Alternative Anticoagulation for Patients with Heparin-Induced Thrombocytopenia on ECMO: A Narrative Review
Abstract
1. Introduction
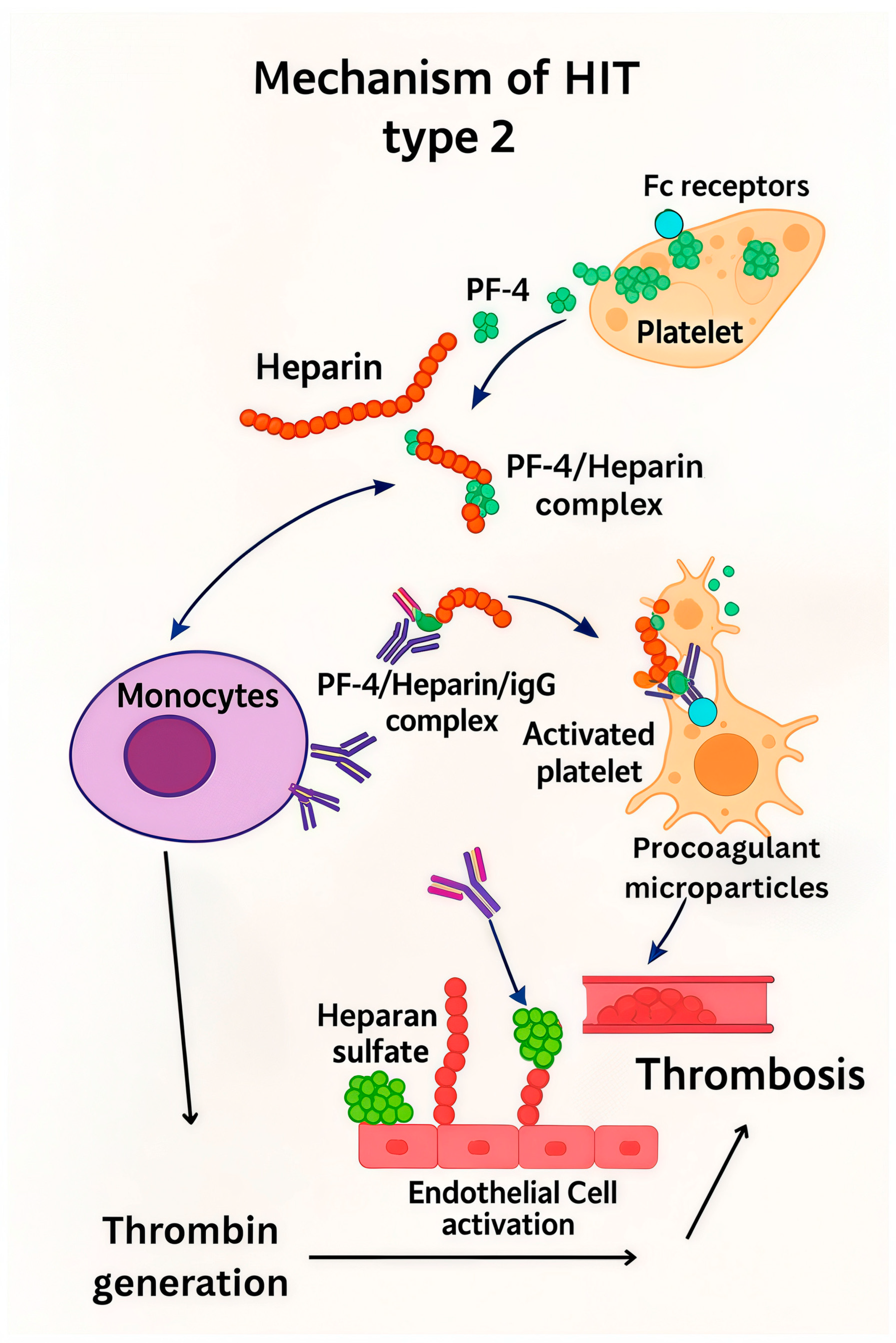
2. Heparin-Induced Thrombocytopenia—HIT
2.1. Types and Incidence
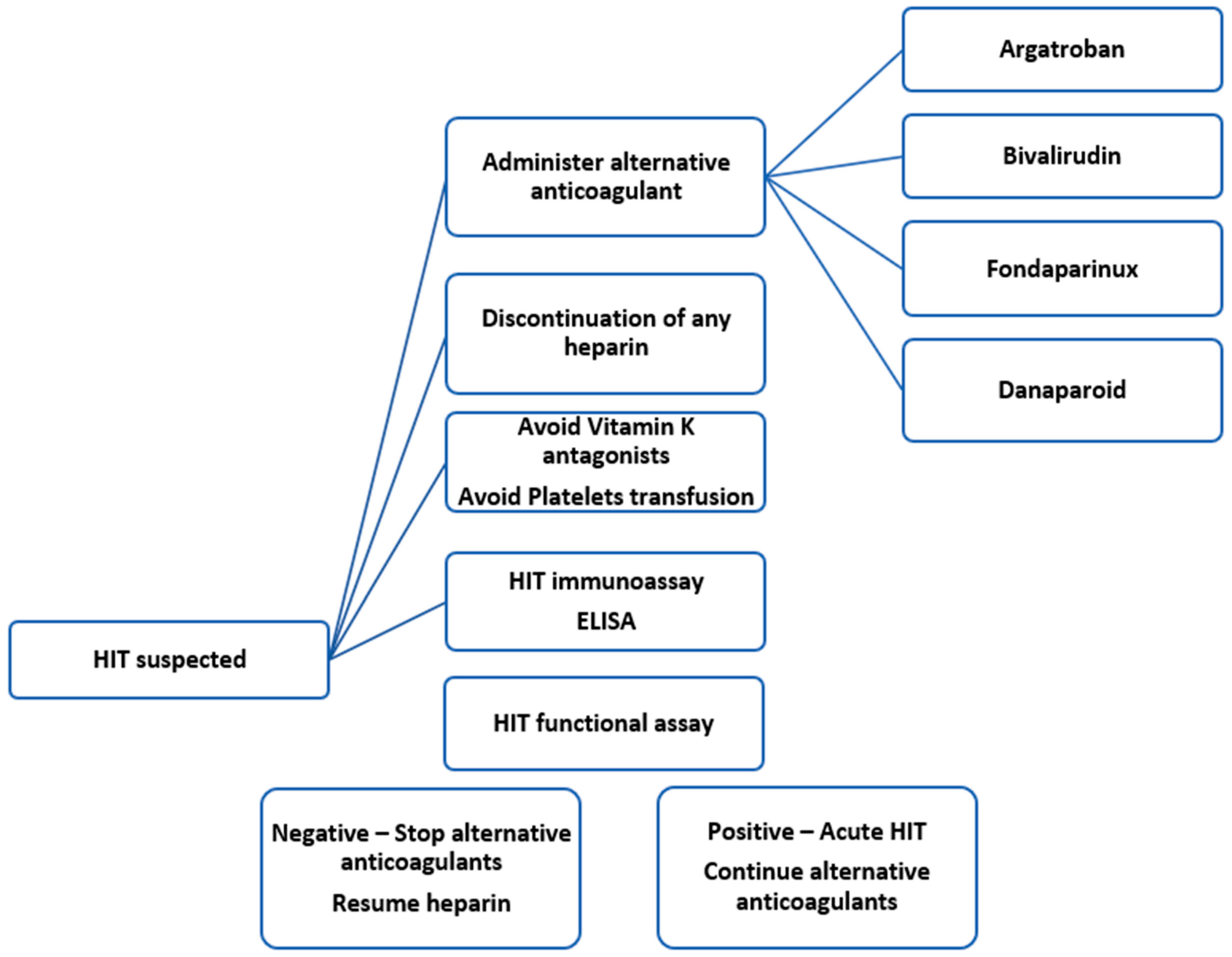
2.2. HIT Diagnostic
3. Alternative Anticoagulants
3.1. Argatroban
3.1.1. Mechanism of Action
3.1.2. Dosing and Monitoring of Argatroban
3.1.3. Clinical Studies
3.2. Bivalirudin
3.2.1. Mechanism of Action
3.2.2. Dosing and Monitoring of Bivalirudin
3.2.3. Clinical Experience
3.3. Lepirudin
3.4. Direct Factor-Xa Inhibitors
| Author (Year Published) | Study Design | No of Patients | Diagnostic Test of HIT | No. of HIT Patients | Type of ECMO, VA, VV | Type of Primary Anticoagulation | Alternative Anticoagulation | Thrombotic Event | Bleeding Event | Mortality | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lüsebrink, E et al., 2023 [16] | Retrospective, single-center | 373 | Detection anti-PF4/heparin antibodies, SRA, HIPA, and/or platelet aggregation test | 13/373 (3.5%) | VA-ECMO in the cardiac intensive care unit | A standardized protocol for anticoagulation was used for all patients with an initial bolus of UFH (5000 IU) and continued IV UFH infusion. Target an aPTT of 60–80 s. | Argatroban | Arterial thrombosis (10% vs. 15%, excluded and confirmed HIT group, p = 0.627), venous thrombosis (8% vs. 15%, excluded and confirmed HIT group p = 0.586) | 28% vs. 31%, p > 0.999 in excluded and confirmed HIT group | In-hospital mortality (43% vs. 38% in confirmed HIT and excluded HIT groups, p > 0.999), mortality after one month (35% vs. 38%, p > 0.999), three months (43% vs. 46%, p > 0.999), and twelve months (53% vs. 46%, p = 0.938) |
| 2 | Lubnow M, et al., 2022 [47] | Retrospective observational single-center study using prospectively collected data from the Regensburg ECMO Registry | 507 | HIT ELISA, HIPA test | 16/507 (3.2%) | VV ECMO therapy for severe respiratory failure and VA ECMO for circulatory failure | UFH is used as the standard of care, with goal aPTT set at 50 s for VV-ECMO and 60 s for VA-ECMO | Argatroban dosing aims for an aPTT of 50 s in HIT-suspected patients and 60 s in those with confirmed HIT | Higher rates of thrombosis in ECMO confirmed HIT | A higher incidence of bleeding in the groups temporarily treated with argatroban | / |
| 3 | Mang S, et al., 2022 [52] | Observational study | 41 | ELISA, HIPA test | 1 out of 41 | Coronavirus disease 2019 (COVID-19) requiring V-V ECMO | / | Argatroban | / | / | / |
| 4 | Arachchillage, et al., 2020 [27] | Single-center, retrospective, observational cohort study | 298 | ELISA, confirmatory tests such as Hemosil AcuStar HIT-IgG, an automated chemiluminescent immunoassay, or a platelet aggregation assay | 19/298 | VA-ECMO (11/142, 7.7%) and VV-ECMO (8/156, 5.1%) | A heparin bolus dose is given at cannulation, followed by heparin infusion during ECMO; the target heparin anti-Xa concentration was 0.2–0.3 U/mL for VV-ECMO and 0.3–0.5 U/mL for VA-ECMO | Argatroban, rate of 0.2 μg/kg/min, and adjusted to maintain an aPTT of 48–78 s. | 89.5% (17/19) | None of the patients with HIT following VA-ECMO had major bleeding. Major bleeding rates in VA-ECMO and VV-ECMO patients were 27.5% (39/142) and 23.7% (37/156), respectively, with bleeding being more common in patients without HIT (p = 0.03). | 6/19, 31.6% HIT group vs. 89/279, 32.2% in patients without HIT on ECMO (p = 0.79). No difference was observed in the mortality rate in patients on ECMO with HIT 1/2, 50% vs. HITT 5/17, 29.4%; p = 0.96 |
| 5 | Pabst D, et al., 2019 [28] | Single-center retrospective study | 455 | SRA | 14/455 | / | Continuous UFH with a goal for an aPTT of 50–60 s | The initial dose of argatroban 2 mcg/kg/min, over 1–3 h at aim to a steady-state aPTT of 50–60 s. The dose was then reduced to 0.5–1 mcg/kg/min to maintain the aPTT in the target range | / | / | Mortality 3/14 (21.4%) |
| 6 | Glick D, et al., 2015 [21] | Retrospective | 119 | Heparin-platelet factor 4 immunoassay, the serotonin release assay | 1/119 | / | Bolus of UFH at ECMO initiation, followed by a recommended infusion of 7.5 U/kg per hour titrated to maintain an aPTT of 40 to 60 s | Argatroban | / | / | Patients suspected of having HIT—significantly higher in-hospital mortality rates (14/23, 61% vs. 31/96, 32%; p = 0.01), reflected the more severe thrombocytopenia in this group, indicating a sicker patient population |
