In Vitro Osteogenic Stimulation of Human Adipose-Derived MSCs on Biofunctional 3D-Printed Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. GGMA Synthesis and 3D Printing
2.2. Cell Culture and Seeding on GGMA-Based Scaffolds
2.3. Cell Viability, Proliferation and Morphological Analysis of hADMSCs Cultured on GGMA-Based Scaffolds
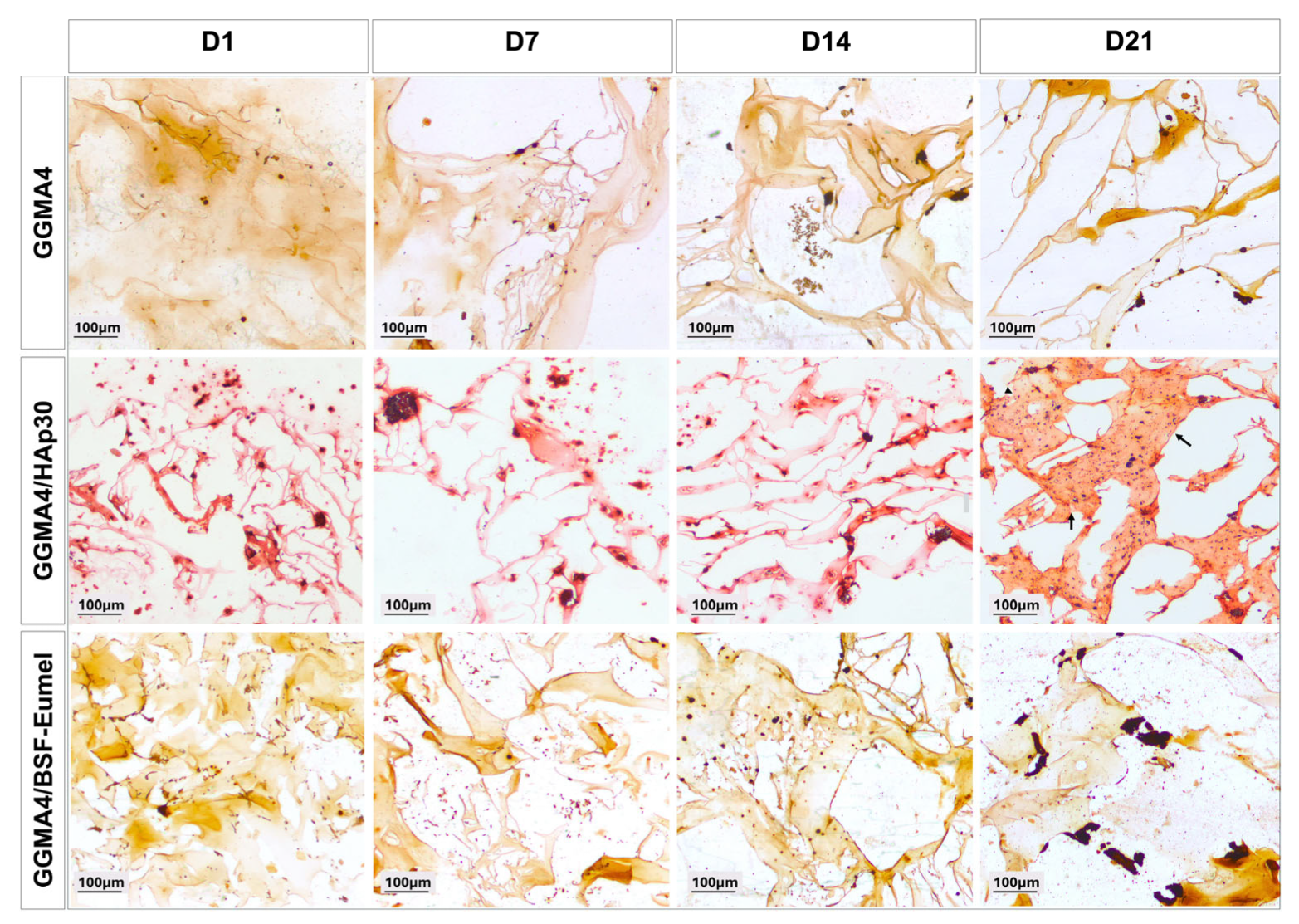
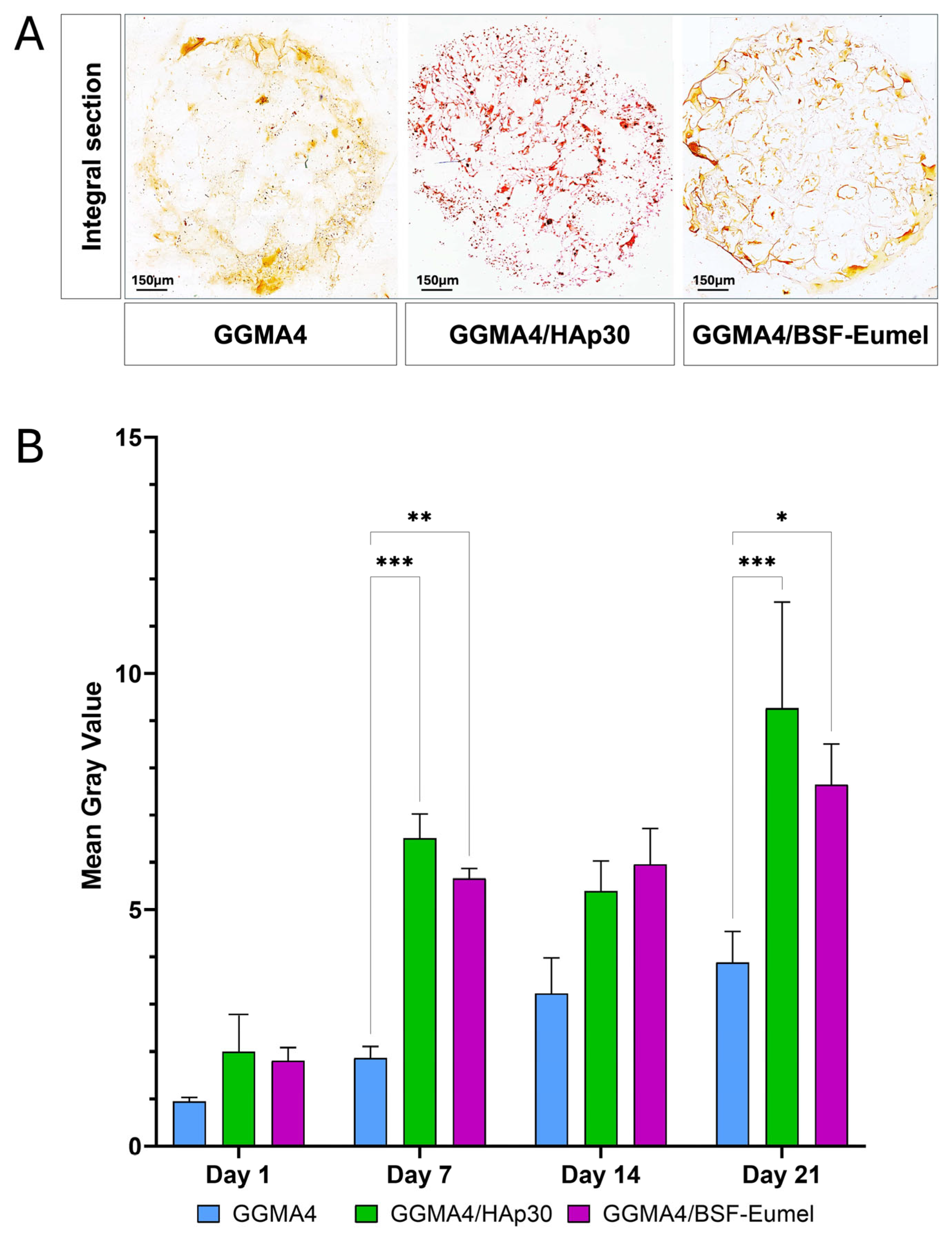
2.4. Extracellular Matrix Mineralization of hADMSCs Cultured on GGMA-Based Scaffolds
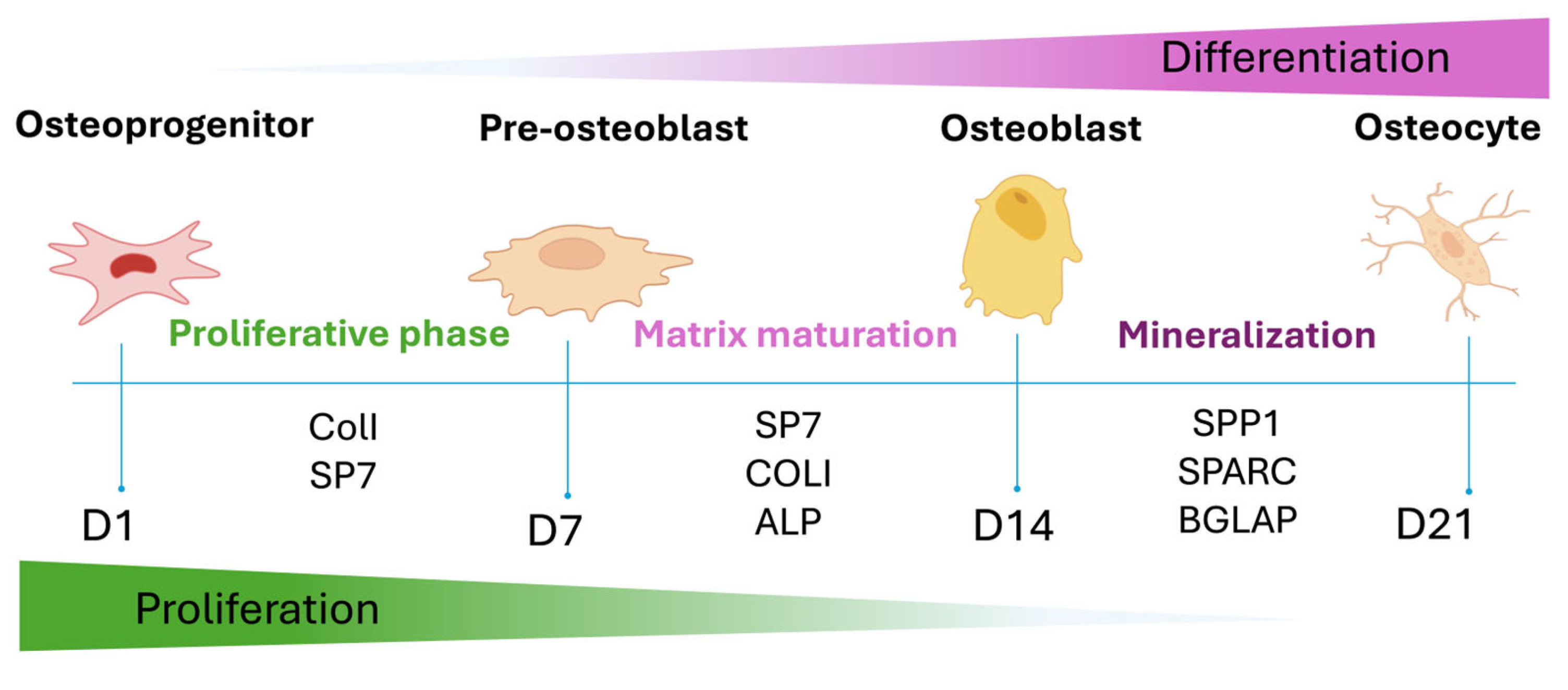
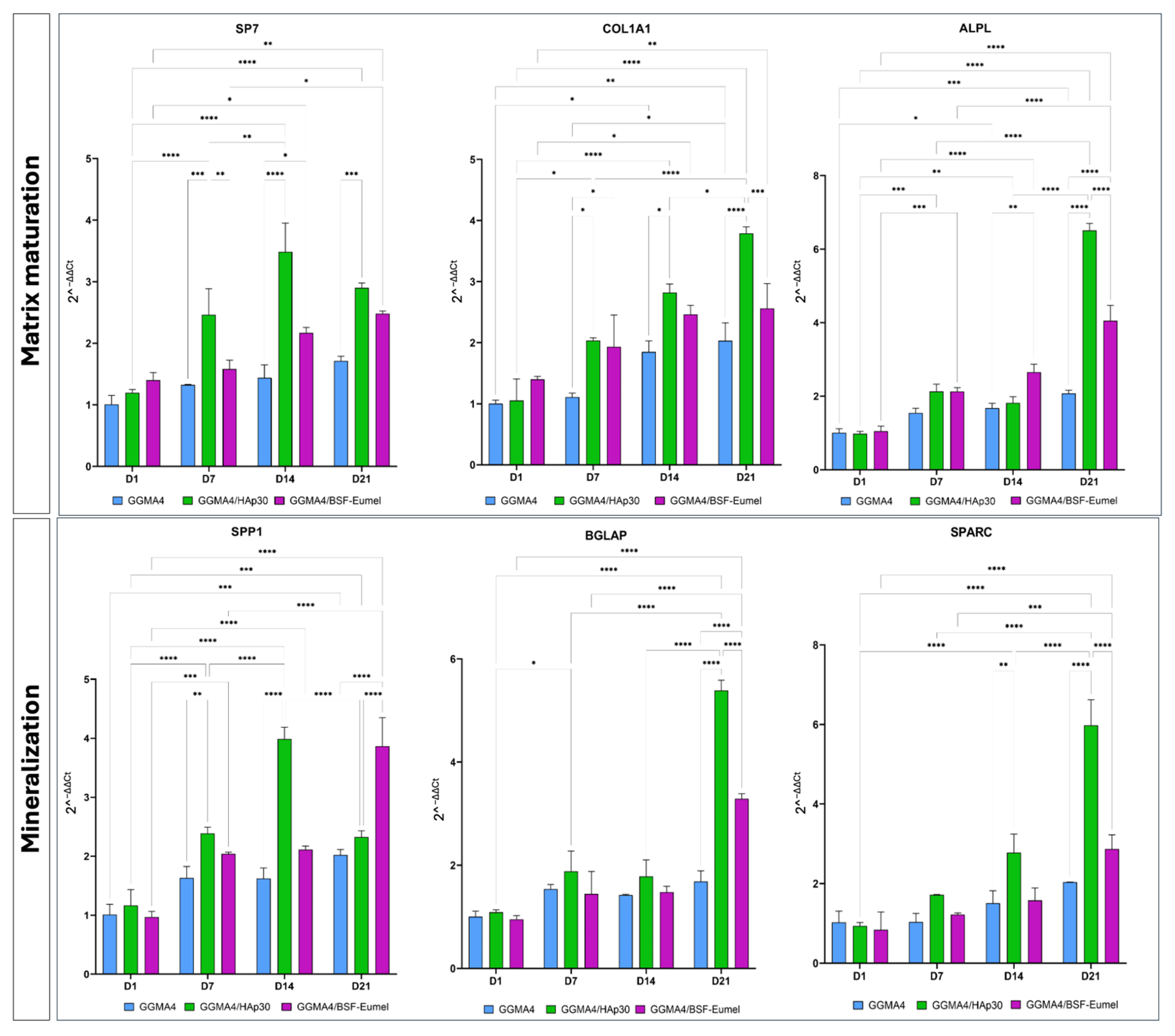
2.5. Gene Expression Analysis of hADMSCs Cultured on GGMA-Based Scaffolds
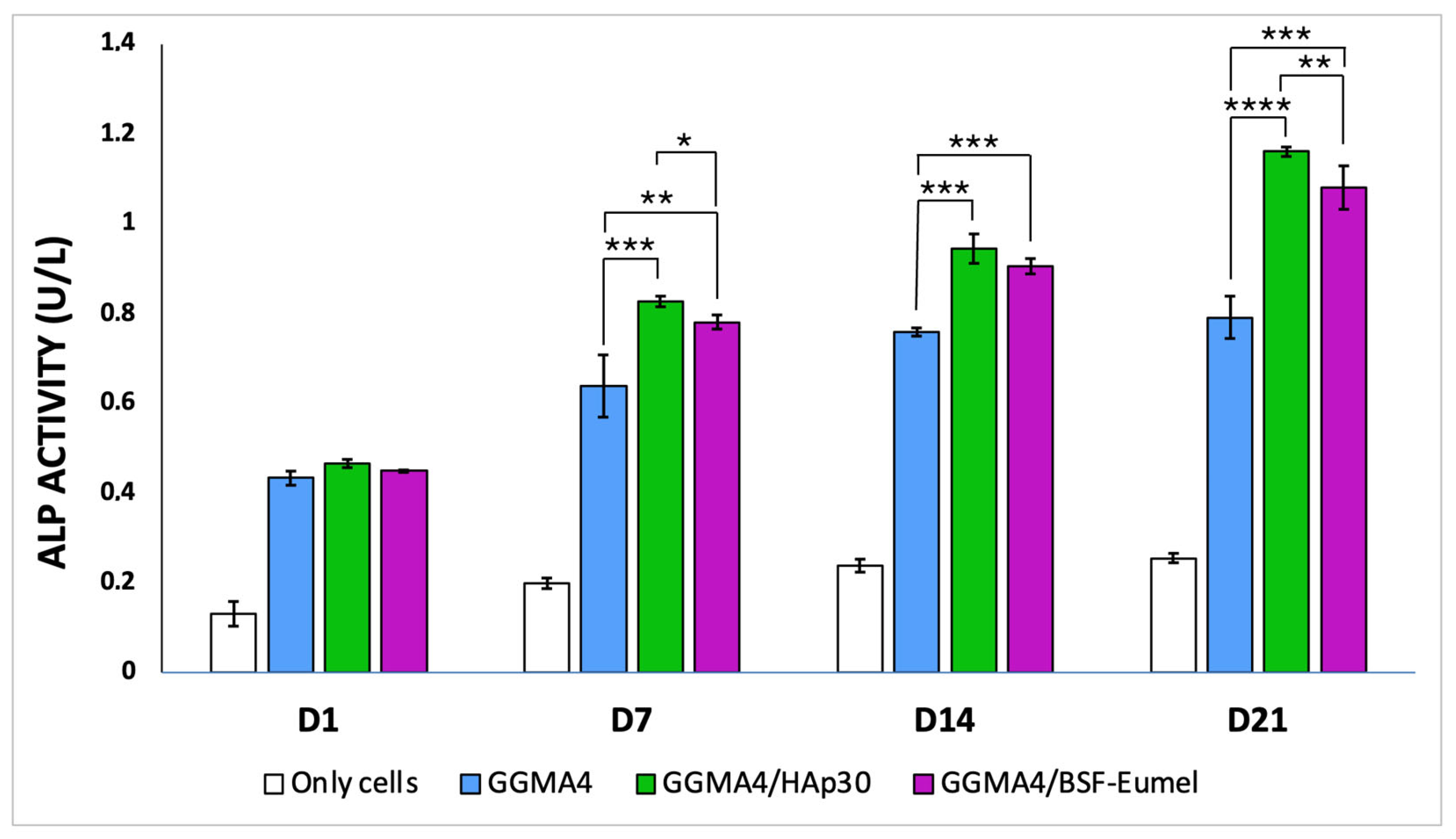
2.6. Quantitative Assessment of Alkaline Phosphatase Activity
2.7. Statistical Analysis
3. Results
3.1. 3D-Printed Scaffold Production and Characterization
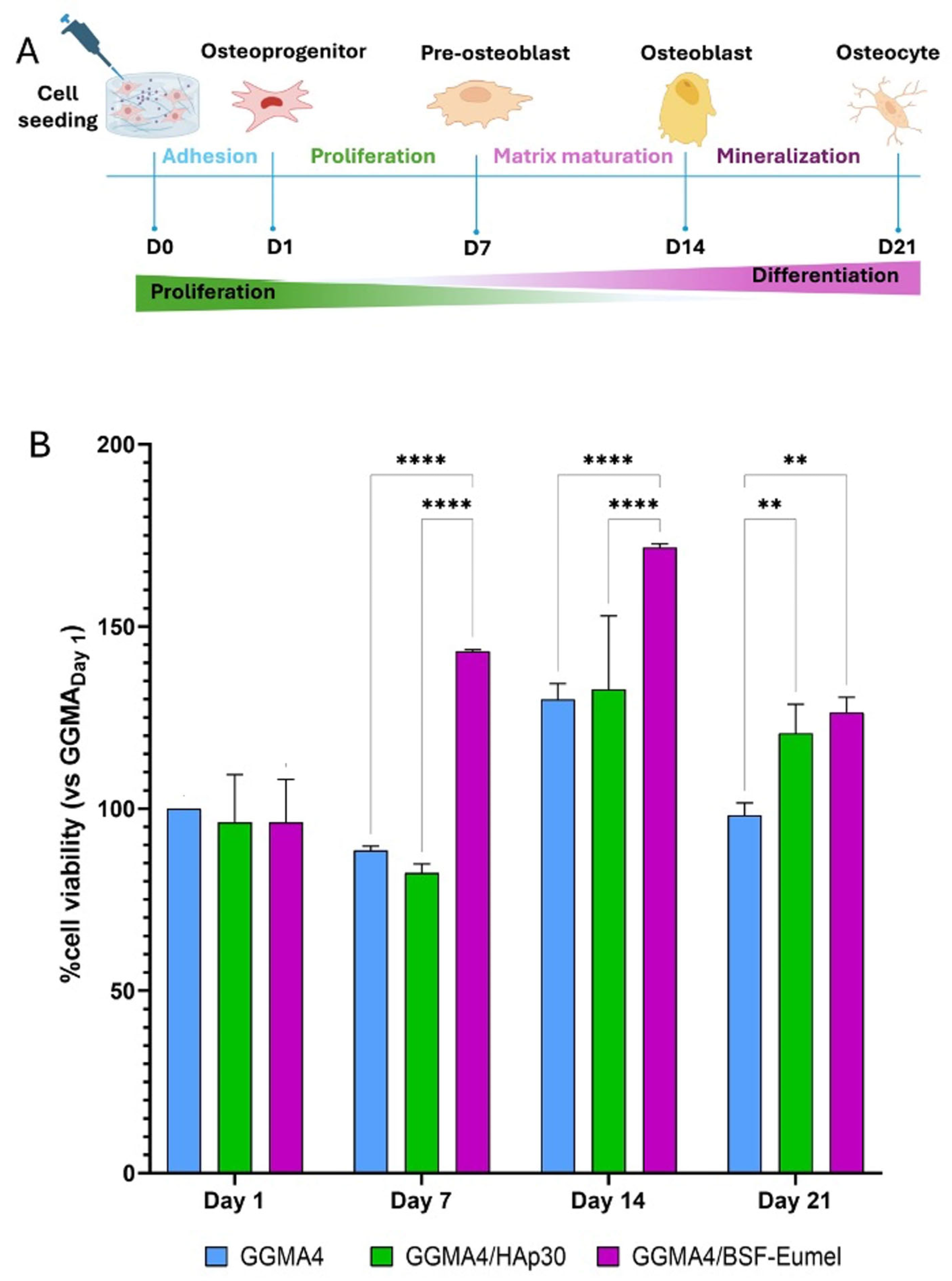
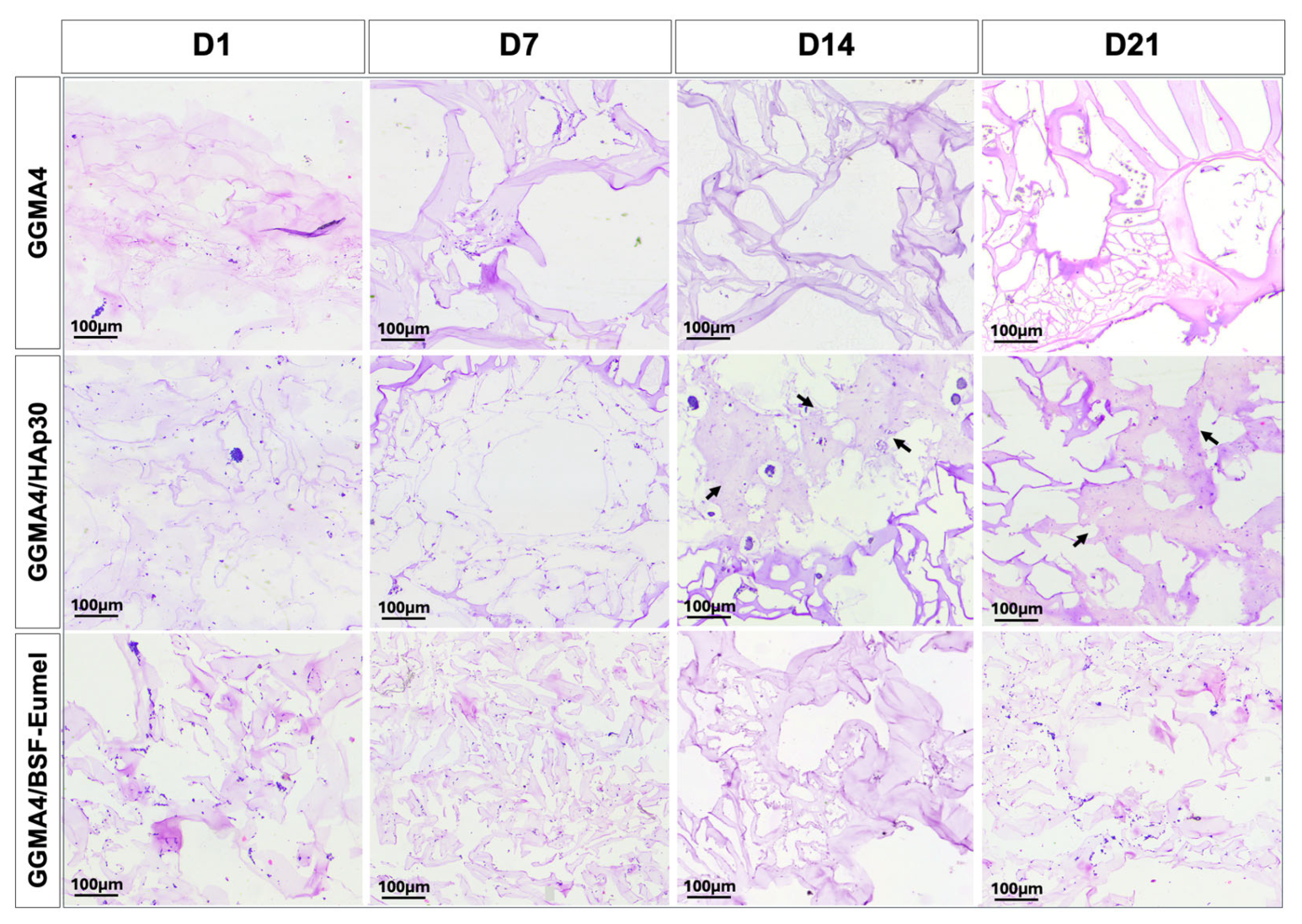
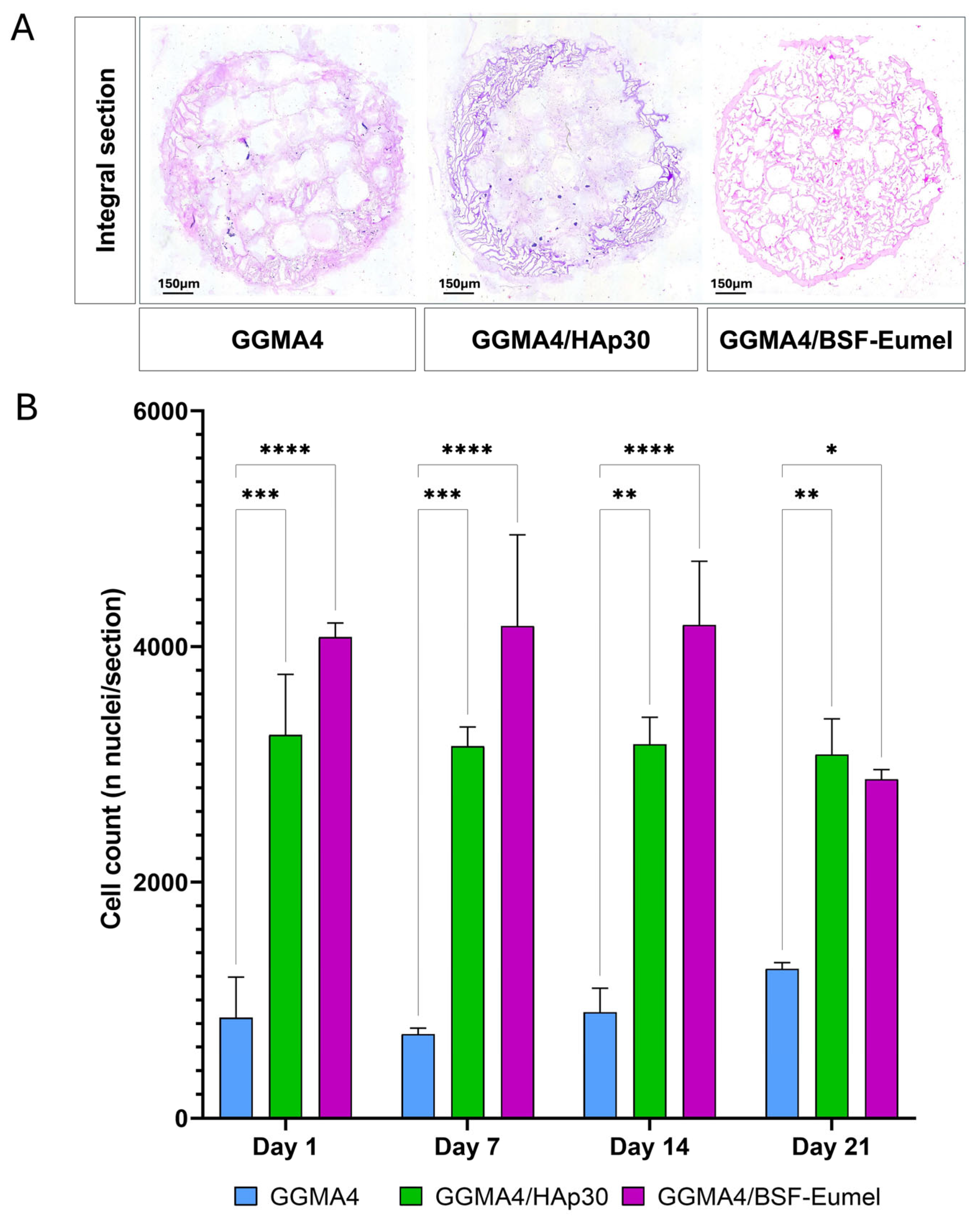
3.2. Osteoconductive Response of hADMSCs Cultured on the Different Types of GGMA Scaffolds
3.3. Osteoinductive Response of hADMSCs Seeded on the Different Types of GGMA-Based Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSF-Eumel | Black Soldier Fly Eumelanin |
| hADMSCs | Human adipose-derived mesenchymal stem cells |
| GG | Gellan gum |
| HAp | Hydroxyapatite |
| BSF-eumel | Eumelanin derived from black soldier fly |
| MSCs | Mesenchymal stem cells |
| BTE | Bone tissue engineering |
| ECM | Extracellular matrix |
| 3D | Three-dimensional |
| CAD | Computer-aided design |
| H&E | Hematoxylin and Eosin |
| AR S | Alizarin Red S |
