Machine Learning-Based Prediction of IVF Outcomes: The Central Role of Female Preprocedural Factors
Abstract
1. Introduction
2. Results
2.1. Univariate Analysis Highlights Distinct Patterns in IVF Outcomes
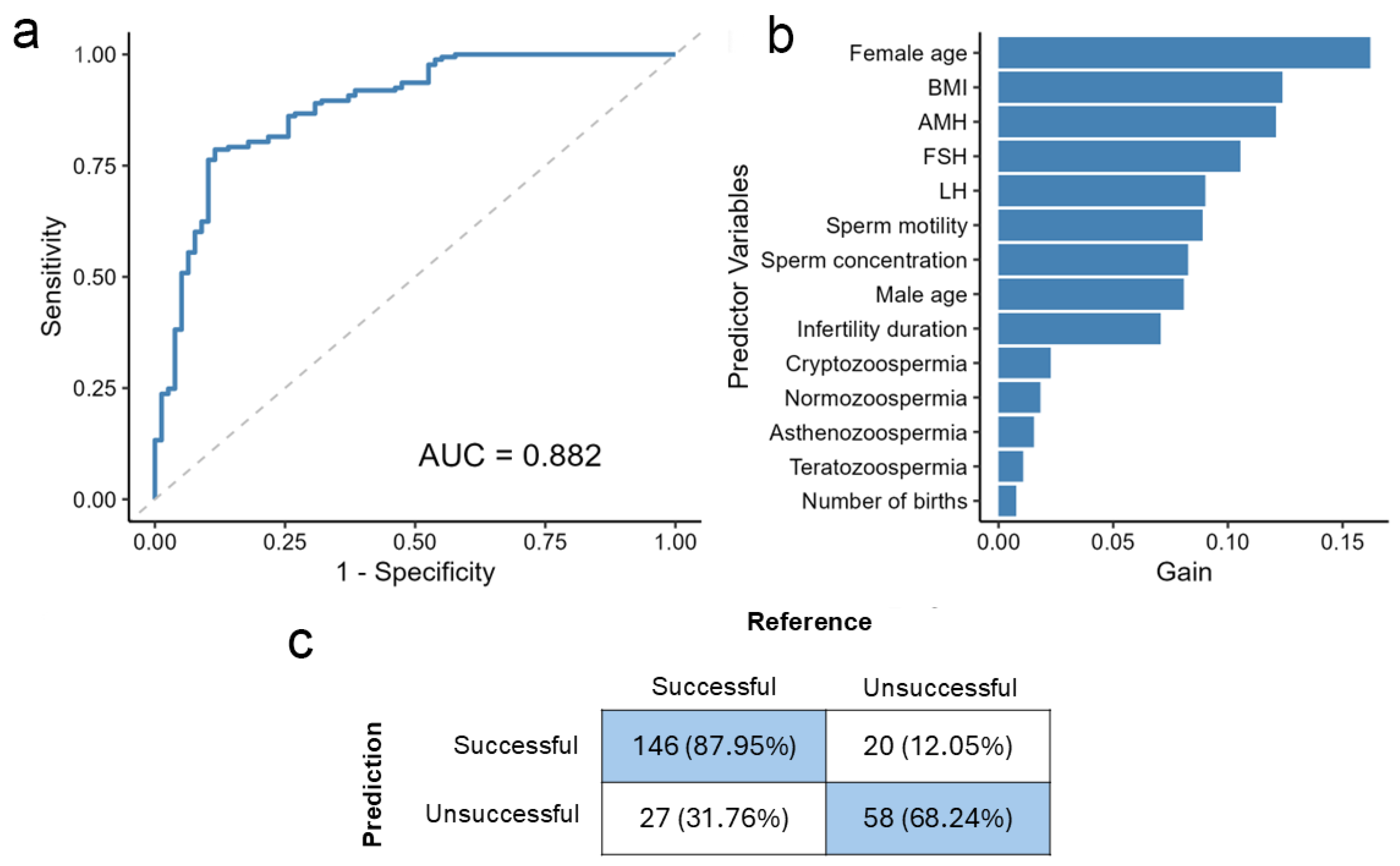
2.2. The Model Achieves High Accuracy in Outcome Prediction Using All Available Clinical Variables
2.3. Removing Negligible Variables Balances Simplicity and Performance
2.4. Feature Metrics Unveil the Impact of Key Predictors on IVF Outcome Classification
2.5. Consistent Performance on an Independent Same-Centre Validation Cohort
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Dataset and Preprocessing
4.3. Univariate Test of Variables
4.4. Balancing the Training Set with SMOTE
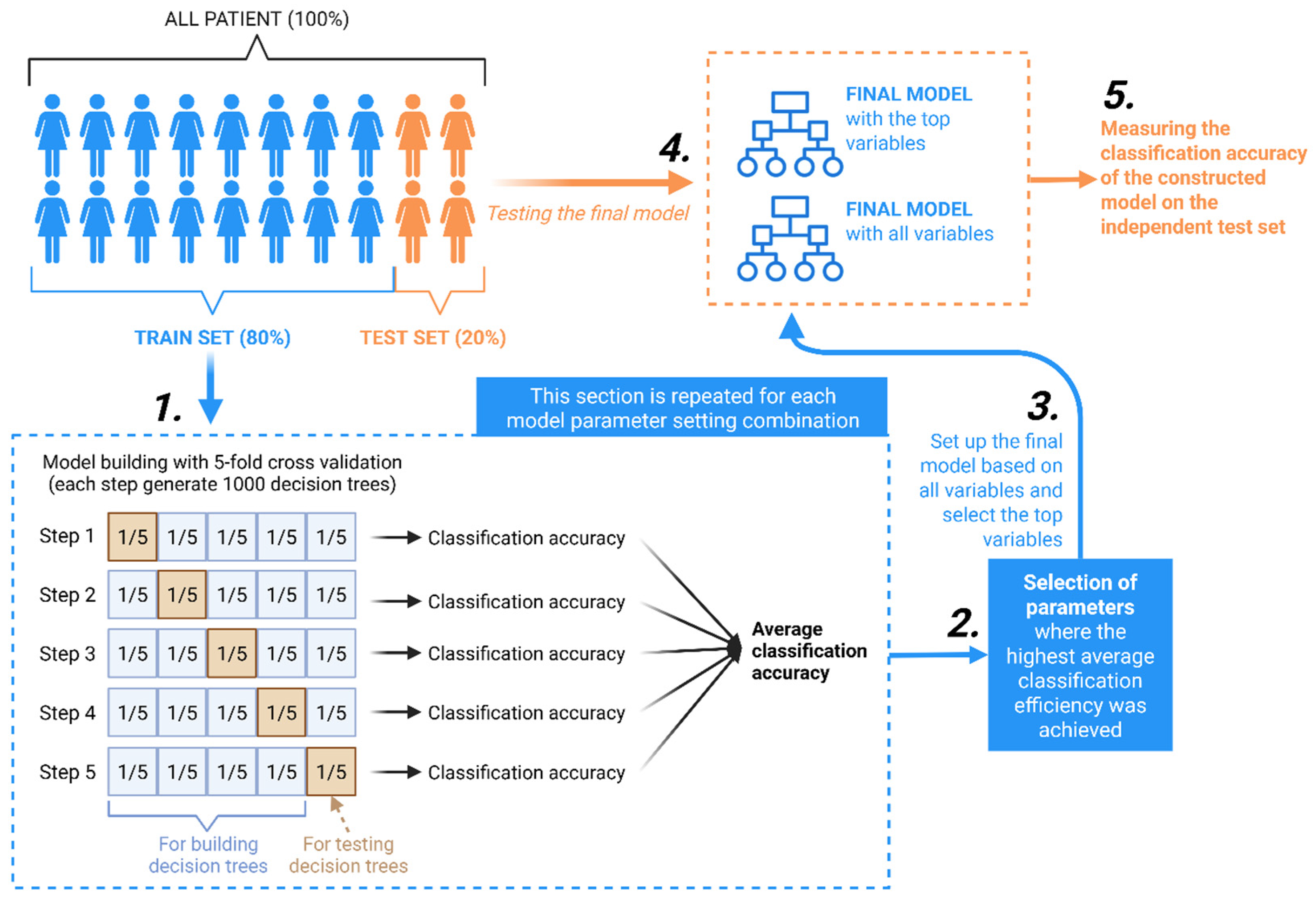
4.5. Model Construction
4.6. Feature Selection and Feature Importance Analysis
4.7. Model Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian Hormone |
| AUC | Area Under the Receiver Operating Characteristic Curve |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DMwR | Data Mining with R |
| FSH | Follicle-Stimulating Hormone |
| HUN-REN | Hungarian Research Network |
| ICSI | Intracytoplasmic Sperm Injection |
| IQR | Interquartile Range |
| IU/L | International Units per Liter |
| IVF | In Vitro Fertilization |
| LH | Luteinizing Hormone |
| Med | Median |
| Min/Max | Minimum/Maximum |
| ML | Machine Learning |
| NIR | No-Information Rate |
| NNGYK | National Public Health and Pharmacy Center |
| NPV | Negative Predictive Value |
| p | p-value |
| PPV | Positive Predictive Value |
| R | R (programming language) |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SMOTE | Synthetic Minority Oversampling Technique |
| SVM | Support Vector Machine |
| XGBoost | Extreme Gradient Boosting |
References
