Cumulative Hydrocortisone Exposure and Early Brain Volumetrics in Very Low Birth Weight Infants: Associations with Neurodevelopmental Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subject Selection
2.2. Administration of HCS in NICU: Hemodynamic Support and BPD Prevention/Treatment
2.3. Data Collection and Definitions
2.4. MRI Acquisition and 3D Volumetric Assessment with Semi-Automatic Segmentation
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Clinical Characteristics of the Study Population
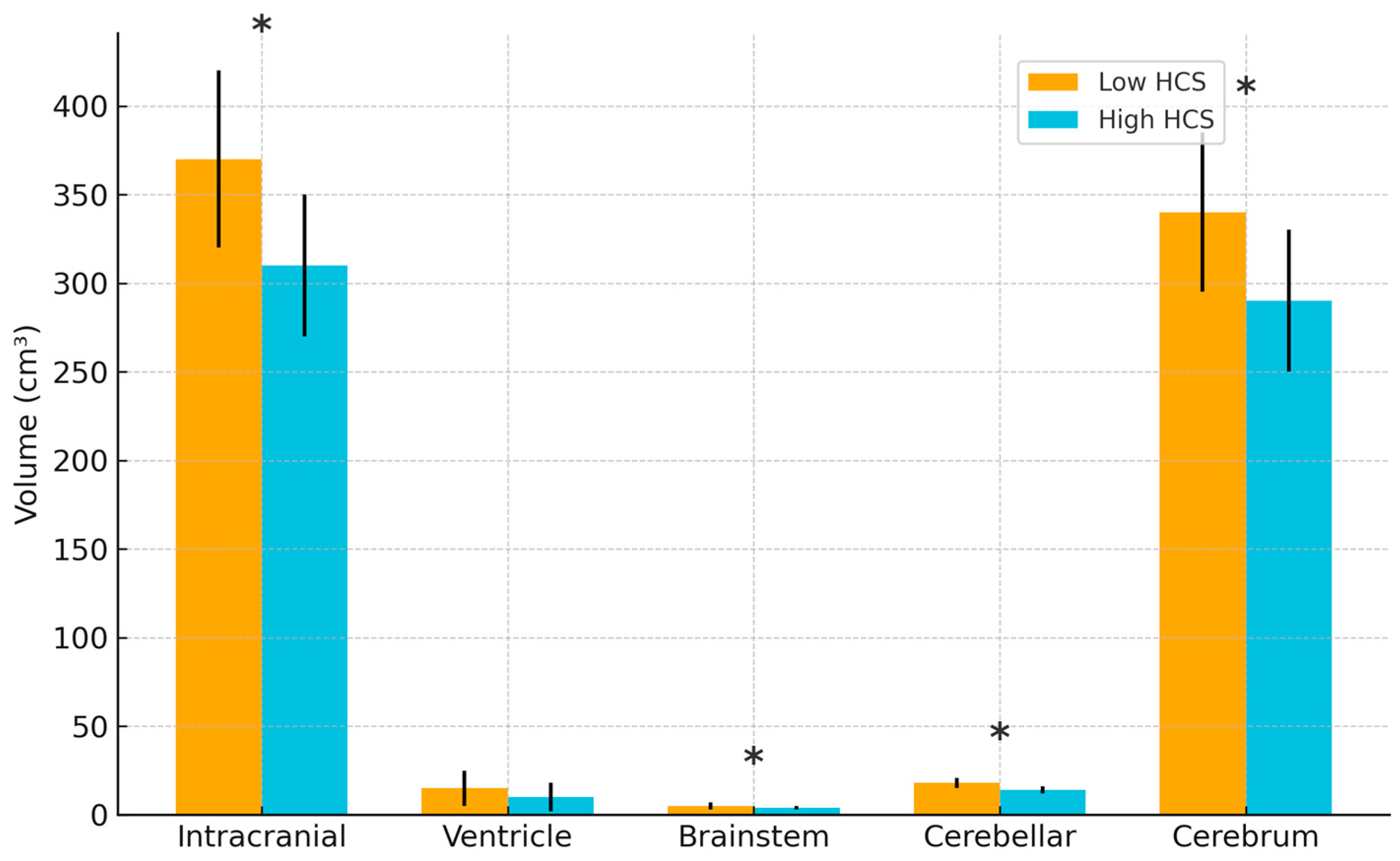
3.2. Association Between Cumulative Hydrocortisone Dose and Brain Volume
3.3. Association Between Cumulative Hydrocortisone Dose and Long-Term Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Brain Volume | Without HCS [N = 23] | HCS for Low BP [N = 49] | Both Low BP and BPD [N = 51] | HCS for BPD [N = 23] |
|---|---|---|---|---|
| Cumulative HCS dose, mg/kg | NA | 78.1 ± 58.9 | 168.9 ± 123.1 | 48.7 ± 54.5 |
| Duration of HCS therapy, days | NA | 18.0 ± 13.6 | 46.9 ± 33.0 | 11.7 ± 12.5 |
| PND of HCS initiation, days | NA | 3.9 ± 6.1 | 3.7 ± 7.4 | 18.0 ± 16.1 |
| PMA at HCS initiation, weeks | NA | 28.8 ± 2.4 | 27.7 ± 1.8 | 31.3 ± 2.7 |
| Brain Volume | Low HCS [N = 58] | High HCS [N = 37] | p |
|---|---|---|---|
| Intracranial volume, cm3 | 371.2 ± 66.1 | 309.7 ± 57.5 | <0.001 |
| Ventricle volume, cm3 | 14.3 ± 33.5 | 11.1 ± 19.7 | 0.531 |
| Brainstem volume, cm3 | 5.3 ± 0.8 | 4.4 ± 0.8 | <0.001 |
| Cerebellar volume, cm3 | 19.6 ± 5.9 | 13.8 ± 5.8 | <0.001 |
| Cerebrum volume, cm3 | 346.4 ± 61.8 | 292.5 ± 52.5 | <0.001 |
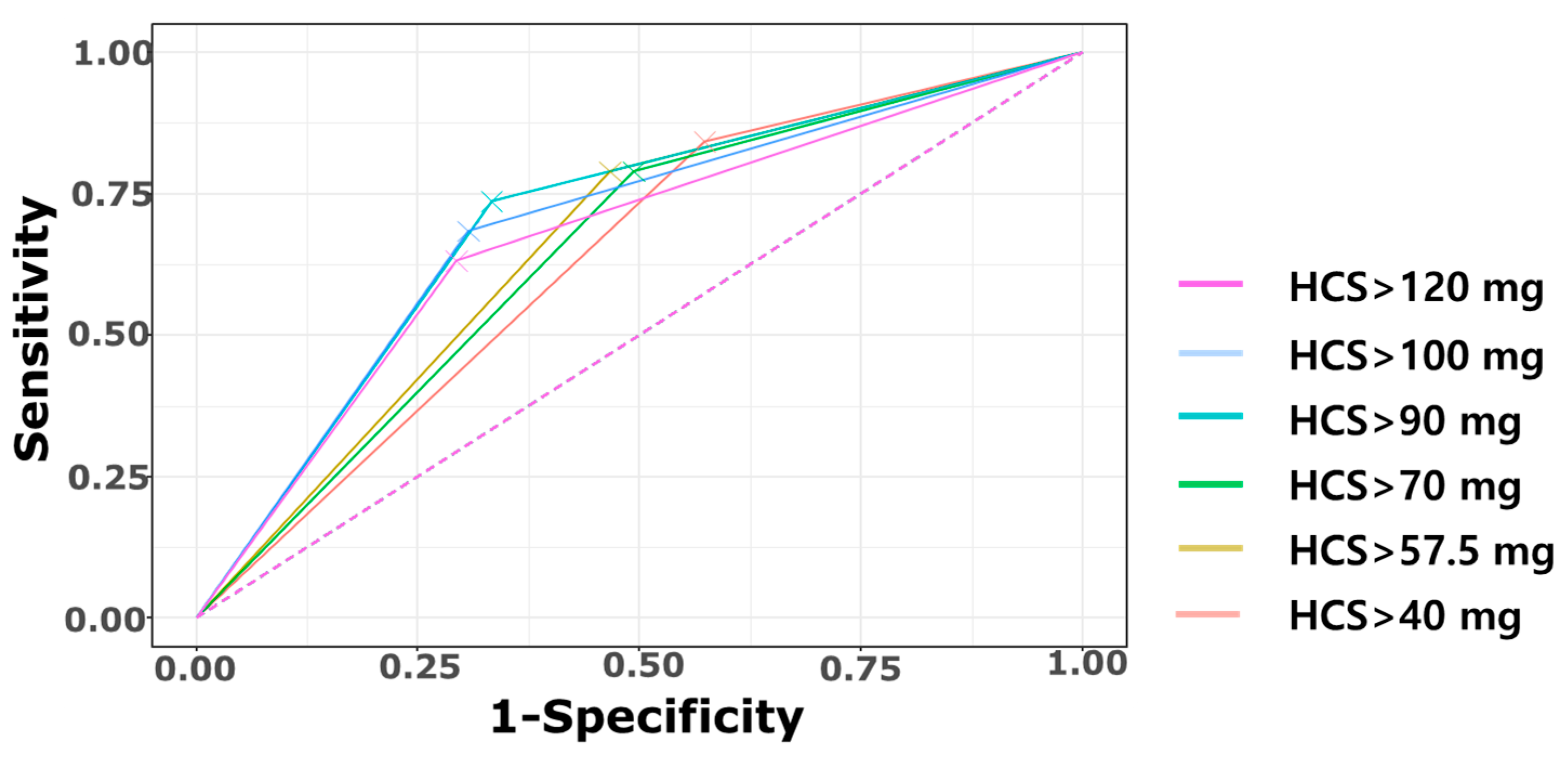
| Cumulative HCS Dose | >40 mg/kg | >57.5 mg/kg | >70 mg/kg | >90 mg/kg | >100 mg/kg | >120 mg/kg |
|---|---|---|---|---|---|---|
| Cerebral palsy | 16/59 (27.1%) | 15/50 (30.0%) | 15/52 (28.8%) | 14/39 (35.9%) | 13/36 (36.1%) | 12/34 (35.3%) |
| TP | 16 | 15 | 15 | 14 | 13 | 12 |
| FN | 3 | 4 | 4 | 5 | 6 | 7 |
| FP | 43 | 35 | 37 | 25 | 23 | 22 |
| TN | 32 | 40 | 38 | 50 | 52 | 53 |
| Sensitivity | 84.2% (16/19) | 78.9% (15/19) | 78.9%(15/19) | 73.7% (14/19) | 68.4% (13/19) | 63.2% (12/19) |
| Specificity | 42.7% (32/75) | 53.3% (40/75) | 50.7% (38/75) | 66.7% (50/75) | 69.3% (52/75) | 70.7% (53/75) |
| PPV | 27.1% (16/59) | 30.0% (15/50) | 28.8% (15/52) | 35.9% (14/39) | 36.1% (13/36) | 35.3% (12/34) |
| NPV | 91.4% (32/35) | 90.9% (40/44) | 90.4% (38/42) | 90.9% (50/55) | 89.7% (52/58) | 88.3% (53/60) |
| Accuracy | 51.0% | 58.5% | 56.4% | 68.1% | 69.1% | 69.1% |
| Youden Index | 0.269 | 0.323 | 0.296 | 0.404 | 0.378 | 0.338 |
| AUC (95% CI) | 0.634 (0.533–0.736) | 0.661 (0.551–0.771) | 0.648 (0.538–0.758) | 0.702 (0.587–0.817) | 0.689 (0.569–0.808) | 0.669 (0.546–0.792) |
| CP | NDI | Head Circumference < 10th | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Maternal characteristics | |||||||||
| Maternal age, years | 1.10 | 0.97–1.24 | 0.124 | 1.02 | 0.93–1.11 | 0.713 | 0.99 | 0.88–1.12 | 0.879 |