| 7 | Arachchillage DJ et al., 2022 [21] | Multicenter observational study | 152 | ELISA, confirmatory tests such as Hemosil AcuStar HIT-IgG, an automated chemiluminescent immunoassay, or a platelet aggregation assay | 16/152 | Consecutive patients (≥18 years) with severe COVID-19 who were supported by VV ECMO | UFH with heparin anti-Xa of 0.2–0.3 IU/mL or equivalent aPTT unless they had major bleeding. For patients with thrombosis at the initiation or during ECMO the targets were increased up to anti-Xa of 0.5–0.7 IU/mL or equivalent aPTT at local clinical discretion. | Argatroban | 10 out 16 patients (62.5%) | / | 3 out of 16 patients |
| 8 | Kimmoun A, et al., 2018 [13] | Retrospective study | 5797 | Positive anti- PF4/heparin antibodies | 21/5797 | VA-ECMO | UFH | Argatroban in 11/21 confirmed HIT patients (52.4%) and danaparoid in 10/21 patients (47.6%). | 7/21 (33.3%) patients—arterial or venous thrombosis | 12/21 patients (57.1%) | / |
| 9 | Vayne C, et al., 2019 [19] | Observational study | 57 | ELISA, SRA | 2 out 57 patients | VA ECMO for at least 5 days | UFH adjusted to maintain aPTT 1.2 and 1.5 for the first 48 h, then, the heparin dose gradually increased to obtain an aPTT ratio between 1.8 and 2.2 | Argatroban | 2 out of 2 | ||
| 10 | Hanna DJ et al., 2022 [62] | Single-center retrospective study | 292 | SRA, ELISA | 12 patients | VA-ECMO, VV -ECMO | UNH bolus administration of 50–100 units/kg at the time of ECMO cannulation plus heparin infusion to maintain an aPTT of 49–67 s (correlating to an anti-factor Xa level of 0.2–0.5 IU/mL) | Bivalirudin titrated to target an aPTT of 46–65 s | 60% (6/12) patients | 8 patients (66.7%)—major bleeding, minor bleeding-2 patients (16.7%) | 60% (6/12) |
| 11 | Giuliano K et al., 2021 [72] | Retrospective cohort study | 144 | Positive PF4/SRA | 13/144 patients (9%) | 80.6% VA ECMO | Heparin infusion, with a goal aPTT of 50–65 s | Bivalirudin titrated with an aPTT target range of 50–65 s. | 0.25 event/patient in HIT positive, 0.22 event/patient in HIT rule-out, 0.32 event/patient in HIT negative | Gastrointestinal bleeding-HIT patients (0.5/patient); HIT negative 0.07 event /patients | Mortality—similar between patients treated with bivalirudin and heparin (69%) and those anticoagulated with heparin alone (62%), 75% in HIT positive |
| 12 | Wood KL, et al., 2020 [85] | Retrospective analysis | 203 | Positive platelet factor 4 test with an optical density value (OD) value exceeding 1.0, SRA | 8/203 | VA-ECMO | Heparin monitored with ACT every 6 h with a target of 180 to 220 s or activated partial thromboplastin time (aPTT) target of 54 to 71 s | bivalirudin | No | No | No |
| 13 | Sullivan J, et al., 2020 [34] | Single-center, observational, retrospective cohort study | 39 | Positive anti-PF4 result with optical density (OD) of 0.4 or higher and positive SRA results | 2/39 (5.1%) | / | Heparin | Bivalirudin and argatroban | No | No | No |
| 14 | Kataria V, et al., 2020 [36] | Retrospective, single-center study | 473 | ELISA with OD greater than or equal to 1.0.; serotonin release assay (SRA) | 9/473 (1.9%) | Fondaparinux, argatroban, or bivalirudin | Clinically significant bleeding, defined as bleeding that caused a hemoglobin drop of 2 g/dL or more, occurred more often in the SRA-positive group (36.8% vs. 5 patients, 55.6%, p = 0.282). | Venous thromboembolism events—more frequent in the SRA-negative group (31.5% vs. 2 patients, 22.2%, p = 0.99 | / | ||
| 15 | Mazzeffi M, et al., 2021 [71] | Observational | 20 | SRA | 2 out 20 (10%) | VA ECMO | Heparin with a goal aPTT between 60 and 80 s | Direct thrombin inhibitors | 1 of 2 patients | / | No |
| 16 | Sokolovic M, et al., 2016 [14] | Retrospective study of a prospectively collected dataset | 96 | SRA, ELISA test (anti-PF4/heparin antibodies) OD values of equal or greater than 0.4 | 8 of 96 | UFH—aim for ACT goal of 160–180 s (antiXa 0.3–0.7 IU/mL) | Argatroban and bivalirudin | 7 of 8 patients | / | / | |
| 17 | Kutleša M, et al., 2017 [79] | Single-center retrospective study of prospective database | 40 | ELISA antibodies PF-4 | 3 out of 40 | VV ECMO | UFH-ACT values targeted at the range between 170–180 s | Fondaparinux (2.5 mg daily) | / | / | / |
| 18 | Kutleša M, et al., 2023 [80] | Single-center retrospective study | 112 | ID-PaGIA Heparin/PF4 Antibody Test; ELISA testing | 39% (44/112) | VV ECMO for COVID-19-induced ARDS | UFH-ACT values targeted at the range between 170–180 s | Fondaparinux (5 mg daily) | / | / | / |