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, D.M.; Grimm, C.K.; West, R.C.; Engelhorn, H.J.; Kile, R.; Reed, L.C.; Swain, J.E.; Katz-Jaffe, M.; Schoolcraft, W.B.; Krisher, R.L.; et al. Maternal Physiology and Blastocyst Morphology Are Correlated with an Inherent Difference in Peri-Implantation Human Embryo Development. Fertil. Steril. 2022, 117, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Zaninovic, N.; Rosenwaks, Z. Artificial Intelligence in Human In Vitro Fertilization and Embryology. Fertil. Steril. 2020, 114, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.S.; Pavlovic, Z.J.; Hariton, E. The Role of Artificial Intelligence and Machine Learning in Assisted Reproductive Technologies. Obstet. Gynecol. Clin. N. Am. 2023, 50, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Curchoe, C.L.; Flores-Saiffe Farias, A.; Mendizabal-Ruiz, G.; Chavez-Badiola, A. Evaluating Predictive Models in Reproductive Medicine. Fertil. Steril. 2020, 114, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Olawade, D.B.; Teke, J.; Adeleye, K.K.; Weerasinghe, K.; Maidoki, M.; Clement David-Olawade, A. Artificial Intelligence in In-Vitro Fertilization (IVF): A New Era of Precision and Personalization in Fertility Treatments. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102903. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Wei, M.; Wang, L.; Su, X.; Zhao, B.; You, Z. Multi-Hop Graph Structural Modeling for Cancer-Related circRNA-miRNA Interaction Prediction. Pattern Recognit. 2026, 170, 112078. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zhao, B.; Su, X.; Li, G.; Hu, L. DeepHIV: A Sequence-Based Deep Learning Model for Predicting HIV-1 Protease Cleavage Sites. IEEE Trans. Comput. Biol. Bioinforma. 2025, 1–8. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on Ovarian Stimulation; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; et al. ESHRE Guideline: Ovarian Stimulation for IVF/ICSI. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B.; Stern, J.E.; Missmer, S.A.; Fujimoto, V.Y.; Leach, R.; A SART Writing Group. Female Obesity Adversely Affects Assisted Reproductive Technology (ART) Pregnancy and Live Birth Rates. Hum. Reprod. 