| Maternal hypertension | NA * | NA | 0.989 | 0.72 | 0.22–2.31 | 0.580 | 0.99 | 0.22–4.54 | 0.991 |
| Delivered by C/S | 0.92 | 0.23–3.68 | 0.902 | 1.05 | 0.40–2.72 | 0.919 | 0.54 | 0.15–1.90 | 0.337 |
| Histologic chorioamnionitis | 1.04 | 0.36–2.95 | 0.946 | 1.38 | 0.64–2.99 | 0.414 | 0.69 | 0.24–2.00 | 0.493 |
| Antenatal steroid | 0.93 | 0.31–2.77 | 0.894 | 1.22 | 0.53–2.85 | 0.640 | 1.38 | 0.41–4.65 | 0.604 |
| Infantile characteristics | |||||||||
| GA, weeks | 0.98 | 0.77–1.26 | 0.899 | 0.9 | 0.75–1.06 | 0.210 | 0.88 | 0.70–1.12 | 0.304 |
| BW, grams | 1.00 | 1.00–1.00 | 0.942 | 1.00 | 1.00–1.00 | 0.259 | 1.00 | 0.99–1.00 | 0.009 |
| 5 min Apgar score | 0.90 | 0.68–1.18 | 0.437 | 0.80 | 0.64–1.00 | 0.057 | 1.04 | 0.79–1.37 | 0.778 |
| Male sex | 2.48 | 0.85–7.21 | 0.096 | 1.74 | 0.84–3.64 | 0.138 | 1.57 | 0.59–4.19 | 0.369 |
| Small for gestational age | 0.41 | 0.05–3.43 | 0.409 | 0.72 | 0.22–2.31 | 0.580 | 3.17 | 0.69–14.6 | 0.138 |
| Short-term Neonatal Outcomes | |||||||||
| Respiratory distress syndrome | NA * | NA | NA | NA * | NA | NA | 0.60 | 0.04–9.94 | 0.718 |
| Treated patent ductus arteriosus | 2.92 | 1.04–8.20 | 0.042 | 2.17 | 1.03–4.68 | 0.045 | 2.58 | 0.93–7.15 | 0.067 |
| Moderate to severe BPD | 2.31 | 0.48–11.03 | 0.296 | 3.00 | 1.15–8.49 | 0.029 | 3.18 | 0.63–16.03 | 0.162 |
| Necrotizing enterocolitis | NA * | NA | NA | 0.68 | 0.16–2.69 | 0.578 | 0.81 | 0.14–4.78 | 0.818 |
| Periventricular leukomalacia | 6.75 | 2.17–21.00 | <0.001 | 3.47 | 1.58–8.02 | 0.003 | 1.78 | 0.65–4.83 | 0.261 |
| Intraventricular hemorrhage ≥ 2 | 1.33 | 0.34–5.18 | 0.678 | 2.92 | 1.21–7.48 | 0.020 | 1.44 | 0.44–4.75 | 0.546 |
| Retinopathy of prematurity | 0.99 | 0.19–5.07 | 0.986 | 1.02 | 0.32–3.37 | 0.972 | 0.43 | 0.08–2.24 | 0.315 |
| Culture-proven sepsis | 0.98 | 0.35–2.71 | 0.965 | 1.30 | 0.62–2.72 | 0.491 | 1.60 | 0.59–4.32 | 0.354 |
| Duration of PN, days | 1.00 | 0.99–1.02 | 0.452 | 1.00 | 0.99–1.01 | 0.471 | 0.99 | 0.98–1.00 | 0.188 |
| Length of stay, days | 1.01 | 1.00–1.02 | 0.09 | 1.02 | 1.01–1.04 | <0.001 | 1.02 | 1.00–1.04 | 0.011 |
| Regional Brain volume | |||||||||
| Intracranial volume | 1.00 | 1.00–1.00 | 0.927 | 1.00 | 1.00–1.00 | 0.755 | 0.99 | 0.99–1.00 | 0.027 |
| Ventricle volume | 1.01 | 0.97–1.05 | 0.762 | 1.02 | 1.00–1.05 | 0.145 | 0.97 | 0.91–1.03 | 0.333 |
| Brainstem volume | 0.85 | 0.59–1.23 | 0.393 | 0.96 | 0.76–1.20 | 0.700 | 0.62 | 0.38–1.01 | 0.054 |
| Cerebellar volume | 1.00 | 0.97–1.04 | 0.946 | 0.99 | 0.97–1.02 | 0.642 | 0.93 | 0.88–1.00 | 0.039 |
| Cerebrum volume | 1.00 | 1.00–1.00 | 0.915 | 1.00 | 1.00–1.00 | 0.774 | 0.99 | 0.99–1.00 | 0.027 |
| HCS > 90 mg/kg | 5.60 | 1.81–17.31 | 0.003 | 3.79 | 1.74–8.61 | 0.001 | 7.42 | 2.49–22.15 | <0.001 |
References
