GBA1 Gene-Associated Transcriptomic Signatures Reveal Risk Genes in Parkinson’s Disease
Abstract
1. Introduction
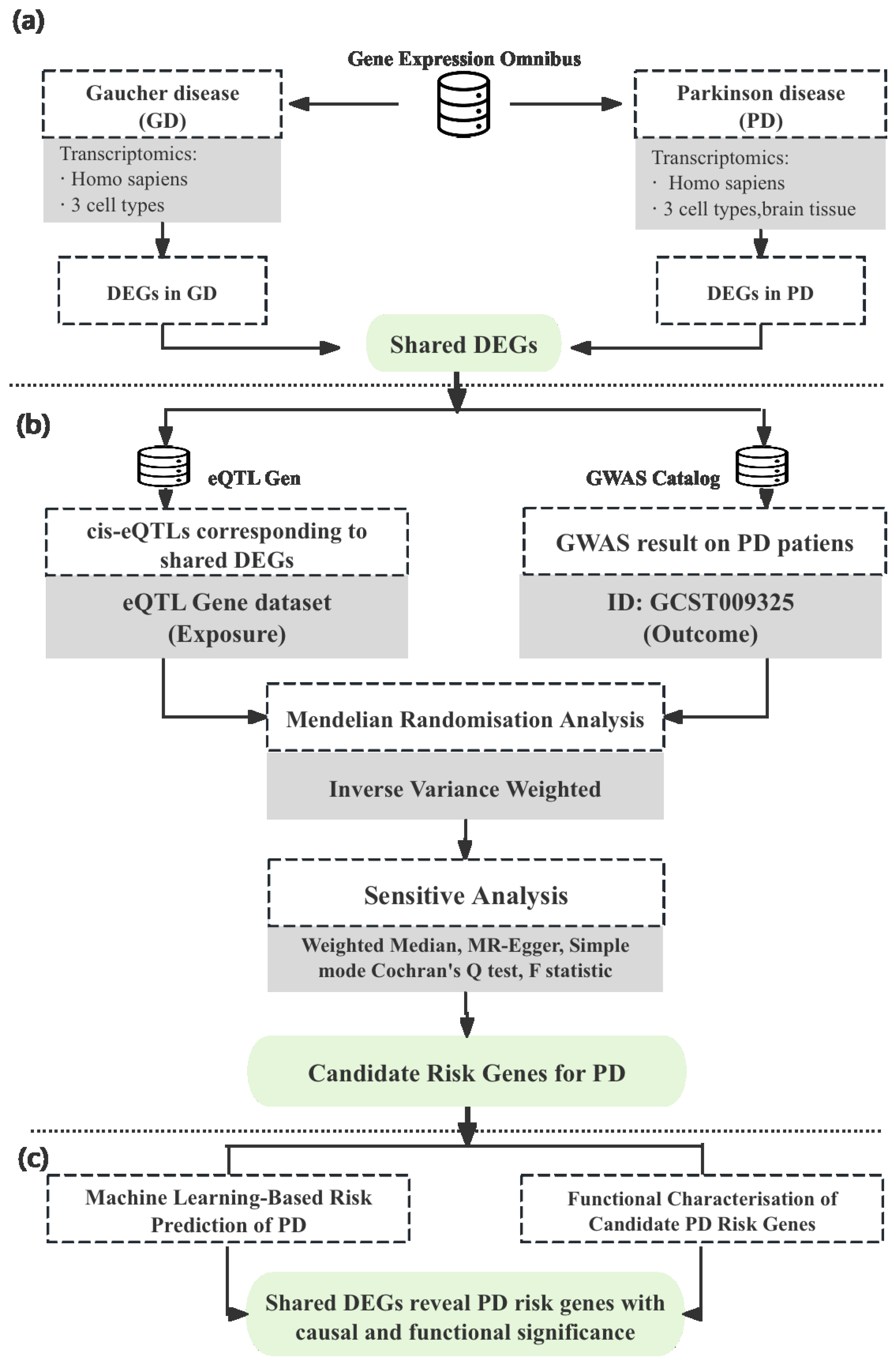
2. Materials and Methods
2.1. Identification of Shared DEGs in Gaucher and Parkinson’s Diseases
2.1.1. Transcriptomic Data Selection
2.1.2. Transcriptomic Data Analysis
2.2. Genetic Risk Association
2.2.1. Identification of cis-eQTL Instruments
2.2.2. Mendelian Randomisation and Sensitive Analyses
2.3. Functional Analysis of Risk Genes
2.3.1. Diagnostic Performance
2.3.2. Metabolic Modelling
3. Results
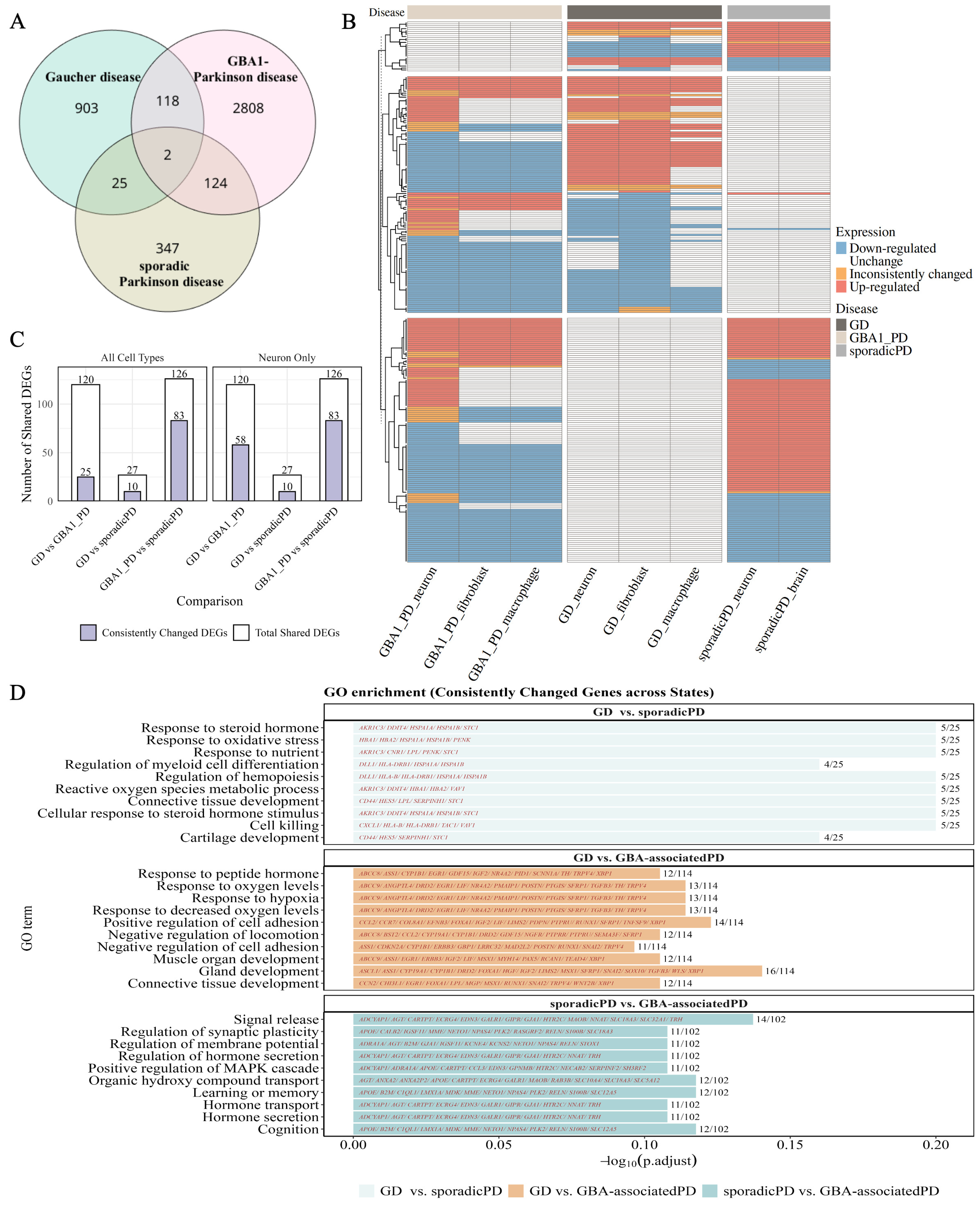
3.1. Identification of Shared Transcriptomic Alterations in Gaucher and Parkinson’s Diseases
3.2. Causal Inference of Shared DEGs in Parkinson’s Disease
3.3. Functional Characterisation of Risk Genes
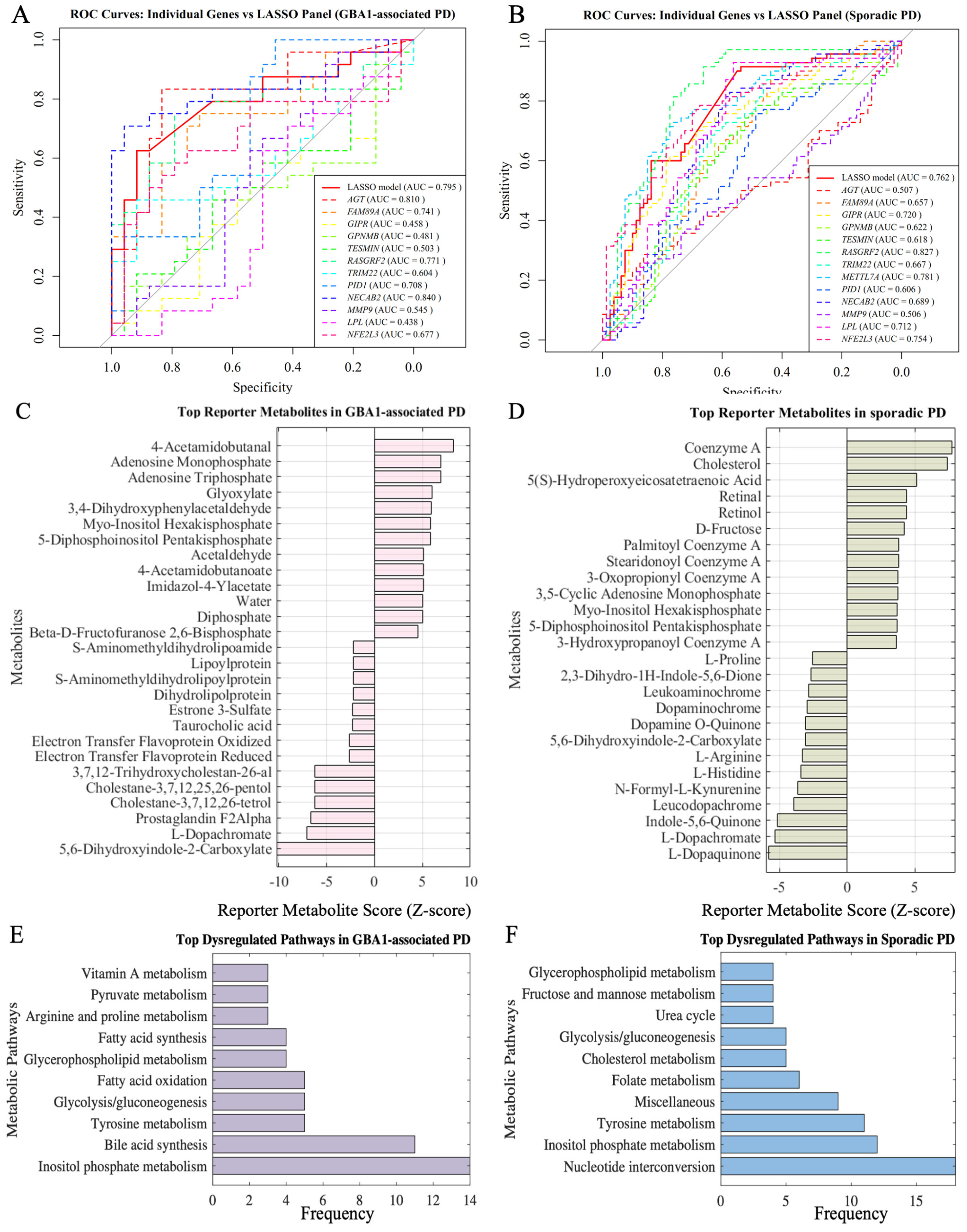
3.3.1. Diagnostic Performance of Risk Genes
3.3.2. Metabolic Impact of Risk Genes in Parkinson’s Disease Subtypes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GD | Gaucher disease |
| PD | Parkinson’s disease |
| DEGs | Differentially expressed genes |
| iPSC | Induced pluripotent stem cell |
| eQTL | Expression quantitative trait loci |
| GWAS | Genome-wide association study |
| SNPs | Single-nucleotide polymorphisms |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| FDR | False discovery rate |
References
- Aerts, J.M.F.G.; Kuo, C.L.; Lelieveld, L.T.; Boer, D.E.C.; van der Lienden, M.J.C.; Overkleeft, H.S.; Artola, M. Glycosphingolipids and lysosomal storage disorders as illustrated by gaucher disease. Curr. Opin. Chem. Biol. 2019, 53, 204–215. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Kallemeijn, W.W.; Wegdam, W.; Joao Ferraz, M.; van Breemen, M.J.; Dekker, N.; Kramer, G.; Poorthuis, B.J.; Groener, J.E.M.; Cox-Brinkman, J.; et al. Biomarkers in the diagnosis of lysosomal storage disorders: Proteins, lipids, and inhibodies. J. Inherit. Metab. Dis. 2011, 34, 605–619. [Google Scholar] [CrossRef]
- Klein, A.D.; Outeiro, T.F. Glucocerebrosidase mutations disrupt the lysosome and now the mitochondria. Nat. Commun. 2023, 14, 6383. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Schueler, U.H.; Kolter, T.; Kaneski, C.R.; Blusztajn, J.K.; Herkenham, M.; Sandhoff, K.; Brady, R.O. Toxicity of glucosylsphingosine (glucopsychosine) to cultured neuronal cells: A model system for assessing neuronal damage in Gaucher disease type 2 and 3. Neurobiol. Dis. 2003, 14, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Soroglu, C.V.; Berkay, E.G. Old disease New reflections: Gaucher, immunity, and inflammation. J. Cell. Mol. Med. 2024, 28, e70087. [Google Scholar] [CrossRef] [PubMed]
- Hertz, E.; Chen, Y.; Sidransky, E. Gaucher disease provides a unique window into Parkinson disease pathogenesis. Nat. Rev. Neurol. 2024, 20, 526–540. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Dinur, T.; Quinn, T.; Sakanaka, K.; Levy, O.; Waters, C.; Fahn, S.; Dorovski, T.; Chung, W.K.; Pauciulo, M.; et al. Comparison of Parkinson risk in Ashkenazi Jewish Gaucher patients and GBA heterozygotes. JAMA Neurol. 2014, 71, 752–757. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Amshalom, I.; Kilarski, L.L.; Bar-Shira, A.; Gana-Weisz, M.; Mirelman, A.; Marder, K.; Bressman, S.; Giladi, N.; Orr-Urtreger, A. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 2015, 84, 880–887. [Google Scholar] [CrossRef]