2011, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Jindal, S.; Kalra, S.; Mersereau, J.; Reindollar, R.; et al. Obesity and Reproduction: A Committee Opinion. Fertil. Steril. 2021, 116, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Bai, L.; mei-Zhou, J.; yu-Wang, X.; Chen, L.; Zhang, J. Analysis of Factors Associated with IUI Pregnancy Outcomes in Elderly and Young Patients. BMC Womens Health 2024, 24, 86. [Google Scholar] [CrossRef] [PubMed]
- Vedelek, V.; Bicskei, P.; Tábi, M.; Lajkó, N.; Ékes, C.; Bereczki, K.; Meixner-Csáti, Z.; Sinka, R.; Vágvölgyi, A.; Zádori, J. Endometrium Development Patterns and BMI Groups among in Vitro Fertilization Patients; Prognostic Aspects. Front. Endocrinol. 2024, 15, 1379109. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Steyerberg, E.W.; Te Velde, E.R.; Lee, A.J.; Bhattacharya, S. Predicting the Chances of a Live Birth after One or More Complete Cycles of In Vitro Fertilisation: Population Based Study of Linked Cycle Data from 113 873 Women. BMJ 2016, 355, i5735. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B.; Wantman, E.; Stern, J.E.; Baker, V.L.; Widra, E.; Coddington, C.C.; Gibbons, W.E.; Ball, G.D. A Prediction Model for Live Birth and Multiple Births within the First Three Cycles of Assisted Reproductive Technology. Fertil. Steril. 2014, 102, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Van Loendersloot, L.L.; Van Wely, M.; Repping, S.; Bossuyt, P.M.M.; Van Der Veen, F. Individualized Decision-Making in IVF: Calculating the Chances of Pregnancy. Hum. Reprod. 2013, 28, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Lawlor, D.A. Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles. PLoS Med. 2011, 8, e1000386. [Google Scholar] [CrossRef] [PubMed]
- Leijdekkers, J.A.; Eijkemans, M.J.C.; Van Tilborg, T.C.; Oudshoorn, S.C.; Van Golde, R.J.T.; Hoek, A.; Lambalk, C.B.; De Bruin, J.P.; Fleischer, K.; Mochtar, M.H.; et al. Cumulative Live Birth Rates in Low-Prognosis Women. Hum. Reprod. 2019, 34, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology; World Health Organization International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary of ART Terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]