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V. Hyperoxia-derived lung damage in preterm infants. Semin. Fetal Neonatal Med. 2010, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Seri, I. Neonatal blood pressure support: The use of inotropes, lusitropes, and other vasopressor agents. Clin. Perinatol. 2012, 39, 221–238. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Policy statement—Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010, 126, 800–808. [Google Scholar] [CrossRef]
- Puia-Dumitrescu, M.; Wood, T.R.; Comstock, B.A.; Law, J.B.; German, K.; Perez, K.M.; Gogcu, S.; Mayock, D.E.; Heagerty, P.J.; Juul, S.E. Dexamethasone, prednisolone, and methylprednisolone use and 2-year neurodevelopmental outcomes in extremely preterm infants. JAMA Netw. Open 2022, 5, e221947. [Google Scholar] [CrossRef]
- Gill, A.; Weindling, A. Randomised controlled trial of plasma protein fraction versus dopamine in hypotensive very low birthweight infants. Arch. Dis. Child. 1993, 69, 284–287. [Google Scholar] [CrossRef]
- Al-Aweel, I.; Pursley, D.M.; Rubin, L.P.; Shah, B.; Weisberger, S.; Richardson, D.K. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J. Perinatol. 2001, 21, 272–278. [Google Scholar] [CrossRef]
- Pejovic, B.; Peco-Antic, A.; Marinkovic-Eric, J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr. Nephrol. 2007, 22, 249–257. [Google Scholar] [CrossRef]
- Stranak, Z.; Semberova, J.; Barrington, K.; O’Donnell, C.; Marlow, N.; Naulaers, G.; Dempsey, E.; HIP consortium. International survey on diagnosis and management of hypotension in extremely preterm babies. Eur. J. Pediatr. 2014, 173, 793–798. [Google Scholar] [CrossRef]
- Batton, B.; Zhu, X.; Fanaroff, J.; Kirchner, H.L.; Berlin, S.; Wilson-Costello, D.; Walsh, M. Blood pressure, anti-hypotensive therapy, and neurodevelopment in extremely preterm infants. J. Pediatr. 2009, 154, 351–357.e1. [Google Scholar] [CrossRef]
- Batton, B.; Li, L.; Newman, N.S.; Das, A.; Watterberg, K.L.; Yoder, B.A.; Faix, R.G.; Laughon, M.M.; Stoll, B.J.; Van Meurs, K.P. Use of antihypotensive therapies in extremely preterm infants. Pediatrics 2013, 131, e1865–e1873. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Thompson, R.J., Jr.; Oehler, J.M.; Brazy, J.E. Influence of acidosis, hypoxemia, and hypotension on neurodevelopmental outcome in very low birth weight infants. Pediatrics 1995, 95, 238–243. [Google Scholar] [PubMed]
- Watkins, A.; West, C.; Cooke, R. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum. Dev. 1989, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Barrington, K.J.; Janaillac, M. Treating hypotension in extremely preterm infants. The pressure is mounting. Arch. Dis. Child.-Fetal Neonatal Ed. 2016, 101, F188–F189. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.J.; Groves, A.M. Inotropes in preterm infants–evidence for and against. Acta Paediatr. 2012, 101, 17–23. [Google Scholar] [CrossRef]
- Dempsey, E.; Barrington, K. Diagnostic criteria and therapeutic interventions for the hypotensive very low birth weight infant. J. Perinatol. 2006, 26, 677–681. [Google Scholar] [CrossRef]