| 19 | Bauer, C. et al., 2008 [84] | Case report | 1 | VA ECMO | UFH 400 IU/h for 4 h, then 300 IU/h (0.5–0.8 U/mL anti-Xa factor activity goal) | No | No | No |
3.5. Other Anticoagulation Approaches
3.6. The Alternative Anticoagulation Methods
4. Future Development and Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECMO | Extracorporeal Membrane Oxygenation |
| CPR | Cardiopulmonary Resuscitation |
| VV-ECMO | Venovenous ECMO |
| VA-ECMO | Venoarterial ECMO |
| DIC | Disseminated Intravascular Coagulation |
| SAPS III | Simplified Acute Physiology Score |
| aPTT | Activated Partial Thromboplastin Time |
| ICU | Intensive Care Unit |
| UFH | Unfractionated heparin |
| AT | Antithrombin |
| ACT | Activated Clotting Time |
| HIT | Heparin-Induced Thrombocytopenia |
| PF4 | Platelet Factor 4 |
| HITT | Heparin-Induced Thrombocytopenia with Thrombosis |
| CPB | Cardiopulmonary Bypass |
| MCS | Mechanical Circulatory Support |
| HEP | HIT Expert Probability |
| LLL | Lilo-Le Louet score |
| ELISA | Enzyme-linked immunosorbent assay |
| C-SRA | C-serotonin release assay |
| HIPA | Heparin-Induced Platelet Activation |
| DTI | Direct Thrombin Inhibitor |
| dTT | Diluted Thrombin Time |
| ECT | Ecarin Clotting Time |
| CAA | Chromogenic Antithrombin Activity |
| VKA | Vitamin K Antagonist |
| BMI | Body Mass Index |
| LV | Left Ventricle |
| ICH | Intracranial Hemorrhage |
| CRRT | Continuous Renal Replacement Therapy |
| ARDS | Acute Respiratory Distress Syndrome |
| PT | Prothrombin Time |
| NM | Nafamostat mesylate |
| NO | Nitric Oxide |
| ELSO | Extracorporeal Life Support Organization |
References
- Rajsic, S.; Breitkopf Jadzic, D.; Popovic Krneta, M.; Tauber, H.; Treml, B. Anticoagulation strategies during extracorporeal membrane oxygenation: A narrative review. J. Clin. Med. 2022, 11, 5147. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Popovic Krneta, M.; Bukumiric, Z. Extracorporeal membrane oxygenation for cardiogenic shock: A meta-analysis of mortality and complications. Ann. Intensive Care 2022, 12, 93. [Google Scholar] [CrossRef]
- Gray, B.W.; Haft, J.W.; Hirsch, J.C.; Annich, G.M.; Hirschl, R.B.; Bartlett, R.H. Extracorporeal life support: Experience with 2,000 patients. ASAIO J. 2015, 61, 2–7. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Oezpeker, U.C.; Bukumirić, Z.; Dobesberger, M.; Treml, B. The role of excessive anticoagulation and missing hyperinflammation in ECMO-associated bleeding. J. Clin. Med. 2022, 11, 2314. [Google Scholar] [CrossRef]
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016, 6, 97. [Google Scholar] [CrossRef]
- Ellouze, O.; Abbad, X.; Constandache, T.; Missaoui, A.; Berthoud, V.; Daily, T.; Aho, S.; Bouchot, O.; Bouhemad, B.; Guinot, P.G. Risk factors of bleeding in patients undergoing venoarterial extracorporeal membrane oxygenation. Ann. Thorac. Surg. 2021, 111, 623–628. [Google Scholar] [CrossRef]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021, 67, 827–844. [Google Scholar] [CrossRef]
- Ghiselli, G. Heparin Binding Proteins as Therapeutic Target: An historical account and current trends. Medicine 2019, 6, 80. [Google Scholar] [CrossRef]
- Boneu, B.; Caranobe, C.; Sie, P. Pharmacokinetics of heparin and low molecular weight heparin. Bailliere’s Clin. Haematol. 1990, 3, 531–544. [Google Scholar] [CrossRef]
- Derbalah, A.; Duffull, S.; Newall, F.; Moynihan, K.; Al-Sallami, H. Revisiting the pharmacology of unfractionated heparin. Clin. Pharmacokinet. 2019, 58, 1015–1028. [Google Scholar] [CrossRef]
- McMichael, A.B.V.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kimmoun, A.; Oulehri, W.; Sonneville, R.; Grisot, P.H.; Zogheib, E.; Amour, J.; Aissaoui, N.; Megarbane, B.; Mongardon, N.; Renou, A.; et al. Prevalence and outcome of heparin-induced thrombocytopenia diagnosed under veno-arterial extracorporeal membrane oxygenation: A retrospective nationwide study. Intensive Care Med. 2018, 44, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, M.; Pratt, A.K.; Vukicevic, V.; Sarumi, M.; Johnson, L.S.; Shah, N.S. Platelet count trends and prevalence of heparin- induced thrombocytopenia in a cohort of extracorporeal membrane oxygenator patients. Crit. Care Med. 2016, 44, e1031–e1037. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. Clinical practice. Heparin-Induced Thrombocytopenia. New Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Scherer, C.; Binzenhöfer, L.; Hoffmann, S.; Höpler, J.; Kellnar, A.; Thienel, M.; Joskowiak, D.; Peterß, S.; Petzold, T.; et al. Heparin-induced thrombocytopenia in patients undergoing venoarterial extracorporeal membrane oxygenation. J. Clin. Med. 2023, 12, 362. [Google Scholar] [CrossRef]