- Lwin, A.; Orvisky, E.; Goker-Alpan, O.; LaMarca, M.E.; Sidransky, E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 2004, 81, 70–73. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, J.; Mizuta, I.; Toyoda, A.; Ashida, R.; Takahashi, Y.; Goto, J.; Fukuda, Y.; year, H.; Iwata, A.; Yamamoto, M.; et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch. Neurol. 2009, 66, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Aharon-Peretz, J.; Rosenbaum, H.; Gershoni-Baruch, R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2004, 351, 1972–1977. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Gegg, M.E.; Burke, D.; Heales, S.J.R.; Cooper, J.M.; Hardy, J.; Wood, N.W.; Schapira, A.H.V. Glucocerebrosidase Deficiency in Substantia Nigra of Parkinson Disease Brains. Ann. Neurol. 2012, 72, 455–463. [Google Scholar] [CrossRef]
- Marano, M.; Zizzo, C.; Malaguti, M.C.; Bacchin, R.; Cavallieri, F.; De Micco, R.; Spagnolo, F.; Bentivoglio, A.R.; Schirinzi, T.; Bovenzi, R.; et al. Increased glucosylsphingosine levels and Gaucher disease in GBA1-associated Parkinson’s disease. Park. Relat. Disord. 2024, 124, 107023. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Tayebi, N.; Kim, W.S.; Sidransky, E.; Cooper, A.; Garner, B.; Halliday, G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain J. Neurol. 2014, 137, 834–848. [Google Scholar] [CrossRef]
- Kumar, M.; Srikanth, M.P.; Deleidi, M.; Hallett, P.J.; Isacson, O.; Feldman, R.A. Acid ceramidase involved in pathogenic cascade leading to accumulation of α-synuclein in iPSC model of GBA1-associated Parkinson’s disease. Hum. Mol. Genet. 2023, 32, 1888–1900. [Google Scholar] [CrossRef]
- Polissidis, A.; Koronaiou, E.; Nikolopoulou, G.; Viel, C.; Nikatou, M.; Bogiongko, M.; Sardi, S.P.; Xilouri, M.; Vekrellis, K.; Stefanis, L. A double-hit in vivo model of GBA viral microRNA-mediated downregulation and human alpha-synuclein overexpression demonstrates nigrostriatal degeneration. Neurobiol. Dis. 2022, 163, 105612. [Google Scholar] [CrossRef]
- Cullen, V.; Sardi, S.P.; Ng, J.; Xu, Y.H.; Sun, Y.; Tomlinson, J.J.; Kolodziej, P.; Kahn, I.; Saftig, P.; Woulfe, J.; et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 2011, 69, 940–953. [Google Scholar] [CrossRef]
- Aflaki, E.; Westbroek, W.; Sidransky, E. The complicated relationship between Gaucher disease and Parkinsonism: Insights from a rare disease. Neuron 2017, 93, 737–746. [Google Scholar] [CrossRef]
- Maor, G.; Cabasso, O.; Krivoruk, O.; Rodriguez, J.; Steller, H.; Segal, D.; Horowitz, M. The contribution of mutant GBA to the development of Parkinson disease in Drosophila. Hum. Mol. Genet. 2016, 25, 2712–2727. [Google Scholar] [CrossRef]
- García-Sanz, P.; Orgaz, L.; Fuentes, J.M.; Vicario, C.; Moratalla, R. Cholesterol and multilamellar bodies: Lysosomal dysfunction in GBA-Parkinson disease. Autophagy 2018, 14, 717–718. [Google Scholar] [CrossRef]
- Schondorf, D.C.; Aureli, M.; McAllister, F.E.; Hindley, C.J.; Mayer, F.; Schmid, B.; Sardi, S.P.; Valsecchi, M.; Hoffmann, S.; Schwarz, L.K.; et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014, 5, 4028. [Google Scholar] [CrossRef]