- Ng, P.; Lam, C.; Fok, T.; Lee, C.; Ma, K.; Chan, I.; Wong, E. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 84, F122–F124. [Google Scholar] [CrossRef]
- Cheong, J.; Doyle, L.; Ehrenkranz, R.; Halliday, H. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Hay, S.; Manley, B.J.; Halliday, H.L. Late (≥7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD001145. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Scott, S.M. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics 1995, 95, 120–125. [Google Scholar] [CrossRef]
- Cummings, J.J.; D’Eugenio, D.B.; Gross, S.J. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N. Engl. J. Med. 1989, 320, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Nanda, D.; Bandiya, P.; Ahmed, J.; Garg, A.; Roehr, C.C.; Nangia, S. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: A systematic review and network meta-analysis. JAMA Pediatr. 2021, 175, e206826. [Google Scholar] [CrossRef] [PubMed]
- Avery, G.B.; Fletcher, A.B.; Kaplan, M.; Brudno, D.S. Controlled trial of dexamethasone in respirator-dependent infants with bronchopulmonary dysplasia. Pediatrics 1985, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Fauser, A.; Pohlandt, F.; Bartmann, P.; Gortner, L. Rapid increase of blood pressure in extremely low birth weight infants after a single dose of dexamethasone. Eur. J. Pediatr. 1993, 152, 354–356. [Google Scholar] [CrossRef]
- Tantivit, P.; Subramanian, N.; Garg, M.; Ramanathan, R.; deLemos, R.A. Low serum cortisol in term newborns with refractory hypotension. J. Perinatol. 1999, 19, 352–357. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Kothadia, J.M.; Klinepeter, K.L.; Goldstein, D.J.; Jackson, B.G.; Weaver, R.G.; Dillard, I.; G, R. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: Outcome of study participants at 1-year adjusted age. Pediatrics 1999, 104, 15–21. [Google Scholar] [CrossRef]
- Gordon, P.V.; Young, M.L.; Marshall, D.D. Focal small bowel perforation: An adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J. Perinatol. 2001, 21, 156–160. [Google Scholar] [CrossRef]
- Stoll, B.J.; Temprosa, M.; Tyson, J.E.; Papile, L.-A.; Wright, L.L.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Lemons, J.A.; Fanaroff, A.A. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics 1999, 104, e63. [Google Scholar] [CrossRef]
- Doyle, L.W.; Ehrenkranz, R.A.; Halliday, H.L. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: A systematic review. Neonatology 2010, 98, 217–224. [Google Scholar] [CrossRef]
- Rios, D.R.; Moffett, B.S.; Kaiser, J.R. Trends in pharmacotherapy for neonatal hypotension. J. Pediatr. 2014, 165, 697–701.e1. [Google Scholar] [CrossRef]
