Effect of Dotinurad on Uric Acid and Hepatorenal Parameters in Steatotic Liver Disease: A Pilot Study in Japanese Patients
Abstract
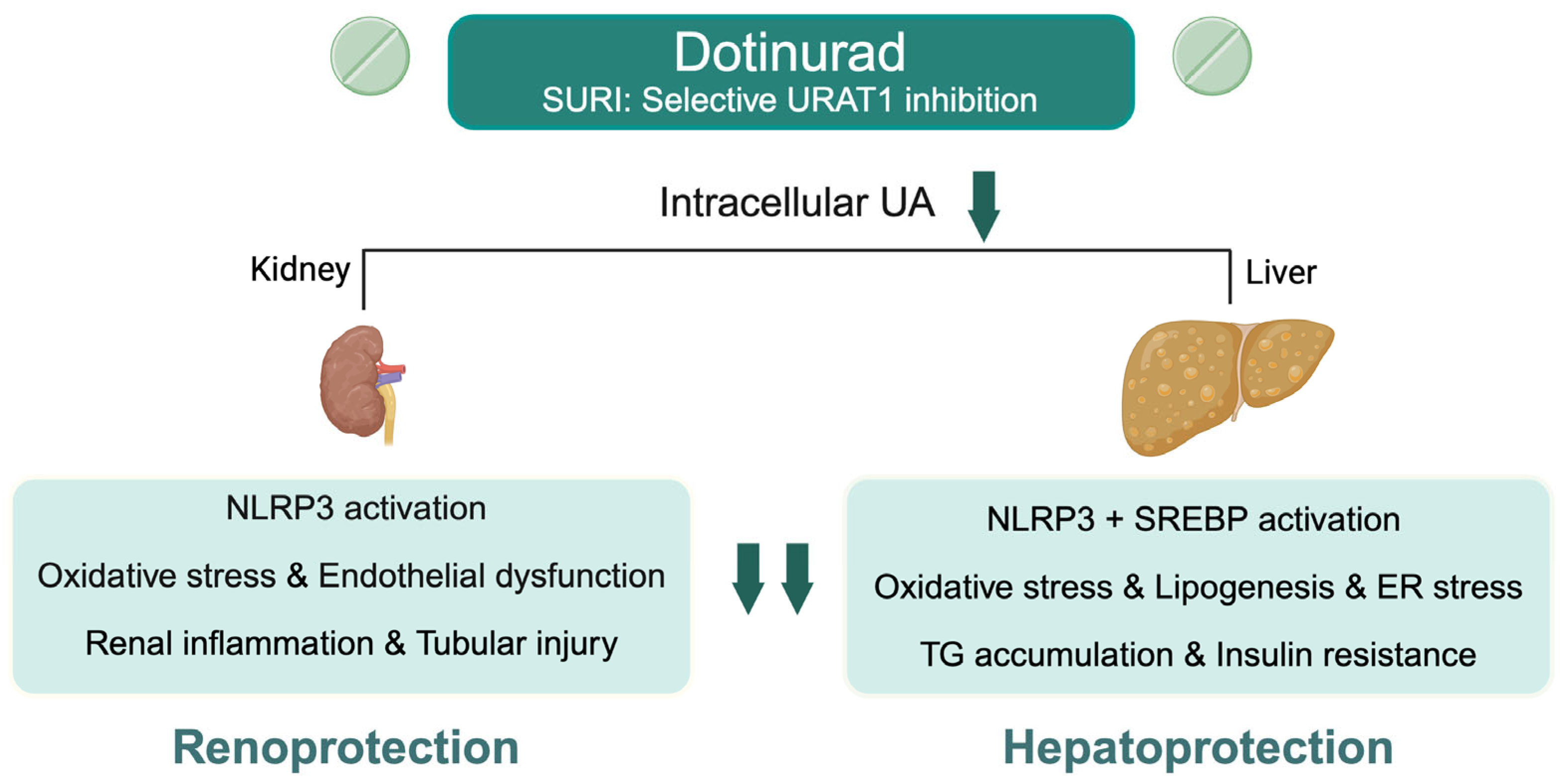
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Examinations
2.2. Statistical Analysis
3. Results
3.1. Clinical Characteristics of HU-SLD Patients Treated with DOT
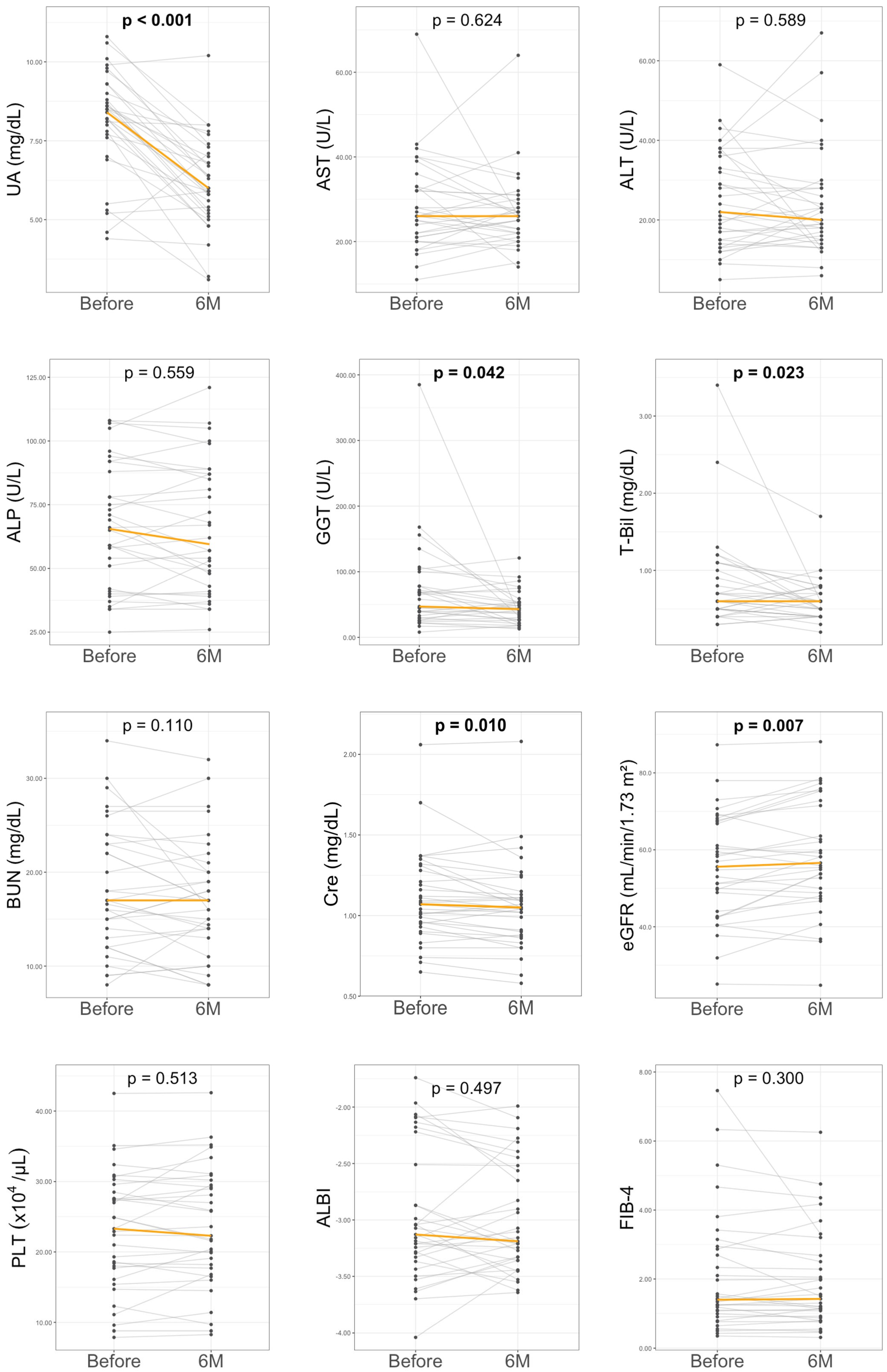
3.2. Six-Month Outcomes of DOT Treatment in HU-SLD
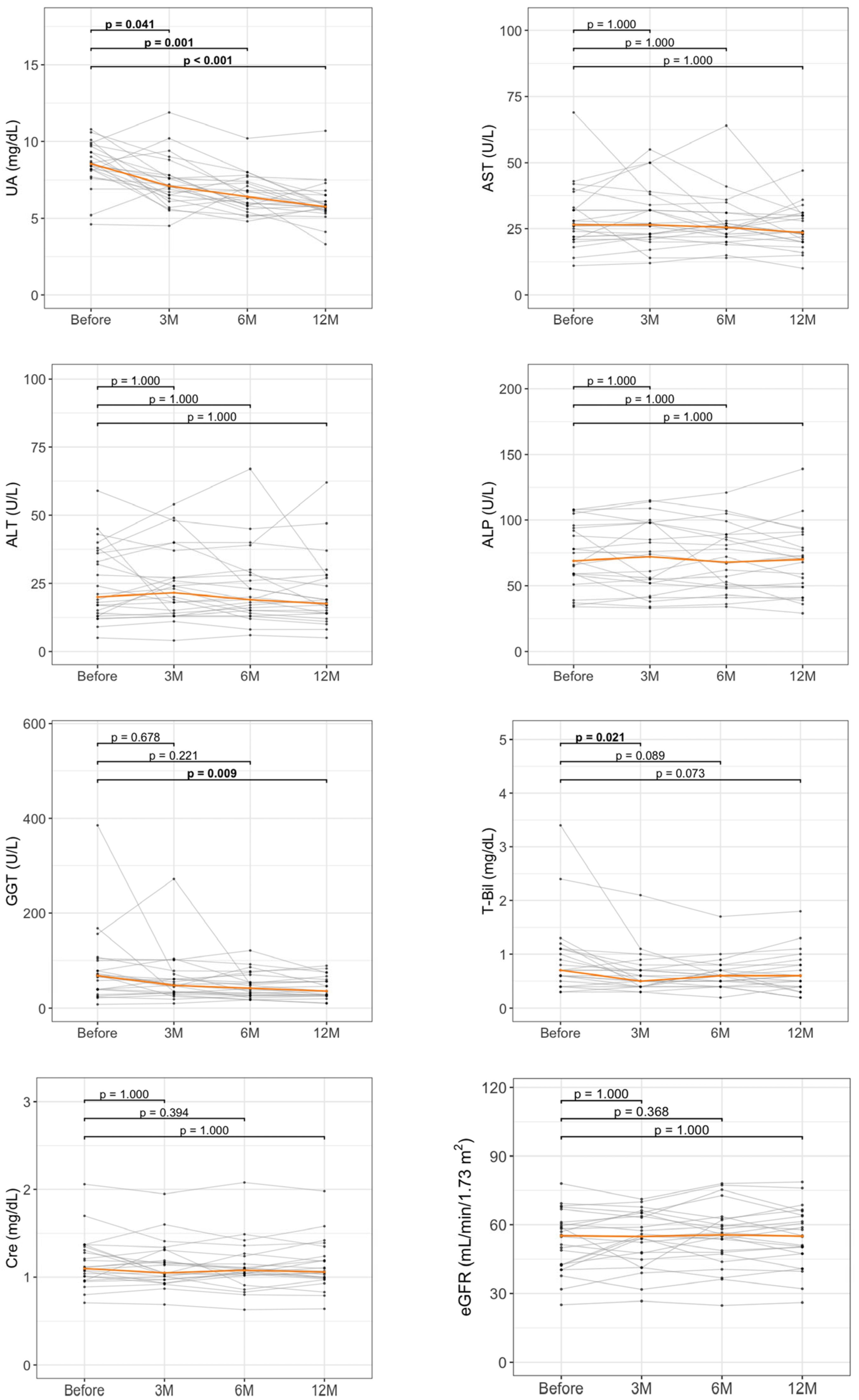
3.3. Longitudinal Changes in Liver Enzymes and UA by 12-Month DOT Therapy
3.4. Comparison of Clinical Factors According to GGT ≥ 30% Improvement
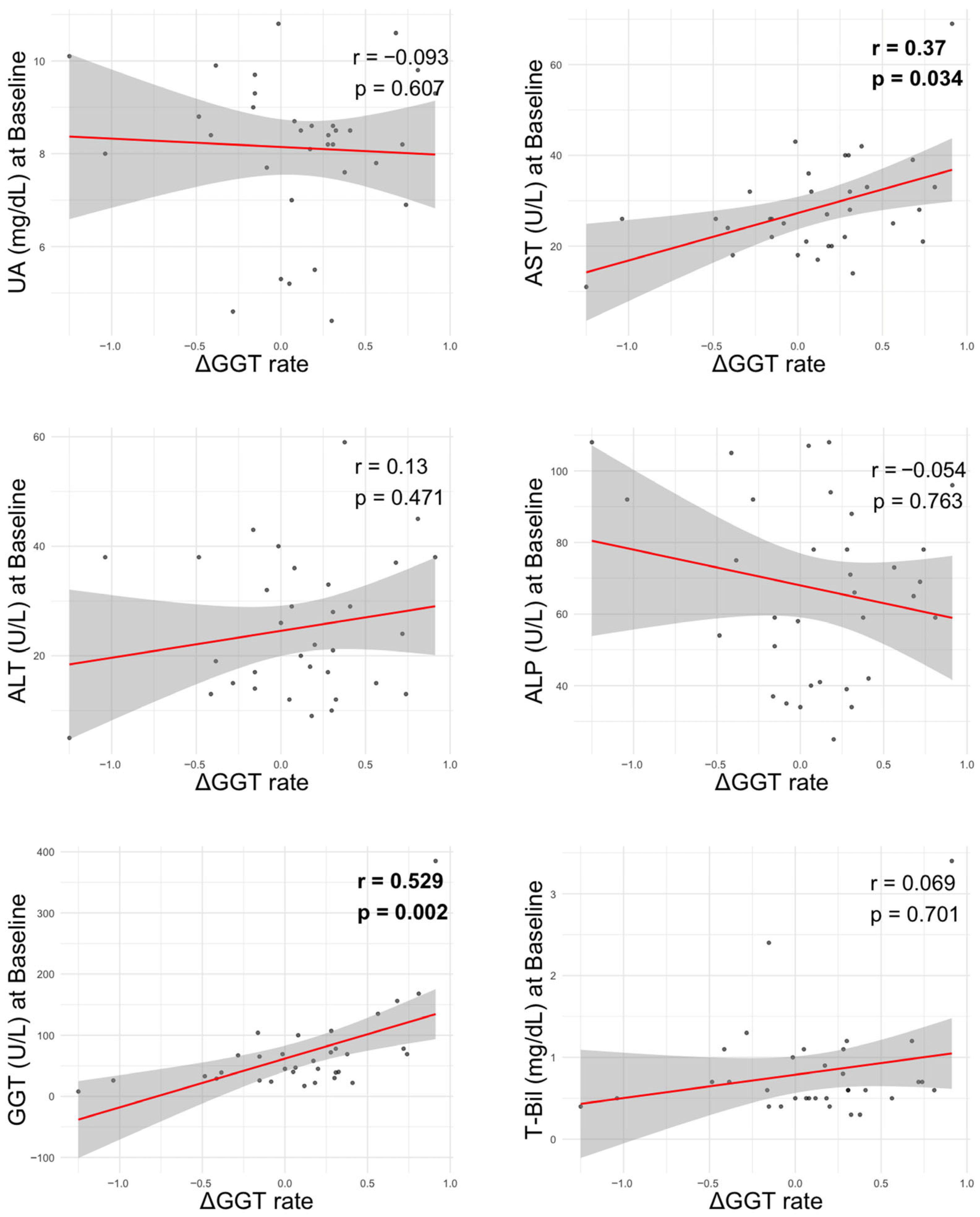
3.5. Correlation Between GGT Improvement and Biochemical Parameters
3.6. Baseline Predictors Changes in GGT and eGFR During DOT Treatment
4. Discussion
4.1. Main Findings
4.2. Context with Published Literature
4.3. Strengths and Limitations
4.4. Future Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction–associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef]
- Tamaki, N.; Kimura, T.; Wakabayashi, S.I.; Umemura, T.; Kurosaki, M.; Loomba, R.; Izumi, N. Long-term clinical outcomes in steatotic liver disease and incidence of liver-related events, cardiovascular events and all-cause mortality. Aliment. Pharmacol. Ther. 2024, 60, 61–69. [Google Scholar] [CrossRef]
- Lai, M.; Lai, J.C.; Allegretti, A.S.; Patidar, K.R.; Cullaro, G. Investigating the Association between Steatotic Liver Disease and CKD in a Nationally Representative Sample. Kidney360 2024, 5, 1844–1852. [Google Scholar] [CrossRef]
- Kimura, T.; Tamaki, N.; Wakabayashi, S.-I.; Tanaka, N.; Umemura, T.; Izumi, N.; Loomba, R.; Kurosaki, M. Colorectal Cancer Incidence in Steatotic Liver Disease (MASLD, MetALD, and ALD). Clin. Gastroenterol. Hepatol. 2025, 23, 2197–2204.e2. [Google Scholar] [CrossRef]