- Rubilar, J.C.; Outeiro, T.F.; Klein, A.D. The lysosomal b-glucocerebrosidase strikes mitochondria: Implications for Parkinson’s therapeutics. Brain 2024, 147, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Tang, B.; Guo, J. Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson’s disease. Transl. Neurodegener. 2024, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, C.; Qiao, H.; Gu, Z.; Gan-Or, Z.; Fon, E.A.; Chan, P. Decreased Penetrance of Parkinson’s Disease in Elderly Carriers of Glucocerebrosidase Gene L444P/R Mutations: A Community-Based 10-Year Longitudinal Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 672–678. [Google Scholar] [CrossRef]
- Võsa, U.; Claringbould, A.; Westra, H.J.; Bonder, M.J.; Deelen, P.; Zeng, B.; Kirsten, H.; Saha, A.; Kreuzhuber, R.; Yazar, S.; et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 2021, 53, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Warrell, J.; Won, H.; Shi, X.; Navarro, F.C.P.; Clarke, D.; Gu, M.; Emani, P.; Yang, Y.T.; et al. Comprehensive functional genomic resource and integrative model for the human brain. Science 2018, 362, eaat8464. [Google Scholar] [CrossRef]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-genome wide association study. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389, Erratum in Eur. J. Epidemiol. 2017, 32, 391–392. https://doi.org/10.1007/s10654-017-0276-5. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Foley, C.N.; Allara, E.; Staley, J.R.; Howson, J.M.M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 2020, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Preciat, G.; Moreno, E.L. Mechanistic model-driven exometabolomic characterisation of human dopamin- ergic neuronal metabolism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Katan, M.B. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1986, 1, 507–508. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ’Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, S.; Lee, J.S.; Han, B. Comparison of Two Meta-Analysis Methods: Inverse-Variance-Weighted Average and Weighted Sum of Z-Scores. Genom. Inform. 2016, 14, 173–180. [Google Scholar] [CrossRef]
- Boehm, F.J.; Zhou, X. Statistical methods for Mendelian randomization in genome-wide association studies: A review. Comput. Struct. Biotechnol. J. 2022, 20, 2338–2351. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Irmady, K.; Hale, C.R.; Qadri, R.; Fak, J.; Simelane, S.; Carroll, T.; Przedborski, S.; Darnell, R.B. Blood transcriptomic signatures associated with molecular changes in the brain and clinical outcomes in Parkinson’s disease. Nat. Commun. 2023, 14, 3956. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Woodard, C.M.; Campos, B.A.; Kuo, S.H.; Nirenberg, M.J.; Nestor, M.W.; Zimmer, M.; Mosharov, E.V.; Sulzer, D.; Zhou, H.; Paull, D.; et al. iPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep. 2014, 9, 1173–1182. [Google Scholar] [CrossRef]
- Patil, K.R.; Nielsen, J. Uncovering transcriptional regulation of metabolism by using metabolic network topology. Proc. Natl. Acad. Sci. USA 2005, 102, 2685–2689. [Google Scholar] [CrossRef] [PubMed]
- Serfecz, J.C.; Saadin, A.; Santiago, C.P.; Zhang, Y.; Bentzen, S.M.; Vogel, S.N.; Feldman, R.A. C5a Activates a Pro-Inflammatory Gene Expression Profile in Human Gaucher iPSC-Derived Macrophages. Int. J. Mol. Sci. 2021, 22, 9912. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, G.; Vivas, E.L.; Matalonga, L.; Berniakovich, I.; Barragán Monasterio, M.; Eguizábal, C.; Gort, L.; González, F.; Ortiz Mellet, C.; García Fernández, J.M.; et al. Neuronopathic Gaucher’s disease: Induced pluripotent stem cells for disease modelling and testing chaperone activity of small compounds. Hum. Mol. Genet. 2013, 22, 633–645. [Google Scholar] [CrossRef]