- Morris, I.P.; Goel, N.; Chakraborty, M. Efficacy and safety of systemic hydrocortisone for the prevention of bronchopulmonary dysplasia in preterm infants: A systematic review and meta-analysis. Eur. J. Pediatr. 2019, 178, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.V.; Kumar, G.; Pullattayil, S.A.K.; Aradhya, A.S.; Suryawanshi, P.; Sahni, M.; Khurana, S.; Saini, S.S.; K, R.; Dhir, S.K. Timing of hydrocortisone therapy in neonates with shock: A systematic review, meta-analysis, and clinical practice guideline. Front. Pediatr. 2025, 13, 1491976. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Trousson, C.; Biran, V.; Leroy, E.; Mohamed, D.; Alberti, C.; Group, P.T. Association between early low-dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA 2017, 317, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Bonsante, F.; Latorre, G.; Iacobelli, S.; Forziati, V.; Laforgia, N.; Esposito, L.; Mautone, A. Early low-dose hydrocortisone in very preterm infants: A randomized, placebo-controlled trial. Neonatology 2007, 91, 217–221. [Google Scholar] [CrossRef]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchée, A.; Bolot, P. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef]
- Onland, W.; Cools, F.; Kroon, A.; Rademaker, K.; Merkus, M.P.; Dijk, P.H.; van Straaten, H.L.; Te Pas, A.B.; Mohns, T.; Bruneel, E. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: A randomized clinical trial. JAMA 2019, 321, 354–363. [Google Scholar] [CrossRef]
- Halbmeijer, N.M.; Onland, W.; Cools, F.; Swarte, R.; Van der Heide-Jalving, M.; Merkus, M.P.; Van Kaam, A.H.; Dijk, P.H.; Mulder-de Tollenaer, S.; Tan, R. Effect of systemic hydrocortisone initiated 7 to 14 days after birth in ventilated preterm infants on mortality and neurodevelopment at 2 years’ corrected age: Follow-up of a randomized clinical trial. JAMA 2021, 326, 355–357. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Walsh, M.C.; Li, L.; Chawla, S.; D’Angio, C.T.; Goldberg, R.N.; Hintz, S.R.; Laughon, M.M.; Yoder, B.A.; Kennedy, K.A. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N. Engl. J. Med. 2022, 386, 1121–1131. [Google Scholar] [CrossRef]
- Kersbergen, K.J.; de Vries, L.S.; van Kooij, B.J.; Išgum, I.; Rademaker, K.J.; van Bel, F.; Hüppi, P.S.; Dubois, J.; Groenendaal, F.; Benders, M.J. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J. Pediatr. 2013, 163, 666–671.e1. [Google Scholar] [CrossRef]
- Rademaker, K.J.; Uiterwaal, C.S.; Groenendaal, F.; Venema, M.M.U.; van Bel, F.; Beek, F.J.; van Haastert, I.C.; Grobbee, D.E.; de Vries, L.S. Neonatal hydrocortisone treatment: Neurodevelopmental outcome and MRI at school age in preterm-born children. J. Pediatr. 2007, 150, 351–357. [Google Scholar] [CrossRef]
- Im, S.-A.; Tomita, E.; Oh, M.Y.; Kim, S.Y.; Kang, H.M.; Youn, Y.-A. Volumetric changes in brain MRI of infants with hypoxic-ischemic encephalopathy and abnormal neurodevelopment who underwent therapeutic hypothermia. Brain Res. 2024, 1825, 148703. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kashimada, K.; Amano, N.; Takasawa, K.; Nakamura-Utsunomiya, A.; Yatsuga, S.; Mukai, T.; Ida, S.; Isobe, M.; Fukushi, M. Clinical guidelines for the diagnosis and treatment of 21-hydroxylase deficiency (2021 revision). Clin. Pediatr. Endocrinol. 2022, 31, 116–143. [Google Scholar] [CrossRef] [PubMed]