- Bilson, J.; Mantovani, A.; Byrne, C.D.; Targher, G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024, 50, 101506. [Google Scholar] [CrossRef]
- Theodorakis, N.; Nikolaou, M. Integrated Management of Cardiovascular-Renal-Hepatic-Metabolic Syndrome: Expanding Roles of SGLT2is, GLP-1RAs, and GIP/GLP-1RAs. Biomedicines 2025, 13, 135. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Yu, C.; Xu, L.; Miao, M. Association of serum uric acid level with non-alcoholic fatty liver disease: A cross-sectional study. J. Hepatol. 2009, 50, 1029–1034. [Google Scholar] [CrossRef]
- Fukuda, T.; Akihisa, T.; Okamoto, T.; Fukaishi, T.; Kawakami, A.; Tanaka, M.; Yamada, T.; Monzen, K. Association of uric acid levels with the development of metabolic dysfunction-associated and metabolic and alcohol-related/associated steatotic liver disease: A study on Japanese participants undergoing health checkups. Endocr. J. 2025, 72, 671–687. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Cachón-Suárez, I.; Garcia-Martín, C.; Short, A.; Bataller, R.; Muga, R. Prevalence and associations of metabolic syndrome in patients with alcohol use disorder. Sci. Rep. 2022, 12, 2625. [Google Scholar] [CrossRef]
- Hernández-Rubio, A.; Sanvisens, A.; Bolao, F.; Pérez-Mañá, C.; García-Marchena, N.; Fernández-Prendes, C.; Muñoz, A.; Muga, R. Association of hyperuricemia and gamma glutamyl transferase as a marker of metabolic risk in alcohol use disorder. Sci. Rep. 2020, 10, 20060. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nagoshi, T.; Takahashi, H.; Oi, Y.; Yoshii, A.; Kimura, H.; Ito, K.; Kashiwagi, Y.; Tanaka, T.D.; Yoshimura, M. URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice. Mol. Metab. 2022, 55, 101411. [Google Scholar] [CrossRef]
- Manley, J.A.; O’Neill, W.C. How echogenic is echogenic? Quantitative acoustics of the renal cortex. Am. J. Kidney Dis. 2001, 37, 706–711. [Google Scholar] [CrossRef]