- Hou, W.C.; Massey, L.A.; Rhoades, D.; Wu, Y.; Ren, W.; Frank, C.; Overkleeft, H.S.; Kelly, J.W. A PIKfyve modulator combined with an integrated stress response inhibitor to treat lysosomal storage diseases. Proc. Natl. Acad. Sci. USA 2024, 121, e2320257121. [Google Scholar] [CrossRef] [PubMed]
- Ługowska, A.; Hetmańczyk-Sawicka, K.; Iwanicka-Nowicka, R.; Fogtman, A.; Cieśla, J.; Purzycka-Olewiecka, J.K.; Sitarska, D.; Płoski, R.; Filocamo, M.; Lualdi, S.; et al. Gene expression profile in patients with Gaucher disease indicates activation of inflammatory processes. Sci. Rep. 2019, 9, 6060. [Google Scholar] [CrossRef]
- Mubariz, F.; Saadin, A.; Lingenfelter, N.; Sarkar, C.; Banerjee, A.; Lipinski, M.M.; Awad, O. Deregulation of mTORC1-TFEB axis in human iPSC model of GBA1-associated Parkinson’s disease. Front. Neurosci. 2023, 17, 1152503. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, O.; Sivapatham, R.; Oron, T.R.; Meyer, M.; Mooney, S.; Rao, M.S.; Zeng, X. Derivation, Characterization, and Neural Differentiation of Integration-Free Induced Pluripotent Stem Cell Lines from Parkinson’s Disease Patients Carrying SNCA, LRRK2, PARK2, and GBA Mutations. PLoS ONE 2016, 11, e0154890. [Google Scholar] [CrossRef]
- Fernandes, H.J.R.; Hartfield, E.M.; Christian, H.C.; Emmanoulidou, E.; Zheng, Y.; Booth, H.; Bogetofte, H.; Lang, C.; Ryan, B.J.; Sardi, S.P.; et al. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s iPSC-Derived Dopamine Neurons. Stem Cell Rep. 2016, 6, 342–356. [Google Scholar] [CrossRef]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef]
- Zagare, A.; Balaur, I.; Rougny, A.; Saraiva, C.; Gobin, M.; Monzel, A.S.; Ghosh, S.; Satagopam, V.P.; Schwamborn, J.C. Deciphering shared molecular dysregulation across Parkinson’s disease variants using a multi-modal network-based data integration and analysis. npj Park. Dis. 2025, 11, 63. [Google Scholar] [CrossRef]
- Usenko, T.; Bezrukova, A.; Basharova, K.; Panteleeva, A.; Nikolaev, M.; Kopytova, A.; Miliukhina, I.; Emelyanov, A.; Zakharova, E.; Pchelina, S. Comparative Transcriptome Analysis in Monocyte-Derived Macrophages of Asymptomatic GBA Mutation Carriers and Patients with GBA-Associated Parkinson’s Disease. Genes 2021, 12, 1545. [Google Scholar] [CrossRef]
- Zaccaria, A.; Antinori, P.; Licker, V.; Kövari, E.; Lobrinus, J.A.; Burkhard, P.R. Multiomic Analyses of Dopaminergic Neurons Isolated from Human Substantia Nigra in Parkinson’s Disease: A Descriptive and Exploratory Study. Cell. Mol. Neurobiol. 2022, 42, 2805–2818. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Tan, M.M.X.; Lawton, M.A.; Jabbari, E.; Reynolds, R.H.; Iwaki, H.; Blauwendraat, C.; Kanavou, S.; Pollard, M.I.; Hubbard, L.; Malek, N.; et al. Genome-Wide Association Studies of Cognitive and Motor Progression in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Boot, B.; Locascio, J.J.; Jansen, I.E.; Winder-Rhodes, S.; Eberly, S.; Elbaz, A.; Brice, A.; Ravina, B.; van Hilten, J.J.; et al. Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann. Neurol. 2016, 80, 674–685. [Google Scholar] [CrossRef]