- NHBPE Program. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am. J. Obstet. Gynecol. 2000, 183, s1–s22. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.-H.; Syu, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1. [Google Scholar] [CrossRef]
- Papile, L.-A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Garner, A. An international classification of retinopathy of prematurity. Arch. Ophthalmol. 1984, 102, 1130–1134. [Google Scholar]
- Engle, W.A. Age terminology during the perinatal period. Pediatrics 2004, 114, 1362–1364. [Google Scholar] [CrossRef]
- Gunel, M.K.; Mutlu, A.; Tarsuslu, T.; Livanelioglu, A. Relationship among the Manual Ability Classification System (MACS), the Gross Motor Function Classification System (GMFCS), and the functional status (WeeFIM) in children with spastic cerebral palsy. Eur. J. Pediatr. 2009, 168, 477–485. [Google Scholar] [CrossRef]
- Noori, S.; Friedlich, P.; Wong, P.; Ebrahimi, M.; Siassi, B.; Seri, I. Hemodynamic changes after low-dosage hydrocortisone administration in vasopressor-treated preterm and term neonates. Pediatrics 2006, 118, 1456–1466. [Google Scholar] [CrossRef]
- Seri, I.; Tan, R.; Evans, J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 2001, 107, 1070–1074. [Google Scholar] [CrossRef]
- Higgins, S.; Friedlich, P.; Seri, I. Hydrocortisone for hypotension and vasopressor dependence in preterm neonates: A meta-analysis. J. Perinatol. 2010, 30, 373–378. [Google Scholar] [CrossRef]
- Scott, S.M.; Watterberg, K.L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 1995, 37, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; McNamara, P.; Jain, A.; Lavoie, P.M.; Wickremasinghe, A.; Merritt, T.A.; Solomon, T.; Sekar, K.; Attridge, J.T.; Swanson, J.R. Catecholamine-resistant hypotension and myocardial performance following patent ductus arteriosus ligation. J. Perinatol. 2015, 35, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Montman, R.; Watterberg, K. ACTH and cortisol response to critical illness in term and late preterm newborns. J. Perinatol. 2008, 28, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Damsted, S.K.; Born, A.; Paulson, O.B.; Uldall, P. Exogenous glucocorticoids and adverse cerebral effects in children. Eur. J. Paediatr. Neurol. 2011, 15, 465–477. [Google Scholar] [CrossRef]
- Bouyssi-Kobar, M.; du Plessis, A.J.; McCarter, R.; Brossard-Racine, M.; Murnick, J.; Tinkleman, L.; Robertson, R.L.; Limperopoulos, C. Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Pediatrics 2016, 138, e20161640. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.-K.; Song, H.; Cheon, J.-E.; Kim, B.N.; Kim, H.-S.; Shin, S.H. Association of brain microstructure and functional connectivity with cognitive outcomes and postnatal growth among early school–aged children born with extremely low birth weight. JAMA Netw. Open 2023, 6, e230198. [Google Scholar] [CrossRef]