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. (Landmark Ed.) 2012, 17, 656–669. [Google Scholar] [CrossRef]
- Komatsu, M.; Kimura, T.; Yazaki, M.; Tanaka, N.; Yang, Y.; Nakajima, T.; Horiuchi, A.; Fang, Z.Z.; Joshita, S.; Matsumoto, A.; et al. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARalpha. Biochim. Biophys. Acta 2015, 1852, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M. Diagnostic criteria for dyslipidemia executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J. Atheroscler. Thromb. 2013, 20, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. A Possible Therapeutic Application of the Selective Inhibitor of Urate Transporter 1, Dotinurad, for Metabolic Syndrome, Chronic Kidney Disease, and Cardiovascular Disease. Cells 2024, 13, 450. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Tanaka, A.; Taguchi, I.; Hisauchi, I.; Yoshida, H.; Shimabukuro, M.; Hongo, H.; Ishikawa, T.; Kadokami, T.; Yagi, S.; Sata, M.; et al. Clinical effects of a selective urate reabsorption inhibitor dotinurad in patients with hyperuricemia and treated hypertension: A multicenter, prospective, exploratory study (DIANA). Eur. J. Med. Res. 2023, 28, 238. [Google Scholar] [CrossRef]
- Amano, H.; Kobayashi, S.; Terawaki, H. Dotinurad restores exacerbated kidney dysfunction in hyperuricemic patients with chronic kidney disease. BMC Nephrol. 2024, 25, 97. [Google Scholar] [CrossRef]
- Motomura, T.; Higashi, M.; Hattori, A.; Akiyama, R.; Kai, H. Efficacy and Safety of Dotinurad in Patients with Advanced Chronic Kidney Disease. Intern. Med. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Takata, T.; Taniguchi, S.; Mae, Y.; Kageyama, K.; Fujino, Y.; Iyama, T.; Hikita, K.; Sugihara, T.; Isomoto, H. Comparative assessment of the effects of dotinurad and febuxostat on the renal function in chronic kidney disease patients with hyperuricemia. Sci. Rep. 2025, 15, 8990. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. The Long-Term Effects of the Selective Inhibitor of Urate Transporter 1, Dotinurad, on Metabolic Parameters and Renal Function in Japanese Patients With Asymptomatic Hyperuricemia. J. Clin. Med. Res. 2025, 17, 320–333. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Lin, Y.; Lu, C.; Li, D.; Sang, J.; He, H.; Liu, X.; Li, Y.; Yu, C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. Hepatol. 2016, 64, 925–932. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, H.S.; Choi, H.S.; Park, J.W.; Jo, I.; Oh, E.S.; Lee, K.Y.; Lee, B.H.; Johnson, R.J.; Kang, D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Nagaya, T.; Tanaka, N.; Suzuki, T.; Sano, K.; Horiuchi, A.; Komatsu, M.; Nakajima, T.; Nishizawa, T.; Joshita, S.; Umemura, T.; et al. Down-regulation of SREBP-1c is associated with the development of burned-out NASH. J. Hepatol. 2010, 53, 724–731. [Google Scholar] [CrossRef]
- Irie, M.; Sohda, T.; Iwata, K.; Kunimoto, H.; Fukunaga, A.; Kuno, S.; Yotsumoto, K.; Sakurai, K.; Iwashita, H.; Hirano, G. Levels of the oxidative stress marker γ-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J. Int. Med. Res. 2012, 40, 924–933. [Google Scholar] [CrossRef]
- De Matteis, C.; Crudele, L.; Di Buduo, E.; Cantatore, S.; Gadaleta, R.M.; Cariello, M.; Suppressa, P.; Antonica, G.; Berardi, E.; Graziano, G.; et al. Hyperhomocysteinemia is linked to MASLD. Eur. J. Intern. Med. 2025, 131, 49–57. [Google Scholar] [CrossRef]
- De Matteis, C.; Novielli, F.; Di Buduo, E.; Arconzo, M.; Gadaleta, R.M.; Cariello, M.; Moschetta, A.; Crudele, L. Atherogenic index of plasma identifies subjects with severe liver steatosis. Sci. Rep. 2025, 15, 9136. [Google Scholar] [CrossRef] [PubMed]
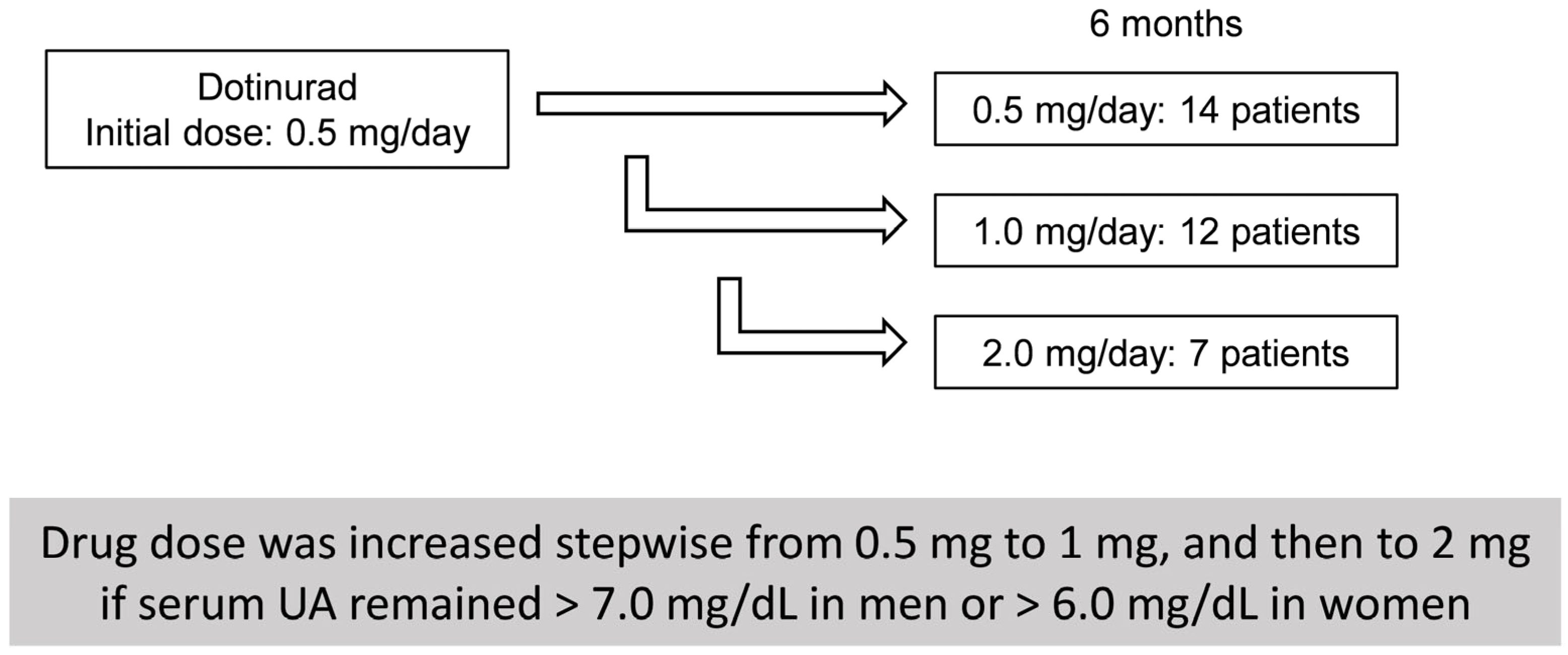
| Before | 6 Months | p-Value | |
|---|---|---|---|