- Ng, J.Y.; D’Souza, M.; Hutani, F.; Choi, P. Management of heparin-induced thrombocytopenia: A contemporary review. J. Clin. Med. 2024, 13, 4686. [Google Scholar] [CrossRef]
- Hvas, A.M.; Favaloro, E.J.; Hellfritzsch, M. Heparin-induced thrombocytopenia: Pathophysiology, diagnosis and treatment. Expert. Rev. Hematol. 2021, 14, 335–346. [Google Scholar] [CrossRef]
- Vayne, C.; May, M.A.; Bourguignon, T.; Lemoine, E.; Guery, E.A.; Rollin, J.; Gruel, Y.; Pouplard, C. Frequency and Clinical Impact of Platelet Factor 4-Specific Antibodies in Patients Undergoing Extracorporeal Membrane Oxygenation. J. Thromb. Haemost. 2019, 119, 1138–1146. [Google Scholar] [CrossRef]
- Choi, J.H.; Luc, J.G.Y.; Weber, M.P.; Reddy, H.G.; Maynes, E.J.; Deb, A.K.; Samuels, L.E.; Morris, R.J.; Massey, H.T.; Loforte, A.; et al. Heparin-induced thrombocytopenia during extracorporeal life support: Incidence, management and outcomes. Ann. Cardiothorac. Surg. 2019, 8, 19–31. [Google Scholar] [CrossRef]
- Glick, D.; Dzierba, A.L.; Abrams, D.; Muir, J.; Eisenberger, A.; Diuguid, D.; Abel, E.; Agerstrand, C.; Bacchetta, M.; Brodie, D. Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J. Crit. Care 2015, 30, 1190–1194. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Greinacher, A.; Koster, A. Heparin-induced thrombocytopenia in patients with ventricular assist devices: Are new prevention strategies required? Ann. Thorac. Surg. 2009, 87, 1633–1640. [Google Scholar] [CrossRef]
- Pollak, U.; Yacobobich, J.; Tamary, H.; Dagan, O.; Manor-Shulman, O. Heparin-induced thrombocytopenia and extracorporeal membrane oxygenation: A case report and review of the literature. J. Extra Corpor. Technol. 2011, 43, 5–12. [Google Scholar] [CrossRef]
- Salter, B.S.; Weiner, M.M.; Trinh, M.A.; Heller, J.; Evans, A.S.; Adams, D.H.; Fischer, G.W. Heparin-induced thrombocytopenia: A comprehensive clinical review. J. Am. Coll. Cardiol. 2016, 67, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Jiritano, F.; Serraino, G.F.; Ten Cate, H.; Fina, D.; Matteucci, M.; Mastroroberto, P.; Lorusso, R. Platelets and extracorporeal membrane oxygenation in adult patients: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Zaaqoq, A.M.; Brammer, R.C.; Chan, C.M.; Shorr, A.F. Heparin-induced thrombocytopenia in extra-corporeal membrane oxygenation: Epidemiology, outcomes, and diagnostic challenges. J. Thromb. Thrombolysis 2022, 53, 499–505. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Laffan, M.; Khanna, S.; Vandenbriele, C.; Kamani, F.; Passariello, M.; Rosenberg, A.; Aw, T.C.; Banya, W.; Ledot, S.; et al. Frequency of thrombocytopenia and heparin-induced thrombocytopenia in patients receiving extracorporeal membrane oxygenation compared with cardiopulmonary bypass and the limited sensitivity of pretest probability score. Crit. Care Med. 2020, 48, e371–e379. [Google Scholar] [CrossRef]
- Pabst, D.; Boone, J.B.; Soleimani, B.; Brehm, C.E. Heparin-induced thrombocytopenia in patients on extracorporeal membrane oxygenation and the role of a heparin-bonded circuit. Perfusion 2019, 34, 584–589. [Google Scholar] [CrossRef]
- Lee, G.M.; Arepally, G.M. Heparin-induced thrombocytopenia. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.J.; Thachil, J.; Anderson, J.A.M.; Baker, P.; Poles, A.; Kitchen, S.; Laffan, M.; Committee, T.B. Diagnosis and management of heparin-induced thrombocytopenia: Third edition. Br. J. Haematol. 2024, 204, 459–475. [Google Scholar] [CrossRef]
- Kram, S.J.; Hamidi, A.; Kram, B.L.; Arepally, G.; Levy, J.H. The predictive value of the 4Ts and HEP score at recommended cutoffs in patients with mechanical circulatory support devices. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1873–1879. [Google Scholar] [CrossRef]
- Lillo-Le Louet, A.; Boutouyrie, P.; Alhenc-Gelas, M.; Le Beller, C.; Gautier, I.; Aiach, M.; Lasne, D. Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J. Thromb. Haemost. 2004, 2, 1882–1888. [Google Scholar] [CrossRef]
- Bolliger, D.; Santer, D.; Tanaka, K.A. Heparin-Induced Thrombocytopenia in Patients With Mechanical Circulatory Support. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1880–1882. [Google Scholar] [CrossRef]