| Variable | Metrics | IVF—Unsuccessful | IVF—Successful | Total | p |
|---|---|---|---|---|---|
| Female age (years) | Min/Max | 19.0/47.0 | 20.0/44.0 | 19.0/47.0 | <0.0001 |
| Med [IQR] | 37.0 [33.0; 41.0] | 34.0 [31.0; 37.0] | 36.0 [32.0; 40.0] | ||
| Mean (SD) | 36.6 (5.1) | 33.9 (4.4) | 35.8 (5.1) | ||
| AMH (pmol/L) | Min/Max | 0.01/17.0 | 0.1/17.0 | 0.01/17.0 | <0.0001 |
| Med [IQR] | 1.6 [0.8; 2.8] | 2.1 [1.3; 3.8] | 1.8 [0.9; 3.3] | ||
| Mean (SD) | 2.2 (2.2) | 2.9 (2.4) | 2.5 (2.3) | ||
| FSH (IU/L) | Min/Max | 0.3/26.3 | 0.3/31.1 | 0.3/31.1 | <0.0001 |
| Med [IQR] | 7.4 [6.0; 9.4] | 6.7 [5.4; 8.2] | 7.2 [5.8; 8.9] | ||
| Mean (SD) | 8.1 (3.3) | 7.1 (2.6) | 7.8 (3.0) | ||
| LH (IU/L) | Min/Max | 0.1/28.0 | 0.1/22.6 | 0.1/28.0 | 0.216 |
| Med [IQR] | 5.7 [4.2; 7.3] | 5.4 [4.1; 7.2] | 5.6 [4.2; 7.3] | ||
| Mean (SD) | 6.0 (2.6) | 5.9 (2.8) | 6.0 (2.7) | ||
| BMI (kg/m2) | Min/Max | 16.6/46.6 | 11.2/44.1 | 11.2/46.6 | 0.491 |
| Med [IQR] | 23.7 [21.2; 27.5] | 23.7 [20.6; 27.9] | 23.7 [21.0; 27.7] | ||
| Mean (SD) | 24.9 (5.1) | 24.7 (5.2) | 24.8 (5.1) | ||
| Infertility duration (years) | Min/Max | 0.5/22.0 | 0.5/15.0 | 0.5/22.0 | 0.095 |
| Med [IQR] | 4.0 [2.0; 6.5] | 3.0 [2.0; 5.0] | 4.0 [2.0; 6.0] | ||
| Mean (SD) | 4.5 (3.2) | 4.3 (2.8) | 4.4 (3.1) | ||
| Number of births | Min/Max | 0/3.0 | 0/3.0 | 0/3.0 | 0.890 |
| Med [IQR] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] | ||
| Mean (SD) | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.5) | ||
| Male age (years) | Min/Max | 24.0/60.0 | 21.0/60.0 | 21.0/60.0 | <0.0001 |
| Med [IQR] | 39.0 [35.0; 44.0] | 37.0 [34.0; 43.0] | 38.0 [34.0; 43.0] | ||
| Mean (SD) | 39.2 (6.3) | 37.4 (5.8) | 38.6 (6.2) | ||
| Sperm concentration (×106/mL) | Min/Max | 0.02/250.0 | 0.02/250.0 | 0.02/250.0 | 0.252 |