| PFA | Paraformaldehyde |
| qRT-PCR | Quantitative real time-PCR |
| SP7 | Osterix |
| Col1A1 | Collagen Type 1 |
| ALPL | Alkaline Phosphatase |
| SPP1 | Osteopontin |
| BGLAP | Osteocalcin |
| SPARC | Osteonectin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DMA | Dynamic mechanical analysis |
References
- Via, A.G.; Frizziero, A. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar] [PubMed]
- Shang, F.; Yu, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2020, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Huang, Y. Adipose-derived mesenchymal stem cells (MSCs) are a superior cell source for bone tissue engineering. Bioact. Mater. 2023, 34, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef]
- Bauso, L.V.; La Fauci, V. Activity of Natural and Synthetic Peptides as Anticancer Agents. Int. J. Mol. Sci. 2024, 25, 7264. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Anandhapadman, A.; Venkateswaran, A. Advances in 3D printing of composite scaffolds for the repairment of bone tissue associated defects. Biotechnol. Prog. 2022, 38, e3234. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y. Advances in 3D printing technology for preparing bone tissue engineering scaffolds from biodegradable materials. Front. Bioeng. Biotechnol. 2024, 12, 1483547. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11 (Suppl. S1), S118–S124. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S. Raising the Bar: Progress in 3D-Printed Hybrid Bone Scaffolds for Clinical Applications: A Review. Cell Transpl. 2024, 33, 9636897241273562. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; De Luca, G. Structural and antibacterial studies of novel ZnO and ZnxMn(1−x)O nanostructured titanium scaffolds for biomedical applications. Biomater. Adv. 2023, 145, 213193. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Sinicropi, M.S. N-Thiocarbazole-based gold nanoparticles: Synthesis, characterization and anti-proliferative activity evaluation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012023. [Google Scholar] [CrossRef]
- Mauro, N.; Calabrese, G. Microporous Fluorescent Poly(D,L-lactide) Acid-Carbon Nanodot Scaffolds for Bone Tissue Engineering Applications. Materials 2024, 17, 449. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Calabrese, G. Evaluation of a Cell-Free Collagen Type I-Based Scaffold for Articular Cartilage Regeneration in an Orthotopic Rat Model. Materials 2020, 13, 2369. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Palermo, N. Physiologic Response Evaluation of Human Foetal Osteoblast Cells within Engineered 3D-Printed Polylactic Acid Scaffolds. Biology 2023, 12, 424. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U. Bioactivation Routes of Gelatin-Based Scaffolds to Enhance at Nanoscale Level Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials 2023, 13, 772. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Abdl Aali, R.A.K.; Al-Sahlany, S.T.G. Gellan Gum as a Unique Microbial Polysaccharide: Its Characteristics, Synthesis, and Current Application Trends. Gels 2024, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, M.; Kim, P.Y. A Gellan Gum, Polyethylene Glycol, Hydroxyapatite Composite Scaffold with the Addition of Ginseng Derived Compound K with Possible Applications in Bone Regeneration. Gels 2024, 10, 257. [Google Scholar] [CrossRef]