- Sullivan, J.; Bak, E.; Sullivan, M.J.; Gurnani, P.K. Predictive value of scoring tools in determining heparin-induced thrombocytopenia in patients on extracorporeal membrane oxygenation. Perfusion 2020, 35, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E. Laboratory diagnosis of heparin-induced thrombocytopenia. Int. J. Lab. Hematol. 2019, 41 (Suppl. 1), 15–25. [Google Scholar] [CrossRef]
- Kataria, V.; Moore, L.; Harrison, S.; Hernandez, O.; Vaughan, N.; Schwartz, G. Evaluation of serotonin release assay and enzyme-linked immunosorbent assay optical density thresholds for heparin-induced thrombocytopenia in patients on extracorporeal membrane oxygenation. Crit. Care Med. 2020, 48, e82–e86. [Google Scholar] [CrossRef]
- Caton, S.; O’Brien, E.; Pannelay, A.J.; Cook, R.G. Assessing the clinical and cost impact of on-demand immunoassay testing for the diagnosis of heparin induced thrombocytopenia. Thromb. Res. 2016, 140, 155–162. [Google Scholar] [CrossRef]
- Pollak, U. Heparin-induced thrombocytopenia complicating extracorporeal membrane oxygenation support: Review of the literature and alternative anticoagulants. J. Thromb. Haemost. 2019, 17, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.; Crowther, M.A.; Rice, L.; Datko, F.; Hook, K.; Propert, K.J.; Kuter, D.J.; Ortel, T.L.; Konkle, B.A.; et al. The HIT expert probability (HEP) score: A novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J. Thromb. Haemost. 2010, 8, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.M.; Chong, B.H.; Cines, D.B.; Greinacher, A.; Gruel, Y.; Linkins, L.A.; Rodner, S.B.; Selleng, S.; Warkentin, T.E.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Heparin-induced thrombocytopenia. Blood Adv. 2018, 2, 3360–3392. [Google Scholar] [CrossRef]
- Linkins, L.-A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia. Chest 2012, 141, e495S–e530S. [Google Scholar] [CrossRef]
- Rougé, A.; Pelen, F.; Durand, M.; Schwebel, C. Argatroban for an alternative anticoagulant in HIT during ECMO. J. Intensive Care 2017, 5, 39. [Google Scholar] [CrossRef]
- Beiderlinden, M.; Treschan, T.; Gölinger, K.; Peters, J. Argatroban in extracorporeal membrane oxygenation. Artif. Organs 2007, 31, 461–465. [Google Scholar] [CrossRef]
- Rajsic, S.; Irsara, C.; Griesmacher, A.; Brunelli, L.; Breitkopf, R.; Innerhofer, N.; Eckhardt, C.; Treml, B. Anticoagulation monitoring during extracorporeal membrane oxygenation: A narrative review. J. Cardiothorac. Vasc. Anesth. 2025, 39, 2446–2459. [Google Scholar] [CrossRef]
- Alberio, L.; Angelillo-Scherrer, A.; Asmis, L.; Casini, A.; Fontana, P.; Graf, L.; Hegemann, I.; Kremer Hovinga, J.A.; Korte, W.; Lecompte, T.; et al. Recommendations on the use of anticoagulants for the treatment of patients with heparin-induced thrombocytopenia in Switzerland. Swiss Med. Wkly. 2020, 150, w20210. [Google Scholar] [CrossRef] [PubMed]
- Gruel, Y.; De Maistre, E.; Pouplard, C.; Mullier, F.; Susen, S.; Roullet, S.; Blais, N.; Le Gal, G.; Vincentelli, A.; Lasne, D.; et al. Diagnosis and management of heparin-induced thrombocytopenia. Anaesth. Crit. Care Pain. Med. 2020, 39, 291–310. [Google Scholar] [CrossRef]
- Lubnow, M.; Berger, J.; Schneckenpointner, R.; Zeman, F.; Lunz, D.; Philipp, A.; Foltan, M.; Lehle, K.; Heimerl, S.; Hart, C.; et al. Prevalence and outcomes of patients developing heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. PLoS ONE 2022, 17, e0272577. [Google Scholar] [CrossRef]
- Arachchillage, D.J.; Rajakaruna, I.; Scott, I.; Gaspar, M.; Odho, Z.; Banya, W.; Vlachou, A.; Isgro, G.; Cagova, L.; Wade, J.; et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br. Soc. Haematol. 2022, 196, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.H.; Lopez, N.D. Argatroban for Heparin-Induced Thrombocytopenia during Venovenous Extracorporeal Membrane Oxygenation with Continuous Venovenous Hemofiltration. J. Extracorpor. Technol. 2017, 49, 115–120. [Google Scholar] [CrossRef]
- Fisser, C.; Winkler, M.; Malfertheiner, M.V.; Philipp, A.; Foltan, M.; Lunz, D.; Zeman, F.; Maier, L.S.; Lubnow, M.; Müller, T. Argatroban versus heparin in patients without heparin-induced thrombocytopenia during venovenous extracorporeal membrane oxygenation: A propensity-score matched study. Crit. Care 2021, 25, 160. [Google Scholar] [CrossRef]
- Lewis, B.E.; Wallis, D.E.; Berkowitz, S.D.; Matthai, W.H.; Fareed, J.; Walenga, J.M.; Bartholomew, J.; Sham, R.; Lerner, R.G.; Zeigler, Z.R.; et al. ARG-911 Study Investigators. Argatroban anticoagulant therapy in patients with heparin- induced thrombocytopenia. Circulation 2001, 103, 1838–1843. [Google Scholar] [CrossRef]