| Age (years) | 59 (53–71) | - | - |
| Male (%) | 29 (87.9%) | - | - |
| HT (%) | 18 (54.5%) | - | - |
| DM (%) | 12 (36.4%) | - | - |
| DL (%) | 20 (60.6%) | - | - |
| MASLD (%) | 20 (60.6%) | - | - |
| MetALD (%) | 1 (3%) | - | - |
| ALD (%) | 12 (36.4%) | - | - |
| Body weight (kg) | 74.3 (65.2–76.6) | 72.2 (65.4–77.6) | 0.221 |
| BMI (kg/m2) | 24.3 (23.8–29.6) | 26.9 (24.6–30.3) | 0.678 |
| ALB (g/dL) | 4.5 (3.8–4.8) | 4.4 (3.8–4.7) | 0.844 |
| AST (U/L) | 26 (21–33) | 26 (22–29) | 0.623 |
| ALT (U/L) | 22 (15–36) | 20 (15–28) | 0.589 |
| ALP (U/L) | 65.5 (41.8–89) | 59.5 (42.5–87) | 0.559 |
| GGT (U/L) | 47 (30–78) | 43 (27–54) | 0.042 |
| T-Bil (mg/dL) | 0.6 (0.5–1.0) | 0.6 (0.4–0.7) | 0.023 |
| PLT (×104/μL) | 23.3 (17.7–28.5) | 22.3 (17.7–29.5) | 0.513 |
| BUN (mg/dL) | 17 (13–23) | 17 (14–20) | 0.110 |
| Cre (mg/dL) | 1.1 (0.9–1.3) | 1.0 (0.9–1.1) | 0.010 |
| eGFR (mL/min/1.73 m2) | 55.6 (44–67.3) | 56.6 (48.8–71.5) | 0.007 |
| TC (mg/dL) | 191 (164–218) | 190 (158–209) | 0.069 |
| TG (mg/dL) | 144 (89–169) | 123 (80–170) | 0.706 |
| LDL-C (mg/dL) | 119 (89–127.2) | 110 (83.8–126.8) | 0.198 |
| HDL-C (mg/dL) | 49.5 (41.5–54.8) | 51 (45.2–55.8) | 0.230 |
| BS (mg/dL) | 123 (107.5–178) | 126.5 (118–134) | 0.964 |
| HbA1c (%) | 6.2 (5.9–7.0) | 6.1 (5.9–6.8) | 0.611 |
| UA (mg/dL) | 8.4 (7.7–9.0) | 6.0 (5.9–6.8) | <0.001 |
| ALBI score | −3.1 (−3.3 to −2.5) | −3.2 (−3.4 to −2.6) | 0.497 |
| FIB-4 index | 1.4 (1.0–2.9) | 1.4 (0.9–2.5) | 0.300 |
| ATX (mg/L) | 1.1 (1.0–1.2) | 1.0 (0.7–1.1) | 0.683 |
| HA (ng/mL) | 23.5 (10.2–81.1) | 36.8 (10.2–75.1) | 0.683 |
| Type IV collagen (ng/mL) | 3.8 (3.5–3.9) | 3.5 (3.5–3.8) | 0.529 |
| Responders (n = 12) | Non-Responders (n = 21) | p-Value | |
|---|---|---|---|
| Age (years) | 60 (52.2–71.8) | 59 (53–71) | 0.881 |
| Male (%) | 9 (75%) | 20 (95.2%) | 0.364 |
| HT (%) | 7 (58.3%) | 11 (52.4%) | 1.000 |