- D’Amora, U.; Soriente, A. Eumelanin from the Black Soldier Fly as Sustainable Biomaterial: Characterisation and Functional Benefits in Tissue-Engineered Composite Scaffolds. Biomedicines 2022, 10, 2945. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. Engl. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- Cecchi, T.; Pezzella, A.; Di Mauro, E.; Cestola, S.; Ginsburg, D.; Luzi, M.; Rigucci, A.; Santato, C. On the antioxidant activity of eumelanin biopigments: A quantitative comparison between free radical scavenging and redox properties. Nat. Prod. Res. 2020, 34, 2465–2473. [Google Scholar] [CrossRef]
- Pezzella, A.; Barra, M. Stem cell-compatible eumelanin biointerface fabricated by chemically controlled solid state polymerization. Mater. Horiz. 2015, 2, 212–220. [Google Scholar] [CrossRef]
- Manini, P.; Lucci, V. Synthetic mycomelanin thin films as emergent bio-inspired interfaces controlling the fate of embryonic stem cells. J. Mater. Chem. B 2020, 8, 4412–4418. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111394. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Calabrese, G. Cycloastragenol as an Exogenous Enhancer of Chondrogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. A Morphological Study. Cells 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Ardizzone, A. Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models. Biomolecules 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Jongprasitkul, H.; Turunen, S.; Parihar, V.S.; Kellomäki, M. Two-step crosslinking to enhance the printability of methacrylated gellan gum biomaterial ink for extrusion-based 3D bioprinting. Bioprinting 2022, 25, e00185. [Google Scholar] [CrossRef]