- Mang, S.; Danziger, G.; Metz, C.; Rixecker, T.; Becker, A.; Omlor, A.J.; Jentgen, C.; Schmoll, C.; Seiler, F.; Reyher, C.; et al. Injection of recombinant tissue plasminogen activator into extracorporeal membrane oxygenators postpones oxygenator exchange in COVID-19. ASAIO J. 2022, 68, 1017–1023. [Google Scholar] [CrossRef]
- Zhong, H.; Zhu, M.L.; Yu, Y.T.; Li, W.; Xing, S.P.; Zhao, X.Y.; Wang, W.J.; Gu, Z.C.; Gao, Y. Management of bivalirudin anticoagulation therapy for extracorporeal membrane oxygenation in heparin-induced thrombocytopenia: A case report and a systematic review. Front. Pharmacol. 2020, 11, 565013. [Google Scholar] [CrossRef]
- Bates, S.M.; Weitz, J.I. The mechanism of action of thrombin inhibitors. J. Invasive Cardiol. 2000, 12 (Suppl. F), 27F–32. [Google Scholar] [PubMed]
- Robson, R.; White, H.; Aylward, P.; Frampton, C. Bivalirudin pharmacokinetics and pharmacodynamics: Effect of renal function, dose, and gender. Clin. Pharmacol. Ther. 2002, 71, 433–439. [Google Scholar] [CrossRef]
- Navaei, A.; Kostousov, V.; Teruya, J. Is it time to switch to bivalirudin for ECMO anticoagulation? Front. Med. 2023, 10, 1237601. [Google Scholar] [CrossRef] [PubMed]
- Netley, J.; Roy, J.; Greenlee, J.; Hart, S.; Todt, M.; Statz, B. Bivalirudin anticoagulation dosing protocol for extracorporeal membrane oxygenation: A retrospective review. J. Extracorpor. Technol. 2018, 50, 161–166. [Google Scholar] [CrossRef]
- Helms, J.; Frere, C.; Thiele, T.; Tanaka, K.A.; Neal, M.D.; Steiner, M.E.; Connors, J.M.; Levy, J.H. Anticoagulation in adult patients supported with extracorporeal membrane oxygenation: Guidance from the Scientific and Standardization Committees on Perioperative and Critical Care Haemostasis and Thrombosis of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2023, 21, 373–396. [Google Scholar] [CrossRef]
- Lopez, N.D.; Seto, S.L.; Barra, M.E.; Roberts, R.J.; Rosovsky, R.P.; Solomon, E.J.; Dalia, A. Evaluation of Bivalirudin During Adult Extracorporeal Membrane Oxygenation: A Retrospective Characterization of Dosing, Efficacy and Bleeding. Hosp. Pharm. 2024, 59, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Berei, T.J.; Lillyblad, M.P.; Wilson, K.J.; Garberich, R.F.; Hryniewicz, K.M. Evaluation of systemic heparin versus bivalirudin in adult patients supported by extracorporeal membrane oxy- genation. ASAIO J. 2018, 64, 623–629. [Google Scholar] [CrossRef]
- Ranucci, M.; Ballotta, A.; Kandil, H.; Isgrò, G.; Carlucci, C.; Baryshnikova, E.; Pistuddi, V.; The Surgical and Clinical Outcome Research Group. Bivalirudin-based versus conventional heparin anticoagulation for postcardi- otomy extracorporeal membrane oxygenation. Crit. Care 2011, 15, R275. [Google Scholar] [CrossRef]
- Hanna, D.J.; Torbic, H.; Militello, M.; Strnad, K.; Krishnan, S.; Hohlfelder, B. Evaluation of anticoagulation with bivalirudin for heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. Int. J. Artif. Organs 2022, 45, 688–694. [Google Scholar] [CrossRef]
- Tsu, L.V.; Dager, W.E. Comparison of bivalirudin dosing strategies using total, adjusted, and ideal body weights in obese patients with heparin-induced thrombocytopenia. Pharmacotherapy 2012, 32, 20–26. [Google Scholar] [CrossRef]
- Kaseer, H.; Soto-Arenall, M.; Sanghavi, D.; Moss, J.; Ratzlaff, R.; Pham, S.; Guru, P. Heparin vs bivalirudin anticoagulation for extracorporeal membrane oxygenation. J. Card. Surg. 2020, 35, 779–786. [Google Scholar] [CrossRef]
- Hasegawa, D.; Sato, R.; Prasitlumkum, N.; Nishida, K.; Keaton, B.; Acquah, S.O.; Im Lee, Y. Comparison of bivalirudin versus heparin for anticoagulation during extracorporeal membrane oxygenation. ASAIO J. 2023, 69, 396–401. [Google Scholar] [CrossRef]
- Hamzah, M.; Jarden, A.M.; Ezetendu, C.; Stewart, R. Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. 2020, 21, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Najam, F.; Pocock, E.S.; Munoz, P.F.; Farrar, K.A.; Yamane, D.P. A comparison of bivalirudin and heparin for patients on extracorporeal membrane oxygenation. Thromb. Res. 2020, 190, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liang, S.; Zhu, J.; Dai, M.; Jia, Z.; Huang, H.; He, Y. The efficacy and safety of bivalirudin versus heparin in the anticoagulation therapy of extracorporeal membrane oxygenation: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 771563. [Google Scholar] [CrossRef] [PubMed]