| Med [IQR] | 40.0 [12.0; 70.0] | 40.0 [14.0; 72.0] | 40.0 [12.0; 70.0] | ||
| Mean (SD) | 46.6 (41.5) | 49.5 (42.7) | 47.5 (41.9) | ||
| Sperm motility (%) | Min/Max | 1.0/90.0 | 0.0/90.0 | 0.0/90.0 | 0.188 |
| Med [IQR] | 45.0 [30.0; 55.0] | 45.0 [30.0; 60.0] | 45.0 [30.0; 55.0] | ||
| Mean (SD) | 42.7 (17.3) | 44.0 (17.2) | 43.1 (17.3) | ||
| Normozoospermia (n; %) | Yes (%) | 393 (66.95%) | 194 (33.05%) | 587 (41.87%) | 0.150 |
| Asthenozoospermia (n; %) | Yes (%) | 460 (69.70%) | 200 (30.30%) | 660 (47.88%) | 0.618 |
| Teratozoospermia (n; %) | Yes (%) | 352 (68.48%) | 162 (31.52%) | 514 (36.66%) | 0.729 |
| Cryptozoospermia (n; %) | Yes (%) | 67 (69.79%) | 29 (30.21%) | 96 (6.85%) | 0.870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bereczki, K.; Bukva, M.; Vedelek, V.; Nádasdi, B.; Kozinszky, Z.; Sinka, R.; Bereczki, C.; Vágvölgyi, A.; Zádori, J. Machine Learning-Based Prediction of IVF Outcomes: The Central Role of Female Preprocedural Factors. Biomedicines 2025, 13, 2768. https://doi.org/10.3390/biomedicines13112768
Bereczki K, Bukva M, Vedelek V, Nádasdi B, Kozinszky Z, Sinka R, Bereczki C, Vágvölgyi A, Zádori J. Machine Learning-Based Prediction of IVF Outcomes: The Central Role of Female Preprocedural Factors. Biomedicines. 2025; 13(11):2768. https://doi.org/10.3390/biomedicines13112768
Chicago/Turabian StyleBereczki, Kristóf, Mátyás Bukva, Viktor Vedelek, Bernadett Nádasdi, Zoltán Kozinszky, Rita Sinka, Csaba Bereczki, Anna Vágvölgyi, and János Zádori. 2025. "Machine Learning-Based Prediction of IVF Outcomes: The Central Role of Female Preprocedural Factors" Biomedicines 13, no. 11: 2768. https://doi.org/10.3390/biomedicines13112768
APA StyleBereczki, K., Bukva, M., Vedelek, V., Nádasdi, B., Kozinszky, Z., Sinka, R., Bereczki, C., Vágvölgyi, A., & Zádori, J. (2025). Machine Learning-Based Prediction of IVF Outcomes: The Central Role of Female Preprocedural Factors. Biomedicines, 13(11), 2768. https://doi.org/10.3390/biomedicines13112768