- D’Amora, U.; Scialla, S.; Fasolino, I.; Ronca, A.; Soriente, A.; De Cesare, N.; Manini, P.; Phua, J.W.; Pezzella, A.; Raucci, M.G.; et al. Eumelanin pigment release from photo-crosslinkable methacrylated gelatin-based cryogels: Exploring the physicochemical properties and antioxidant efficacy in wound healing. Biomater. Adv. 2025, 170, 214214. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Silva-Correia, J.; Zavan, B. Biocompatibility evaluation of ionic- and photo-crosslinked methacrylated gellan gum hydrogels: In vitro and in vivo study. Adv. Healthc. Mater. 2013, 2, 568–575. [Google Scholar] [CrossRef]
- Pereira, D.R.; Canadas, R.F. Injectable gellan-gum/hydroxyapatite-based bilayered hydrogel composites for osteochondral tissue regeneration. Appl. Mater. Today 2018, 2, 309–321. [Google Scholar] [CrossRef]
- Manda, M.G.; Da Silva, L.P. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 479–490. [Google Scholar] [CrossRef]
- Liu, W.; Cheong, N. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. [Google Scholar] [CrossRef]
- Radha, G.; Manjubaashini, N. Nano-hydroxyapatite/natural polymer composite scaffolds for bone tissue engineering: A brief review of recent trend. In Vitro Models 2023, 2, 125–151. [Google Scholar] [CrossRef]