- Patra, K.; Greene, M.; Silvestri, J. Neurodevelopmental impact of hydrocortisone exposure in extremely low birth weight infants: Outcomes at 1 and 2 years. J. Perinatol. 2015, 35, 77–81. [Google Scholar] [CrossRef]
- Watterberg, K.L.; Shaffer, M.L.; Mishefske, M.J.; Leach, C.L.; Mammel, M.C.; Couser, R.J.; Abbasi, S.; Cole, C.H.; Aucott, S.W.; Thilo, E.H. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics 2007, 120, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Tyson, J.E.; Heyne, R.J.; Hintz, S.R.; Vohr, B.R.; Bann, C.M.; Das, A.; Bell, E.F.; Debsareea, S.B.; Stephens, E. Use of term reference infants in assessing the developmental outcome of extremely preterm infants: Lessons learned in a multicenter study. J. Perinatol. 2023, 43, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Onland, W.; de Waal, K.; Kok, J. Postnatale dexamethason en neurologische uitkomst: Een meta-analyse. Tijdschr. Kindergeneeskd. 2005, 73, 144–151. [Google Scholar] [CrossRef]
- Taniguchi, A.; Chrétien, B.; Maeda, T.; Ueda, K.; Miura, R.; Tanaka, R.; Suzuki, T.; Muramatsu, Y.; Kataoka, E.; Kato, E. Total hydrocortisone dosage in extremely low birth weight infants and neurodevelopment up to school age. Pediatr. Res. 2025, 1–7. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef]
- Hüppi, P.S.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1998, 43, 224–235. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Tollefson, G.D.; Charles, C.; Zipursky, R.; Sharma, T.; Kahn, R.S.; Keefe, R.S.; Green, A.I.; Gur, R.E.; McEvoy, J. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry 2005, 62, 361–370. [Google Scholar] [CrossRef]
- Cheong, J.L.; Burnett, A.C.; Lee, K.J.; Roberts, G.; Thompson, D.K.; Wood, S.J.; Connelly, A.; Anderson, P.J.; Doyle, L.W.; Group, V.I.C.S. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J. Pediatr. 2014, 164, 737–743.e1. [Google Scholar] [CrossRef]
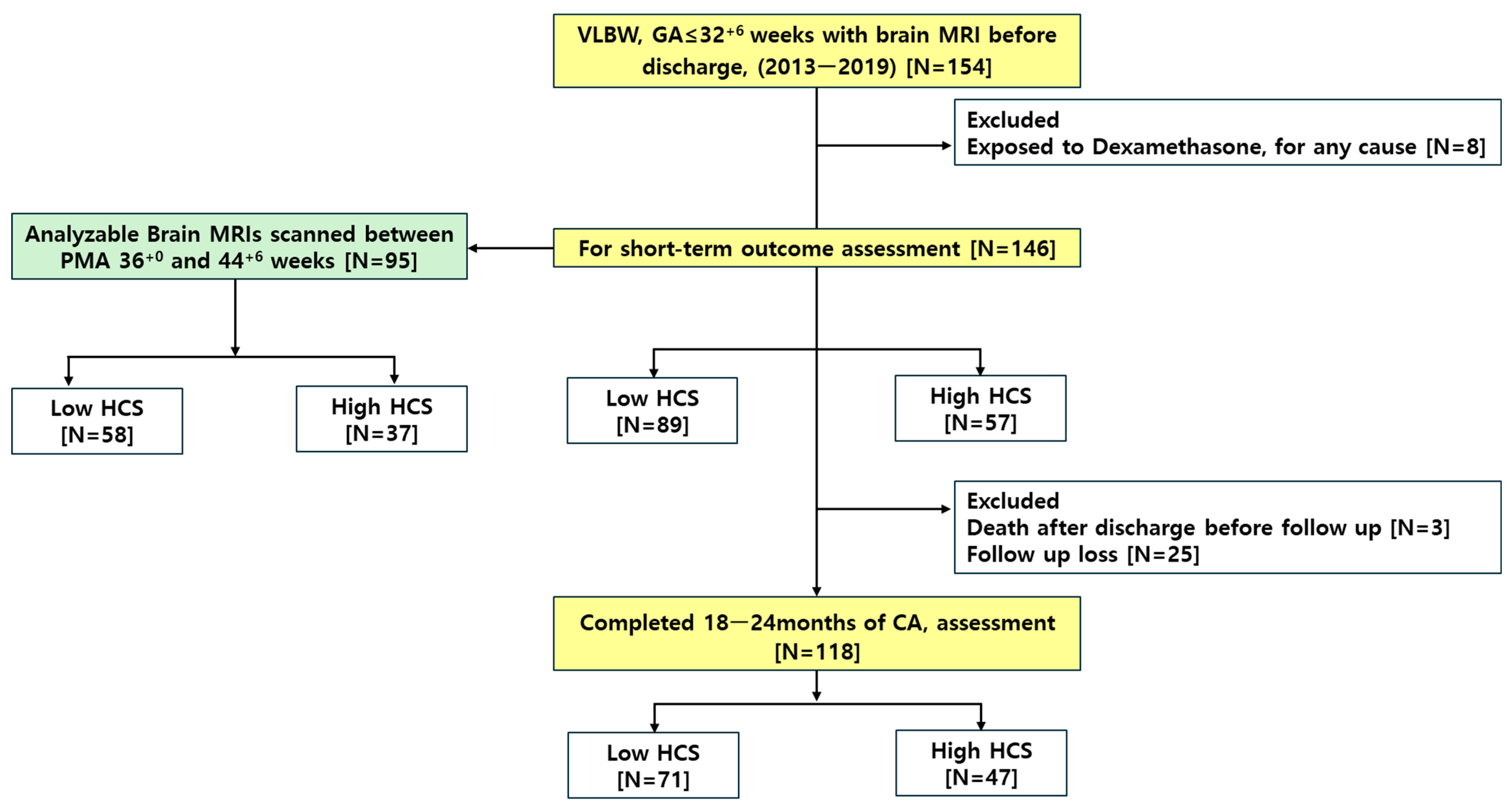
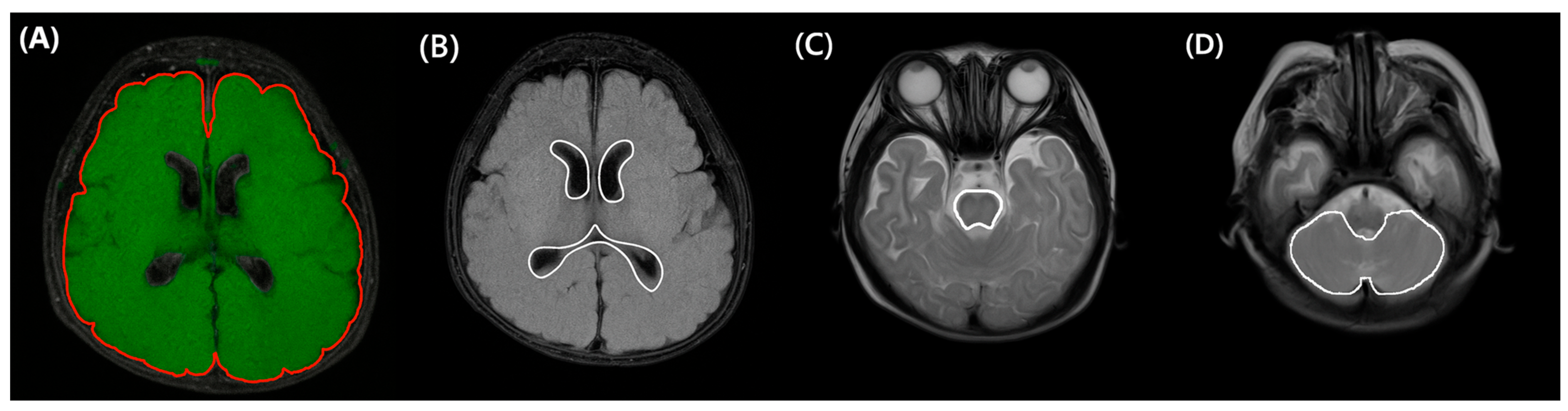
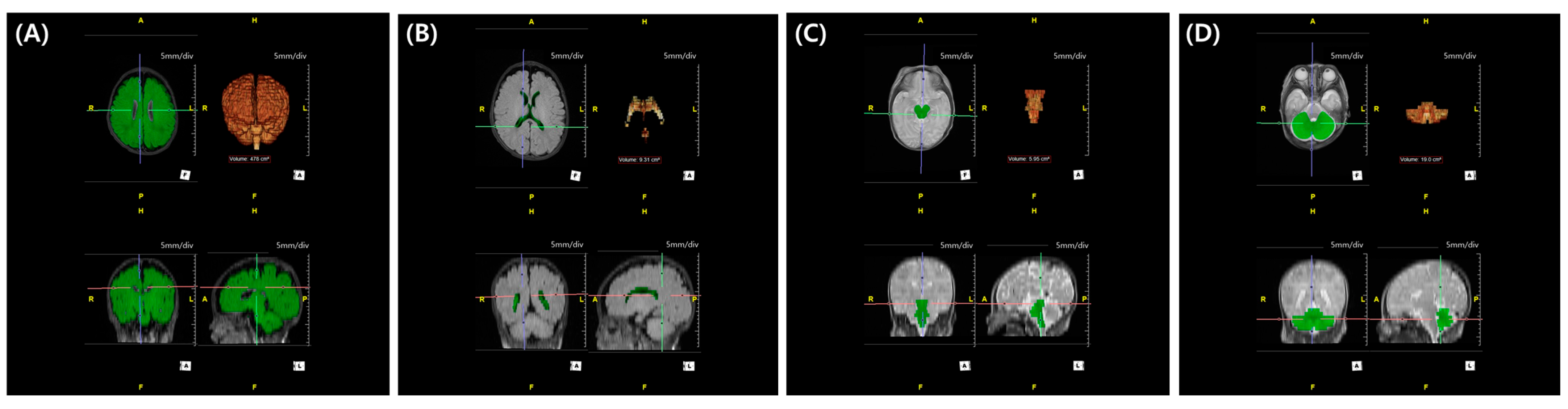
| Characteristics | Low HCS [N = 89] | High HCS [N = 57] | p |
|---|---|---|---|
| Maternal Characteristics | |||
| Maternal age, years | 33.5 ± 3.9 | 33.2 ± 4.7 | 0.705 |
| Maternal HDP | 10 (11.2%) | 8 (14.0%) | 0.807 |
| Delivery mode, C/S | 73 (82.0%) | 47 (82.5%) | >0.999 |
| HCA | 33 (37.1%) | 21 (36.8%) | >0.999 |
| Antenatal steroid | 63 (75.0%) | 40 (76.9%) | 0.961 |
| Infantile Characteristics | |||
| Gestational age, weeks | 28.6 ± 2.1 | 27.2 ± 2.0 | <0.001 |
| Birth weight, grams | 1133.7 ± 220.9 | 917.7 ± 248.9 | <0.001 |
| 5 min Apgar Score | 5.9 ± 1.7 | 5.1 ± 2.0 | 0.011 |
| Male | 42 (47.2%) | 31 (54.4%) | 0.497 |