| DM (%) | 8 (66.7%) | 4 (19%) | 0.010 |
| DL (%) | 8 (66.7%) | 12 (57.1%) | 0.719 |
| MASLD (%) | 6 (50%) | 14 (66.7%) | 0.465 |
| ALD (%) | 5 (41.7%) | 7 (33.3%) | 0.716 |
| BMI (kg/m2) | 24.4 (22.8–26.9) | 26.6 (23.1–29.6) | 0.673 |
| ALB (g/dL) | 4.4 (4.2–4.6) | 4.5 (3.6–4.8) | 0.822 |
| AST (U/L) | 32.5 (27.2–39.2) | 25 (20–27) | 0.036 |
| ALT (U/L) | 26 (14.5–37.2) | 20 (15–33) | 0.613 |
| ALP (U/L) | 67.5 (59–74.2) | 59 (40–92) | 0.940 |
| GGT (U/L) | 73.5 (39.8–140.2) | 45 (26–67) | 0.043 |
| T-Bil (mg/dL) | 0.6 (0.6–0.8) | 0.6 (0.5–1.0) | 0.836 |
| PLT (×104/μL) | 22.9 (18.3–27.8) | 24.9 (15.4–29.6) | 1.000 |
| BUN (mg/dL) | 18 (14.2–24.6) | 16 (13–23) | 0.431 |
| Cre (mg/dL) | 1.0 (0.9–1.1) | 1.1 (1.0–1.3) | 0.184 |
| eGFR (mL/min/1.73 m2) | 59.1 (47.9–67.8) | 54.8 (44–66.8) | 0.837 |
| TC (mg/dL) | 197 (153–223) | 186 (161.5–204.8) | 0.869 |
| TG (mg/dL) | 140 (88.5–188) | 152 (91–169) | 1.000 |
| LDL-C (mg/dL) | 115 (85–131.5) | 122.5 (104.2–133.8) | 0.288 |
| HDL-C (mg/dL) | 54.5 (42–58.8) | 45.5 (41–49.8) | 0.142 |
| BS (mg/dL) | 140.5 (124–162) | 111 (103–139.5) | 0.232 |
| HbA1c (%) | 6.0 (5.9–6.5) | 5.8 (5.4–6.4) | 0.326 |
| UA (mg/dL) | 8.3 (7.8–8.8) | 8.4 (7.7–9) | 0.896 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Age | 0.005 | (−0.007–0.017) | 0.372 | |||
| Male | −0.208 | (−0.731–0.314) | 0.422 | −0.344 | (−0.744–0.056) | 0.088 |
| HT | 0.135 | (−0.208–0.477) | 0.429 | |||
| DM | 0.327 | (−0.011–0.664) | 0.058 | |||
| DL | 0.007 | (−0.346–0.359) | 0.969 | |||
| MASLD | −0.070 | (−0.421–0.282) | 0.689 | |||
| ALD | 0.012 | (−0.346–0.370) | 0.947 | |||
| BMI | −0.009 | (−0.046–0.029) | 0.644 | |||
| ALB | 0.160 | (−0.13–0.45) | 0.268 | |||
| AST | 0.020 | (0.006–0.034) | 0.008 | |||
| ALT | 0.007 | (−0.007–0.021) | 0.302 | −0.011 | (−0.024 to −0.001) | 0.078 |
| ALP | −0.004 | (−0.011–0.003) | 0.282 | −0.008 | (−0.013 to −0.002) | 0.007 |