- Cilia, R.; Tunesi, S.; Marotta, G.; Cereda, E.; Siri, C.; Tesei, S.; Zecchinelli, A.L.; Canesi, M.; Mariani, C.B.; Meucci, N.; et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 2016, 80, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Mejia-Santana, H.; Mirelman, A.; Saunders-Pullman, R.; Raymond, D.; Palmese, C.; Caccappolo, E.; Ozelius, L.; Orr-Urtreger, A.; Clark, L.; et al. Neuropsychological performance in LRRK2 G2019S carriers with Parkinson’s disease. Park. Relat. Disord. 2015, 21, 106–110. [Google Scholar] [CrossRef]
- Hüttenrauch, M.; Ogorek, I.; Klafki, H.; Otto, M.; Stadelmann, C.; Weggen, S.; Wiltfang, J.; Wirths, O. Glycoprotein NMB: A novel Alzheimer’s disease associated marker expressed in a subset of activated microglia. Acta Neuropathol. Commun. 2018, 6, 108. [Google Scholar] [CrossRef]
- Tanaka, H.; Shimazawa, M.; Kimura, M.; Takata, M.; Tsuruma, K.; Yamada, M.; Takahashi, H.; Hozumi, I.; Niwa, J.i.; Iguchi, Y.; et al. The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis. Sci. Rep. 2012, 2, 573. [Google Scholar] [CrossRef]
- Moloney, E.B.; Moskites, A.; Ferrari, E.J.; Isacson, O.; Hallett, P.J. The glycoprotein GPNMB is selectively elevated in the substantia nigra of Parkinson’s disease patients and increases after lysosomal stress. Neurobiol. Dis. 2018, 120, 1–11. [Google Scholar] [CrossRef]
- Kramer, G.; Wegdam, W.; Donker-Koopman, W.; Ottenhoff, R.; Gaspar, P.; Verhoek, M.; Nelson, J.; Gabriel, T.; Kallemeijn, W.; Boot, R.G.; et al. Elevation of glycoprotein nonmetastatic melanoma protein B in type 1 Gaucher disease patients and mouse models. FEBS Open Bio 2016, 6, 902–913. [Google Scholar] [CrossRef]
- Zigdon, H.; Savidor, A.; Levin, Y.; Meshcheriakova, A.; Schiffmann, R.; Futerman, A.H. Identification of a Biomarker in Cerebrospinal Fluid for Neuronopathic Forms of Gaucher Disease. PLoS ONE 2015, 10, e0120194. [Google Scholar] [CrossRef]
- Marques, A.R.A.; Gabriel, T.L.; Aten, J.; van Roomen, C.P.A.A.; Ottenhoff, R.; Claessen, N.; Alfonso, P.; Irún, P.; Giraldo, P.; Aerts, J.M.F.G.; et al. Gpnmb Is a Potential Marker for the Visceral Pathology in Niemann-Pick Type C Disease. PLoS ONE 2016, 11, e0147208. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ortiz, M.E.; Seo, Y.; Posavi, M.; Carceles Cordon, M.; Clark, E.; Jain, N.; Charan, R.; Gallagher, M.D.; Unger, T.L.; Amari, N.; et al. GPNMB confers risk for Parkinson’s disease through interaction with α-synuclein. Science 2022, 377, eabk0637. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, G.; He, Y.; He, G.; Zhang, P.; Shen, X.; Zhang, W.; Chen, S.; Cui, S.; Tan, Y. The Association Analysis of GPNMB rs156429 With Clinical Manifestations in Chinese Population With Parkinson’s Disease. Front. Genet. 2020, 11, 952. [Google Scholar] [CrossRef]
- Canafoglia, L.; Robbiano, A.; Pareyson, D.; Panzica, F.; Nanetti, L.; Giovagnoli, A.R.; Venerando, A.; Gellera, C.; Franceschetti, S.; Zara, F. Expanding sialidosis spectrum by genome-wide screening. Neurology 2014, 82, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.i.; Kino, Y.; Yanaizu, M.; Ishida, T.; Saito, Y. Microglia express GPNMB in the brains of Alzheimer’s disease and Nasu-Hakola disease. Intractable Rare Dis. Res. 2019, 8, 120–128. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef]
- Tamargo, I.A.; Bader, M.; Li, Y.; Yu, S.J.; Wang, Y.; Talbot, K.; DiMarchi, R.D.; Pick, C.G.; Greig, N.H. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp. Neurol. 2017, 288, 176–186. [Google Scholar] [CrossRef]
- Verma, A.; Goyal, A. Beyond insulin: The Intriguing role of GLP-1 in Parkinson’s disease. Eur. J. Pharmacol. 2024, 982, 176936. [Google Scholar] [CrossRef]