| Small for gestational age | 9 (10.1%) | 10 (17.5%) | 0.294 |
| Short-term Neonatal Outcomes | |||
| Respiratory distress syndrome | 83 (93.3%) | 55 (96.5%) | 0.642 |
| Treated patent ductus arteriosus | 25 (28.1%) | 28 (49.1%) | 0.016 |
| Moderate to severe BPD | 62 (69.7%) | 57 (100.0%) | <0.001 |
| Necrotizing enterocolitis | 7 (7.9%) | 6 (10.5%) | 0.800 |
| Periventricular leukomalacia | 25 (28.1%) | 28 (49.1%) | 0.016 |
| Intraventricular hemorrhage ≥ grade 2 | 65 (73.0%) | 45 (78.9%) | 0.541 |
| Treated retinopathy of prematurity | 10 (11.2%) | 5 (8.8%) | 0.842 |
| Culture proven sepsis | 29 (32.6%) | 32 (56.1%) | 0.008 |
| Duration of ventilator, d | 20.4 ± 27.5 | 62.9 ± 38.8 | <0.001 |
| Duration of PN, d | 49.4 ± 44.3 | 39.6 ± 28.8 | 0.104 |
| Length of stay, d | 72.0 ± 32.4 | 112.3 ± 52.0 | <0.001 |
| Low HCS | High HCS | p | ||
|---|---|---|---|---|
| Neurodevelopmental outcome | Cerebral palsy | 5/55 (9.1%) | 14/39 (35.9%) | 0.003 |
| Motor impairment | 24/57 (42.1%) | 25/42 (59.5%) | 0.131 | |
| Language impairment | 19/57 (33.3%) | 25/42 (59.5%) | 0.017 | |
| Cognitive impairment | 17/57 (29.8%) | 17/42 (40.5%) | 0.374 | |
| NDI | 13/55 (23.6%) | 34/63 (54.0%) | 0.002 | |
| Growth outcome | FU Body weight < 10th | 14/58 (24.1%) | 17/39 (43.6%) | 0.073 |
| FU Height < 10th | 10/54 (18.5%) | 16/38 (42.1%) | 0.025 | |
| FU Head circumference < 10th | 8/41 (19.5%) | 18/28 (64.3%) | <0.001 | |
| Low HCS [N = 71] | High HCS [N = 47] | aOR | 95% CI | p | |
|---|---|---|---|---|---|
| CPa | 5/55(9.1%) | 14/39(35.9%) | 5.44 | 1.45–23.96 | 0.016 |
| NDIb | 13/55(23.6%) | 34/63(54.0%) | 2.58 | 0.94–7.42 | 0.071 |
| HC < 10%c | 8/41(19.5%) | 18/28(64.3%) | 4.45 | 1.34–15.71 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Oh, M.-Y.; Tomita, E.; Im, S.-A.; Youn, Y.-A.; Kim, S.Y. Cumulative Hydrocortisone Exposure and Early Brain Volumetrics in Very Low Birth Weight Infants: Associations with Neurodevelopmental Outcomes. Biomedicines 2025, 13, 2765. https://doi.org/10.3390/biomedicines13112765
Kim MS, Oh M-Y, Tomita E, Im S-A, Youn Y-A, Kim SY. Cumulative Hydrocortisone Exposure and Early Brain Volumetrics in Very Low Birth Weight Infants: Associations with Neurodevelopmental Outcomes. Biomedicines. 2025; 13(11):2765. https://doi.org/10.3390/biomedicines13112765
Chicago/Turabian StyleKim, Min Soo, Moon-Yeon Oh, Emi Tomita, Soo-Ah Im, Young-Ah Youn, and Sae Yun Kim. 2025. "Cumulative Hydrocortisone Exposure and Early Brain Volumetrics in Very Low Birth Weight Infants: Associations with Neurodevelopmental Outcomes" Biomedicines 13, no. 11: 2765. https://doi.org/10.3390/biomedicines13112765
APA StyleKim, M. S., Oh, M.-Y., Tomita, E., Im, S.-A., Youn, Y.-A., & Kim, S. Y. (2025). Cumulative Hydrocortisone Exposure and Early Brain Volumetrics in Very Low Birth Weight Infants: Associations with Neurodevelopmental Outcomes. Biomedicines, 13(11), 2765. https://doi.org/10.3390/biomedicines13112765