- Rivosecchi, R.M.; Arakelians, A.R.; Ryan, J.; Murray, H.; Padmanabhan, R.; Gomez, H.; Phillips, D.; Sciortino, C.; Arlia, P.; Freeman, D.; et al. Comparison of anticoagulation strategies in patients requiring venovenous extracorporeal membrane oxygenation: Heparin versus bivalirudin. Crit. Care Med. 2021, 49, 1129–1136. [Google Scholar] [CrossRef]
- Ranucci, M. Bivalirudin and post-cardiotomy ECMO: A word of caution. Crit. Care 2012, 16, 427. [Google Scholar] [CrossRef]
- Mazzeffi, M.; Clark, M.; Grazioli, A.; Dugan, C.; Rector, R.; Dalton, H.; Madathil, R.; Menaker, J.; Herr, D.; Tanaka, K. Platelet factor-4 concentration in adult veno-arterial ECMO patients. Perfusion 2021, 36, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.; Bigelow, B.F.; Etchill, E.W.; Velez, A.K.; Ong, C.S.; Choi, C.W.; Bush, E.; Cho, S.M.; Whitman, G.J.R. Extracorporeal membrane oxygenation complications in heparin- and bivalirudin-treated patients. Crit. Care Explor. 2021, 3, e0485. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Ansell, J.E. Direct thrombin inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 581–592, Erratum in Br. J. Clin. Pharmacol. 2011, 72, 718. [Google Scholar] [CrossRef]
- Dager, W.E.; Gosselin, R.C.; Yoshikawa, R.; Owings, J.T. Lepirudin in heparin-induced thrombocytopenia and extracorporeal membranous oxygenation. Ann. Pharmacother. 2004, 38, 598–601. [Google Scholar] [CrossRef]
- Kelton, J.G.; Arnold, D.M.; Bates, S.M. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N. Engl. J. Med. 2013, 368, 737–744. [Google Scholar] [CrossRef]
- Yin, Q.; Han, L.; Wang, Y.; Kang, F.; Cai, F.; Wu, L.; Zheng, X.; Li, L.; Dong, L.E.; Dong, L.; et al. Unlocking the potential of fondaparinux: Guideline for optimal usage and clinical suggestions (2023). Front. Pharmacol. 2024, 15, 1352982. [Google Scholar] [CrossRef]
- Parlar, A.I.; Sayar, U.; Cevirme, D.; Yuruk, M.A.; Mataraci, I. Successful use of fondaparinux in a patient with heparin-induced thrombocytopenia while on extracorporeal membrane oxygenation after mitral valve redo surgery. Int. J. Artif. Organs 2014, 37, 344–347. [Google Scholar] [CrossRef]
- Osawa, E.A.; Brandão, A.; Dias Américo, A.; Maciel, A.T. Fondaparinux for systemic anticoagulation during continuous hemofiltration in a patient with heparin-induced thrombocytopenia and limb ischemia—A case report. Hematol. Transfus. Cell Ther. 2022, 44, 108–111. [Google Scholar] [CrossRef]
- Kutleša, M.; Novokmet, A.; Josipović Mraović, R.; Baršić, B. Venovenous extracorporeal membrane oxygenation for ARDS: Outcome analysis of a Croatian referral center for respiratory ECMO. Wien. Klin. Wochenschr. 2017, 129, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Kutleša, M.; Santini, M.; Krajinović, V.; Papić, N.; Gjurašin, B.; Krznarić, J.; Kotarski, V. Extracorporeal membrane oxygenation in COVID-19: Results of the Croatian extracorporeal membrane oxygenation referral center. Int. J. Artif. Organs 2023, 46, 248–251. [Google Scholar] [CrossRef]
- Wilde, M.I.; Markham, A. Danaparoid. A review of its pharmacology and clinical use in the management of heparin-induced thrombocytopenia. Drugs 1997, 54, 903–924. [Google Scholar] [CrossRef]
- Leo, A.; Winteroll, S. Laboratory diagnosis of heparin-induced thrombocytopenia and monitoring of alternative anticoagulants. Clin. Diagn. Lab. Immunol. 2003, 10, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Davidson, S.; Keeling, D. Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: Second edition. Br. J. Haematol. 2012, 159, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Vichova, Z.; Ffrench, P.; Hercule, C.; Jegaden, O.; Bastien, O.; Lehot, J.J. Extracorporeal membrane oxygenation with danaparoid sodium after massive pulmonary embolism. Anesth. Analg. 2008, 106, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.L.; Ayers, B.; Gosev, I.; Kumar, N.; Melvin, A.L.; Barrus, B.; Prasad, S. Venoarterial-extracorporeal membrane oxygenation without routine systemic anticoagulation decreases adverse events. Ann. Thorac. Surg. 2020, 109, 1458–1466. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.W.; Kim, G.S.; Jeong, I.S. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med. J. 2017, 53, 110–117. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. ELSO Anticoagulation Guideline. Available online: https://www.elso.org/ecmo-resources/elso-ecmo-guidelines.aspx (accessed on 29 September 2025).
