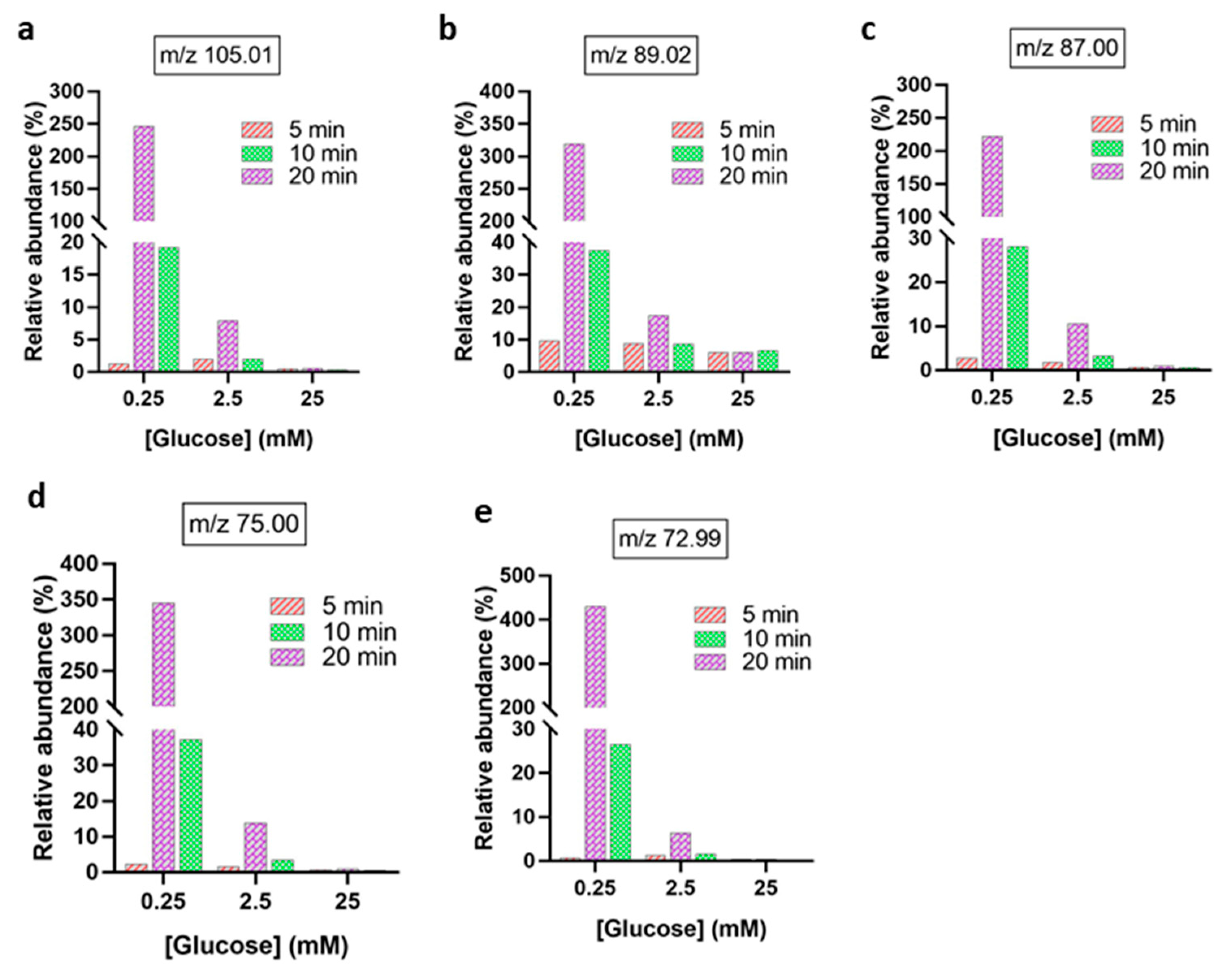
| m/z Values | MS/MS Values | Generic Names [Molecular Ions] |
|---|---|---|
| 195.04 | 177.03, 129.01, 99.00, 87.00, 85.00, 75.00, 59.01, and 57.00 | D-Gluconic acid [C6H12O7 − H]− |
| 193.03 | 165.03, 147.02, 131.03, 113.02, 103.00, 89.02, 85.02, 73.00, 71.01, 59.01, and 45.00 | D-Glucuronic acid [C6H10O7 − H]− |
| 177.03 | 159.02, 129.01, 117.01, 99.00, 87.00, 71.01, and 59.01 | 2-Keto-D-Glucose (Glucono-1,4-lactone) a [C6H10O6 − H]− |
| 163.02 | 145.01, 103.00, 101.02, 75.00, 73.00, 71.01, 59.01, and 45.00 | Aldehydo-arabinuronate [C5H7O6 − H]− |
| 161.04 | 103.00, 99.00, 87.00, 73.00, 71.01, 59.01, and 43.02 | 3-Deoxyglucosone (3DG) [C6H10O5 − H]− |
| 143.03 | 125.03, 99.00, 97.00, and 71.01 | 3,4-Dideoxyglucosone-3-ene (3,4DGE) [C6H8O4 − H]− |
| 95.01 | 95.08, 80.00, and 77.00 | Furaldehyde [C5H4O2 − H]− |
| 85.02 | 85.10, 69.00, and 53,00 | Crotonate [C4H5O2 − H]− |
| 71.01 | 71.01 and 41.00 | Methylglyoxal [C3H4O2 − H]− |
| 43.10 | 43.10 | Acetaldehyde [C2H4O − H]− |
| m/z Values | Chemical Formula | Controls and Plasma Treatments * | ||
|---|---|---|---|---|
| Control (a) (%) | Control (b) (%) | Plasma Treatment (%) 0.25, 2.5, 25 mM | ||
| 61.98 | NO3− | 1 | ~0.5 | 14, 4, ~0.5 |
| 60.99 | HCO3− | 0 | 6 | ~0.5, 0, 0 |
| 59.98 | CO3•− | 0 | 2 | 6, 1, ~0.5 |
| 44.99 | HCO2− | 25 | 25 | 5, 10, 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, M.; Masur, K.; Bekeschus, S.; Wende, K. Gas Plasma-Induced Oxidative Transformation of Glucose. Biomedicines 2025, 13, 2833. https://doi.org/10.3390/biomedicines13112833
Ahmadi M, Masur K, Bekeschus S, Wende K. Gas Plasma-Induced Oxidative Transformation of Glucose. Biomedicines. 2025; 13(11):2833. https://doi.org/10.3390/biomedicines13112833
Chicago/Turabian StyleAhmadi, Mohsen, Kai Masur, Sander Bekeschus, and Kristian Wende. 2025. "Gas Plasma-Induced Oxidative Transformation of Glucose" Biomedicines 13, no. 11: 2833. https://doi.org/10.3390/biomedicines13112833
APA StyleAhmadi, M., Masur, K., Bekeschus, S., & Wende, K. (2025). Gas Plasma-Induced Oxidative Transformation of Glucose. Biomedicines, 13(11), 2833. https://doi.org/10.3390/biomedicines13112833