- Nowell, J.; Blunt, E.; Edison, P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol. Psychiatry 2023, 28, 217–229. [Google Scholar] [CrossRef]
- Vaccari, C.; Grotto, D.; Pereira, T.d.V.; Camargo, J.L.V.d.; Lopes, L.C. GLP-1 and GIP receptor agonists in the treatment of Parkinson’s disease: Translational systematic review and meta-analysis protocol of clinical and preclinical studies. PLoS ONE 2021, 16, e0255726. [Google Scholar] [CrossRef]
- Gürol, G.; Demiralp, D.Ö.; Yılmaz, A.K.; Akman, Ö.; Ateş, N.; Karson, A. Comparative proteomic approach in rat model of absence epilepsy. J. Mol. Neurosci. MN 2015, 55, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Deng, J.; Huang, W.; Liu, L.; Chen, Y.; Jiang, Y.; Liu, G. FAM89A and IFI44L for distinguishing between viral and bacterial infections in children with febrile illness. Pediatr. Investig. 2021, 5, 195–202. [Google Scholar] [CrossRef]
- Gómez-Carballa, A.; Cebey-López, M.; Pardo-Seco, J.; Barral-Arca, R.; Rivero-Calle, I.; Pischedda, S.; Currás-Tuala, M.J.; Gómez-Rial, J.; Barros, F.; Martinón-Torres, F.; et al. A qPCR expression assay of IFI44L gene differentiates viral from bacterial infections in febrile children. Sci. Rep. 2019, 9, 11780. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Irizar, H.; Thygesen, J.H.; Kuchenbaecker, K.; Pain, O.; Adams, R.A.; Zartaloudi, E.; Harju-Seppänen, J.; Austin-Zimmerman, I.; Wang, B.; et al. Transcriptome-wide association study reveals two genes that influence mismatch negativity. Cell Rep. 2021, 34, 108868. [Google Scholar] [CrossRef]
- Oji, A.; Isotani, A.; Fujihara, Y.; Castaneda, J.M.; Oura, S.; Ikawa, M. Tesmin, Metallothionein-Like 5, is Required for Spermatogenesis in Mice†. Biol. Reprod. 2020, 102, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Maremanda, K.P.; Khan, S.; Jena, G. Zinc protects cyclophosphamide-induced testicular damage in rat: Involvement of metallothionein, tesmin and Nrf2. Biochem. Biophys. Res. Commun. 2014, 445, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.D.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Kim, J.H.; Matsubara, T.; Lee, J.; Fenollar-Ferrer, C.; Han, K.; Kim, D.; Jia, S.; Chang, C.J.; Yang, H.; Nagano, T.; et al. Lysosomal SLC46A3 modulates hepatic cytosolic copper homeostasis. Nat. Commun. 2021, 12, 290. [Google Scholar] [CrossRef]
- Navarro-Romero, A.; Fernandez-Gonzalez, I.; Riera, J.; Montpeyo, M.; Albert-Bayo, M.; Lopez-Royo, T.; Castillo-Sanchez, P.; Carnicer-Caceres, C.; Arranz-Amo, J.A.; Castillo-Ribelles, L.; et al. Lysosomal lipid alterations caused by glucocerebrosidase deficiency promote lysosomal dysfunction, chaperone-mediated-autophagy deficiency, and alpha-synuclein pathology. npj Park. Dis. 2022, 8, 1–15. [Google Scholar] [CrossRef]
- Du, T.T.; Wang, L.; Duan, C.L.; Lu, L.L.; Zhang, J.L.; Gao, G.; Qiu, X.B.; Wang, X.M.; Yang, H. GBA deficiency promotes SNCA/a synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy 2015, 11, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Steward, L.J.; Ge, J.; Barnes, N.M. Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br. J. Pharmacol. 1996, 118, 414–420. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Rodríguez-Pallares, J.; Valenzuela, R.; Muñoz, A.; Guerra, M.J.; Baltatu, O.C.; Labandeira-Garcia, J.L. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: Implications for progression of Parkinson’s disease. Eur. J. Neurosci. 2010, 32, 1695–1706. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M.; Diaz-Corrales, F.J.; Miyoshi, K.; Ogawa, N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004, 1029, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pallares, J.; Rey, P.; Parga, J.A.; Muñoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008, 31, 58–73. [Google Scholar] [CrossRef]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Labandeira, C.M.; Guerra, M.J.; Rodriguez-Perez, A.I. The role of the brain renin-angiotensin system in Parkinson’s disease. Transl. Neurodegener. 2024, 13, 22. [Google Scholar] [CrossRef]
- Kobiec, T.; Otero-Losada, M.; Chevalier, G.; Udovin, L.; Bordet, S.; Menéndez-Maissonave, C.; Capani, F.; Pérez-Lloret, S. The Renin–Angiotensin System Modulates Dopaminergic Neurotransmission: A New Player on the Scene. Front. Synaptic Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Aschrafi, A.; Berndt, A.; Kowalak, J.A.; Gale, J.R.; Gioio, A.E.; Kaplan, B.B. Angiotensin II Mediates the Axonal Trafficking of Tyrosine Hydroxylase and Dopamine β-Hydroxylase mRNAs and enhances Norepinephrine Synthesis in Primary Sympathetic Neurons. J. Neurochem. 2019, 150, 666–677. [Google Scholar] [CrossRef]
- Wu, S.A.; Kersten, S.; Qi, L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol. Metab. TEM 2021, 32, 48–61. [Google Scholar] [CrossRef]
- Liu, P.; Ying, Y.; Zhao, Y.; Mundy, D.I.; Zhu, M.; Anderson, R.G.W. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 2004, 279, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Zehmer, J.K.; Bartz, R.; Bisel, B.; Liu, P.; Seemann, J.; Anderson, R.G.W. Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets. J. Cell Sci. 2009, 122, 3694–3702. [Google Scholar] [CrossRef]
- Zehmer, J.K.; Bartz, R.; Liu, P.; Anderson, R.G.W. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J. Cell Sci. 2008, 121, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.W.; Albers, K.; Schlein, C.; Sass, F.; Krott, L.M.; Schmale, H.; Gordts, P.L.; Scheja, L.; Heeren, J. PID1 regulates insulin-dependent glucose uptake by controlling intracellular sorting of GLUT4-storage vesicles. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1592. [Google Scholar] [CrossRef]
- Bueno, D.; Narayan Dey, P.; Schacht, T.; Wolf, C.; Wüllner, V.; Morpurgo, E.; Rojas-Charry, L.; Sessinghaus, L.; Leukel, P.; Sommer, C.; et al. NECAB2 is an endosomal protein important for striatal function. Free Radic. Biol. Med. 2023, 208, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Stacey, D.; Bilbao, A.; Maroteaux, M.; Jia, T.; Easton, A.C.; Longueville, S.; Nymberg, C.; Banaschewski, T.; Barker, G.J.; Büchel, C.; et al. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc. Natl. Acad. Sci. USA 2012, 109, 21128–21133. [Google Scholar] [CrossRef]
- Roussotte, F.F.; Gutman, B.A.; Hibar, D.P.; Jahanshad, N.; Madsen, S.K.; Jack, C.R.; Weiner, M.W.; Thompson, P.M. A single nucleotide polymorphism associated with reduced alcohol intake in the RASGRF2 gene predicts larger cortical volumes but faster longitudinal ventricular expansion in the elderly. Front. Aging Neurosci. 2013, 5, 93. [Google Scholar] [CrossRef]
- Pagani, I.; Poli, G.; Vicenzi, E. TRIM22. A Multitasking Antiviral Factor. Cells 2021, 10, 1864. [Google Scholar] [CrossRef]