- Helmi, S.A.; Rohani, L. Enhanced Osteogenic Differentiation of Pluripotent Stem Cells via γ-Secretase Inhibition. Int. J. Mol. Sci. 2021, 22, 5215. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Nayar, S. Synthesis of nanosized and microporous precipitated hydroxyapatite in synthetic polymers and biopolymers. J. Am. Ceram. Soc. 2003, 86, 357–359. [Google Scholar] [CrossRef]
- Afzal, F.; Pratap, J.; Ito, K.; Ito, Y.; Stein, J.L.; Van Wijnen, A.J.; Stein, G.S.; Lian, J.B.; Javed, A. Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J. Cell. Physiol. 2005, 204, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Min. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef]
- Arya, P.N.; Saranya, I.; Selvamurugan, N. Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation. World J. Stem Cells 2024, 16, 102–113. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Bayram, S.; Dengiz, C.; Gerçek, Y.C.; Cetin, I.; Topcul, M.R. Bioproduction, structure elucidation and in vitro antiproliferative effect of eumelanin pigment from Streptomyces parvus BSB49. Arch. Microbiol. 2020, 202, 2401–2409. [Google Scholar] [CrossRef]
- Ren, W.H.; Xin, S.; Yang, K.; Yu, Y.B.; Li, S.M.; Zheng, J.J.; Huang, K.; Zeng, R.C.; Yang, X.X.; Gao, L.; et al. Strontium-Doped Hydroxyapatite Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoporotic Rats through the CaSR-JAK2/STAT3 Signaling Pathway. Adv. NanoBiomed Res. 2022, 2, 2200018. [Google Scholar] [CrossRef]
- Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari, M.L.A.D.; Rahadiansyah, E.; Ardianto, C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals 2021, 14, 615. [Google Scholar] [CrossRef]
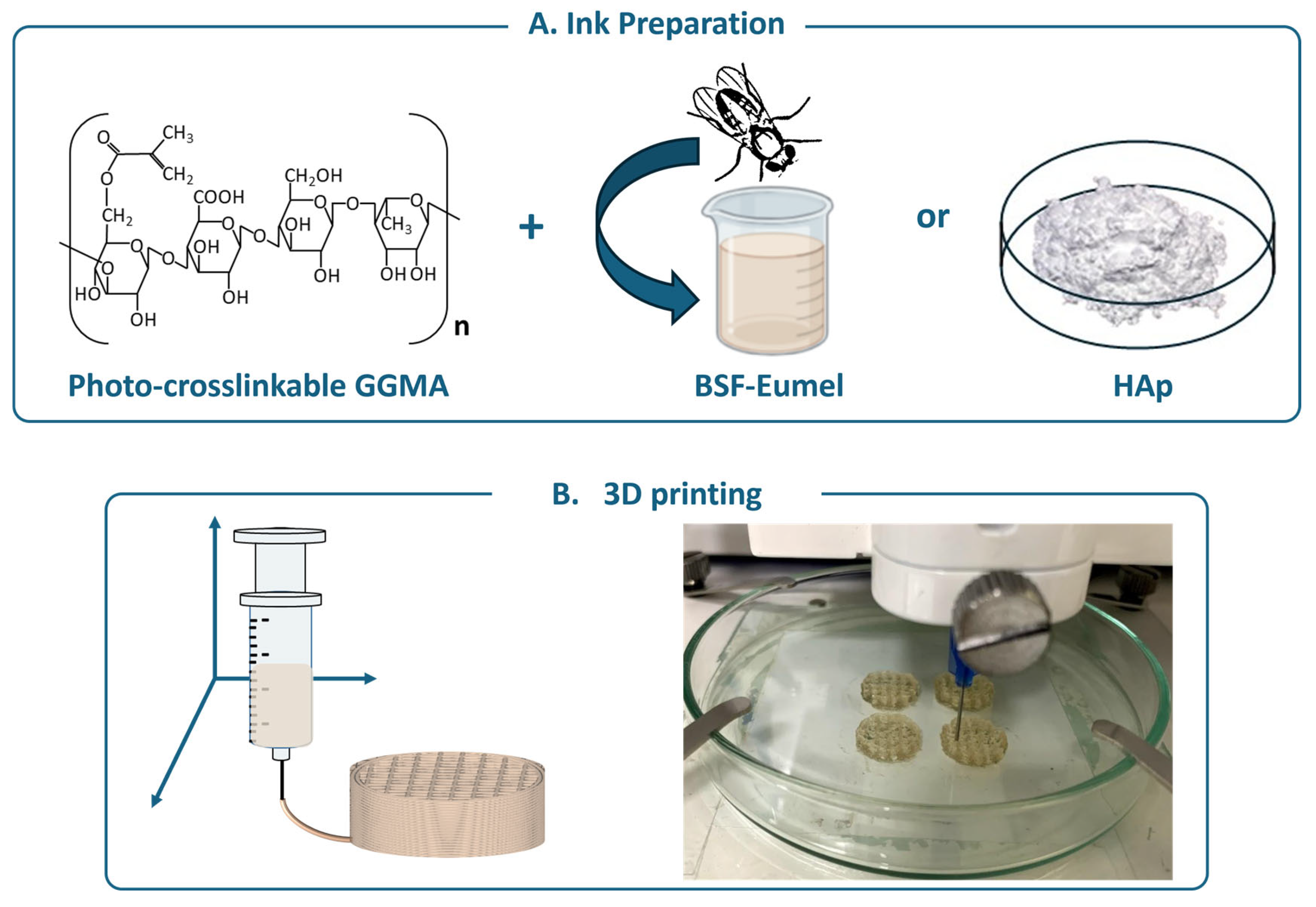
| Ink | GGMA (% w/v) | BSF-Eumel (mg/mL) | HAp (% w/wGGMA) | Nomenclature |