| GGT | 0.004 | (0.002–0.006) | <0.001 | 0.005 | (0.003–0.007) | <0.001 |
| T-Bil | 0.170 | (−0.106–0.447) | 0.218 | |||
| PLT | −0.008 | (−0.028–0.013) | 0.459 | |||
| BUN | 0.018 | (−0.007–0.044) | 0.143 | 0.028 | (0.010–0.047) | 0.004 |
| Cre | −0.001 | (−0.619–0.616) | 0.996 | |||
| eGFR | −0.002 | (−0.015–0.01) | 0.697 | |||
| UA | −0.016 | (−0.123–0.092) | 0.77 | −0.047 | (−0.130–0.035) | 0.247 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Age | 0.000 | (−0.003–0.002) | 0.853 | |||
| Male | 0.047 | (−0.059–0.152) | 0.377 | −0.123 | (−0.249–0.002) | 0.054 |
| HT | 0.024 | (−0.046–0.094) | 0.488 | |||
| DM | −0.010 | (−0.083–0.062) | 0.771 | −0.054 | (−0.120–0.011) | 0.100 |
| DL | 0.009 | (−0.062–0.081) | 0.789 | 0.072 | (−0.002–0.147) | 0.055 |
| MASLD | 0.017 | (−0.054–0.088) | 0.628 | |||
| ALD | −0.020 | (−0.092–0.053) | 0.584 | |||
| BMI | −0.051 | (−0.108–0.006) | 0.079 | |||
| ALB | −0.004 | (−0.007 to −0.002) | 0.003 | −0.057 | (−0.119–0.005) | 0.069 |
| AST | −0.002 | (−0.005–0.001) | 0.129 | −0.011 | (−0.017 to −0.005) | 0.002 |
| ALT | 0.000 | (−0.001–0.002) | 0.567 | 0.002 | (−0.001–0.006) | 0.156 |
| ALP | 0.000 | (−0.001–0.000) | 0.173 | |||
| GGT | −0.029 | (−0.086–0.027) | 0.299 | 0.001 | (0.000–0.002) | 0.066 |
| T-Bil | −0.002 | (−0.007–0.002) | 0.253 | |||
| PLT | −0.003 | (−0.008–0.002) | 0.253 | −0.006 | (−0.011 to −0.001) | 0.018 |
| BUN | −0.048 | (−0.173–0.076) | 0.432 | |||
| Cre | 0.001 | (−0.001–0.004) | 0.273 | |||
| eGFR | 0.002 | (−0.02–0.024) | 0.826 | |||
| UA | −0.051 | (−0.108–0.006) | 0.079 | 0.008 | (−0.012–0.029) | 0.393 |
| Author | No | Study Design | Pre UA (mg/dL) | 3M UA (mg/dL) | 6M UA (mg/dL) | Pre eGFR (mL/min/1.73 m2) | 3M eGFR (mL/min/1.73 m2) | 6M eGFR (mL/min/1.73 m2) |
|---|---|---|---|---|---|---|---|---|
| Tanaka, et al. 2023 [21] | 50 | Prospective | 8.3 | 5.2 | 5.2 | 47.8 | 47.2 | 46.9 |