- Hernandez-Anzaldo, S.; Brglez, V.; Hemmeryckx, B.; Leung, D.; Filep, J.G.; Vance, J.E.; Vance, D.E.; Kassiri, Z.; Lijnen, R.H.; Lambeau, G.; et al. Novel Role for Matrix Metalloproteinase 9 in Modulation of Cholesterol Metabolism. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e004228. [Google Scholar] [CrossRef]
- Waku, T.; Kobayashi, A. Pathophysiological Potentials of NRF3-Regulated Transcriptional Axes in Protein and Lipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 12686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sam, R.; Sharma, P.; Chen, L.; Do, J.; Sidransky, E. Glucocerebrosidase as a therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 2020, 24, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Toffoli, M.; Schapira, A.H.V. Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues. Pharmacol. Ther. 2023, 246, 108419. [Google Scholar] [CrossRef]
- Atashrazm, F.; Hammond, D.; Perera, G.; Dobson-Stone, C.; Mueller, N.; Pickford, R.; Kim, W.S.; Kwok, J.B.; Lewis, S.J.G.; Halliday, G.M.; et al. Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci. Rep. 2018, 8, 15446. [Google Scholar] [CrossRef]

| Disease | Dataset | Cell Type | Data Type | Reference |
|---|---|---|---|---|
| GSE183484 | iPSCs-macrophages | Expression profiling by array | [47] | |
| GSE41243 | iPSCs-fibroblasts | Expression profiling by array | [48] | |
| GSE21899 | iPSCs-fibroblasts | Expression profiling by array | - | |
| Gaucher disease | GSE263252 | Primary fibroblasts | Expression profiling by high-throughput sequencing | [49] |
| GSE124283 | Primary fibroblasts | Expression profiling by array | [50] | |
| GSE13675 | Chemical-induced MSCs | Expression profiling by array | - | |
| GSE118511 | iPSCs neurons | Expression profiling by high-throughput sequencing | [51] | |
| GSE78152 | iPSC neurons | Expression profiling by array | [52] | |
| GSE62642 | iPSC neurons | Expression profiling by array | [45] | |
| GSE53424 | iPSC neurons | Expression profiling by array | [53] | |
| GSE24378 | iPSC neurons | Expression profiling by high-throughput sequencing | [54] | |
| Parkinson’s Disease | GSE208781 | iPSC organoids | Expression profiling by high-throughput sequencing | [55] |
| GSE99142 | iPSC fibroblasts | Expression profiling by array | - | |
| GSE184956 | Macrophages | Expression profiling by high-throughput sequencing | [56] | |
| GSE205450 | Post-mortem brain tissue | Expression profiling by high-throughput sequencing | [42] | |
| GSE169755 | Post-mortem brain tissue | Expression profiling by high-throughput sequencing | [57] | |
| GSE20186 | Post-mortem brain tissue | Expression profiling by high-throughput sequencing | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Luo, X.; Fleming, R.M.T. GBA1 Gene-Associated Transcriptomic Signatures Reveal Risk Genes in Parkinson’s Disease. Biomedicines 2025, 13, 2799. https://doi.org/10.3390/biomedicines13112799
Liu Y, Luo X, Fleming RMT. GBA1 Gene-Associated Transcriptomic Signatures Reveal Risk Genes in Parkinson’s Disease. Biomedicines. 2025; 13(11):2799. https://doi.org/10.3390/biomedicines13112799
Chicago/Turabian StyleLiu, Yanjun, Xi Luo, and Ronan M. T. Fleming. 2025. "GBA1 Gene-Associated Transcriptomic Signatures Reveal Risk Genes in Parkinson’s Disease" Biomedicines 13, no. 11: 2799. https://doi.org/10.3390/biomedicines13112799
APA StyleLiu, Y., Luo, X., & Fleming, R. M. T. (2025). GBA1 Gene-Associated Transcriptomic Signatures Reveal Risk Genes in Parkinson’s Disease. Biomedicines, 13(11), 2799. https://doi.org/10.3390/biomedicines13112799