|---|---|---|---|---|
| GGMA | 4 | - | - | GGMA4 |
| GGMA/BSF-Eumel | 0.3 | - | GGMA4/BSF-Eumel | |
| GGMA/HAp | - | 30 | GGMA4/HAp30 |
| Printing Parameters | ||||||
|---|---|---|---|---|---|---|
| Scaffolds | Needle (mm) | Layer Thickness (mm) | Print Speed (mm/s) | Temperature (°C) | Pattern | Infill (%) |
| GGMA | 0.41 | 0.3 | 3 | 35 | Grid | 45 |
| GGMA/BSF-Eumel | ||||||
| GGMA/HAp30 | ||||||
| Post Processing | |||||
|---|---|---|---|---|---|
| UV Crosslinking | CaCl2 Crosslinking | ||||
| Wavelength (nm) | Power Source (J/cm2) | Time (min) | CaCl2 (% w/v) | Time (min) | Temperature (°C) |
| 365 | 10 | 10 | 0.05 | 10 | 25 |
| Gene | Forward | Reverse |
|---|---|---|
| SP7 | TGCTTGAGGAGGAAGTTCACTATG | TGCCCAGAGTTGTTGAGTCC |
| COL1A1 | CCGGAAACAGACAAGCAACCCAAA | AAAGGAGCAGAAAGGGCAGCATTG |
| ALPL | GACCCTTGACCCCCACAAT | CGCCTCGTACTGCATGTCCCCT |
| SPP1 | AGTTTCGCAGACCTGACATCCAGT | TTCATAACTGTCCTTCCCACGGCT |
| BGLAP | GGCAGCGAGGTAGTGAAGAG | GATGTGGTCAGCCAACTCGT |
| SPARC | TTCTGCCTGGAGACAAGGTGCTAA | TCTGTTACTTCCCTTTGCCCACCT |
| GAPDH | TGTGAACGGATTTGGCCGTA | ACTGTGCCGTTGAATTTGCC |
| Sample | Storage Modulus (E’, kPa) [20] | Swelling Behavior (Q, mg/mg) [20] | Stability (Relative Weight, WR%) [20] | DPPH Scavenging Activity (SADPPH %) |
|---|---|---|---|---|
| GGMA4 | 9.3 kPa | Highest ≈ 28.4 ± 2.9 (after 24 h), de-swelling after 7 days | Gradual ~10% weight loss over 28 days; moderate stability | 9.0 ± 1.3 |
| GGMA4/HAp30 | 32.5 kPa (≈3.5 × GGMA4) | Lowest ≈ 19.8 ± 1.3 after 24 h) due to reduced chain mobility | Slight initial burst loss then stable; total ~10% mass loss after 28 days | 8.4 ± 0.6 |