| Amano, et al. 2024 [22] | 35 | Retrospective | 8.1 | 6.7 | - | 31.8 | 35.5 | - |
| Motomura, et al. 2025 [23] | 14 | Retrospective | 8.2 | - | 6.2 | 24.9 | - | 24.4 |
| Takata, et al. 2025 [24] | 29 | Retrospective | 8.4 | 6.5 | - | 33.9 | 36.2 | - |
| Yanai, et al. 2025 [25] | 73 | Retrospective | 6.8 | - | 5.8 | 61.2 | - | 59.2 |
| Present study | 33 | Retrospective | 8.4 | 7.1 | 6.0 | 55.6 | 56.8 | 56.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamijo, Y.; Iwadare, T.; Kimura, T.; Fujita, K.; Okumura, T.; Wakabayashi, S.-i.; Kobayashi, H.; Yamazaki, T.; Tanaka, N.; Kunimoto, H. Effect of Dotinurad on Uric Acid and Hepatorenal Parameters in Steatotic Liver Disease: A Pilot Study in Japanese Patients. Biomedicines 2025, 13, 2716. https://doi.org/10.3390/biomedicines13112716
Kamijo Y, Iwadare T, Kimura T, Fujita K, Okumura T, Wakabayashi S-i, Kobayashi H, Yamazaki T, Tanaka N, Kunimoto H. Effect of Dotinurad on Uric Acid and Hepatorenal Parameters in Steatotic Liver Disease: A Pilot Study in Japanese Patients. Biomedicines. 2025; 13(11):2716. https://doi.org/10.3390/biomedicines13112716
Chicago/Turabian StyleKamijo, Yuma, Takanobu Iwadare, Takefumi Kimura, Kaede Fujita, Taiki Okumura, Shun-ichi Wakabayashi, Hiroyuki Kobayashi, Tomoo Yamazaki, Naoki Tanaka, and Hideo Kunimoto. 2025. "Effect of Dotinurad on Uric Acid and Hepatorenal Parameters in Steatotic Liver Disease: A Pilot Study in Japanese Patients" Biomedicines 13, no. 11: 2716. https://doi.org/10.3390/biomedicines13112716
APA StyleKamijo, Y., Iwadare, T., Kimura, T., Fujita, K., Okumura, T., Wakabayashi, S.-i., Kobayashi, H., Yamazaki, T., Tanaka, N., & Kunimoto, H. (2025). Effect of Dotinurad on Uric Acid and Hepatorenal Parameters in Steatotic Liver Disease: A Pilot Study in Japanese Patients. Biomedicines, 13(11), 2716. https://doi.org/10.3390/biomedicines13112716