| GGMA4/BSF-Eumel | 12.7 kPa (similar to GGMA4, no significant increase) | 26.9 ± 0.6 (after 24 h); sustained swelling up to 28 days | Highest stability: minimal weight loss due to negatively charged eumelanin groups enabling ionic stabilization | 27.5 ± 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munaò, S.; D’Amora, U.; Bauso, L.V.; Ronca, A.; Manini, P.; Pezzella, A.; Raucci, M.G.; Ambrosio, L.; Calabrese, G. In Vitro Osteogenic Stimulation of Human Adipose-Derived MSCs on Biofunctional 3D-Printed Scaffolds. Biomedicines 2025, 13, 2755. https://doi.org/10.3390/biomedicines13112755
Munaò S, D’Amora U, Bauso LV, Ronca A, Manini P, Pezzella A, Raucci MG, Ambrosio L, Calabrese G. In Vitro Osteogenic Stimulation of Human Adipose-Derived MSCs on Biofunctional 3D-Printed Scaffolds. Biomedicines. 2025; 13(11):2755. https://doi.org/10.3390/biomedicines13112755
Chicago/Turabian StyleMunaò, Serena, Ugo D’Amora, Luana Vittoria Bauso, Alfredo Ronca, Paola Manini, Alessandro Pezzella, Maria Grazia Raucci, Luigi Ambrosio, and Giovanna Calabrese. 2025. "In Vitro Osteogenic Stimulation of Human Adipose-Derived MSCs on Biofunctional 3D-Printed Scaffolds" Biomedicines 13, no. 11: 2755. https://doi.org/10.3390/biomedicines13112755
APA StyleMunaò, S., D’Amora, U., Bauso, L. V., Ronca, A., Manini, P., Pezzella, A., Raucci, M. G., Ambrosio, L., & Calabrese, G. (2025). In Vitro Osteogenic Stimulation of Human Adipose-Derived MSCs on Biofunctional 3D-Printed Scaffolds. Biomedicines, 13(11), 2755. https://doi.org/10.3390/biomedicines13112755