- Nakamura, M.; Imamura, T.; Hida, Y.; Izumida, T.; Nakagaito, M.; Nagura, S.; Doi, T.; Kinugawa, K. Anticoagulation with nafamostat mesilate during impella support: A case report. Medicina 2025, 61, 309. [Google Scholar] [CrossRef]
- Sanfilippo, F.; Currò, J.M.; La Via, L.; Dezio, V.; Martucci, G.; Brancati, S.; Murabito, P.; Pappalardo, F.; Astuto, M. Use of nafamostat mesilate for anticoagulation during extracorporeal membrane oxygenation: A systematic review. Artif. Organs 2022, 46, 2371–2381. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Jang, J.H.; Kim, S.H.; Hong, S.Y.; Heo, W.; Lee, D.H.; Choi, H.S.; Kim, K.H.; Jang, H.J. The role of nafamostat mesilate as a regional anticoagulant during extracorporeal membrane oxygenation. Acute Crit. Care 2022, 37, 177–184. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, J.B.; Choo, S.J.; Chung, C.H.; Lee, J.W.; Jung, S.H. Anticoagulation during extracorporeal membrane oxygenation; nafamostat mesilate versus heparin. Ann. Thorac. Surg. 2016, 102, 534–539. [Google Scholar] [CrossRef]
- Jacobson, J. Nitric oxide: Platelet protectant properties during cardiopulmonary bypass/ECMO. J. Extra-Corpor. Technol. 2002, 34, 144–147. [Google Scholar] [CrossRef]
- Bartlett, R.; Arachichilage, D.J.; Chitlur, M.; Hui, S.R.; Neunert, C.; Doyle, A.; Retter, A.; Hunt, B.J.; Lim, H.S.; Saini, A.; et al. The History of Extracorporeal Membrane Oxygenation and the Development of Extracorporeal Membrane Oxygenation Anticoagulation. Semin. Thromb. Hemost. 2024, 50, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Obstals, F.; Vorobii, M.; Riedel, T.; de los Santos Pereira, A.; Bruns, M.; Singh, S.; Rodriguez-Emmenegger, C. Improving hemocompatibility of membranes for extracorporeal membrane oxygenators by grafting nonthrombogenic polymer brushes. Macromol. Biosci. 2018, 18, 1700359. [Google Scholar] [CrossRef]
- Zhang, M.; Pauls, J.P.; Bartnikowski, N.; Haymet, A.B.; Chan, C.H.H.; Suen, J.Y.; Schneider, B.; Ki, K.K.; Whittaker, A.K.; Dargusch, M.S.; et al. Anti-thrombogenic surface coatings for extracorporeal membrane oxygenation: A narrative review. ACS Biomater. Sci. Eng. 2021, 7, 4402–4419. [Google Scholar] [CrossRef]
- Larm, O.; Larsson, R.; Olsson, P. A new non-thrombogenic surface prepared by selective covalent binding of heparin via a modified reducing terminal residue. Biomater. Med. Devices Artif. Organs 1983, 11, 161–173. [Google Scholar] [CrossRef]
- Pappalardo, F.; Maj, G.; Scandroglio, A.; Sampietro, F.; Zangrillo, A.; Koster, A. Bioline heparin-coated ECMO with bivalirudin anticoagulation in a patient with acute heparin-induced thrombocytopenia: The immune reaction appeared to continue unabated. Perfusion 2009, 24, 135–137. [Google Scholar] [CrossRef]
- Steinlechner, B.; Kargl, G.; Schlömmer, C.; Holaubek, C.; Scheriau, G.; Eichinger, S.; Gratz, J.; Rössler, B. Can heparin-coated ECMO cannulas induce thrombocytopenia in COVID-19 patients? Case Rep. Immunol. 2021, 2021, 6624682. [Google Scholar] [CrossRef]
- Koster, A.; Loebe, M.; Sodian, R.; Potapov, E.V.; Hansen, R.; Müller, J.; Mertzlufft, F.; Crystal, G.J.; Kuppe, H.; Hetzer, R. Heparin antibodies and thromboembolism in heparin-coated and noncoated ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2001, 121, 331–335. [Google Scholar] [CrossRef]
- Laurance Lequier, G.A.; Omar Al-Ibrahim, M.B.; Dan Brodie, T.B.; Shannon Buckvold, L.C.; Steve Conrad, D.C.; Heidi Dalton, J.F.; Bill Harris, R.M.; Paden, M.; Natalie Rintoul, L.R.; Phil Spinella, J.T.; et al. ELSO Anticoagulation Guideline; ELSO: Ann Arbor, MI, USA, 2014. [Google Scholar]
- M’Pembele, R.; Roth, S.; Metzger, A.; Nucaro, A.; Stroda, A.; Polzin, A.; Hollmann, M.W.; Lurati Buse, G.; Huhn, R. Evaluation of clinical outcomes in patients treated with heparin or direct thrombin inhibitors during extracorporeal membrane oxygenation: A systematic review and meta-analysis. Thromb. J. 2022, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.R.; Murphree, C.R.; Zonies, D.; Meyer, A.D.; McCarty, O.J.T.; Deloughery, T.G.; Shatzel, J.J. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: A systematic review. ASAIO J. 2021, 67, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Fina, D.; Matteucci, M.; Jiritano, F.; Meani, P.; Kowalewski, M.; Ballotta, A.; Ranucci, M.; Lorusso, R. Extracorporeal membrane oxygenation without systemic anticoagulation: A case-series in challenging conditions. J. Thorac. Dis. 2020, 12, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- De Paulis, S.; Cavaliere, F. Anticoagulation management in high bleeding-risk ECMO in adults. Front. Cardiovasc. Med. 2022, 9, 884063. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, C.; Walter, J.M.; Karim, A.; Thakkar, S.; Saine, M.; Odell, D.D.; Kim, S.; Tomic, R.; Wunderink, R.G.; Budinger, G.R.S.; et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann. Thorac. Surg. 2020, 110, 1209–1215. [Google Scholar] [CrossRef] [PubMed]

| Anticoagulation Agent | Inhibition Site | Monitoring | Onset/Half-Life | Usual Dose | Elimination Routes |
|---|---|---|---|---|---|
| Unfractionated Heparin | Factor Xa and thrombin inhibition, predominantly inactivating thrombin | Anti-FXa, ACT, aPTT, TT | Half-life: 60–90 min | Bolus 50–100 IU/kg, continious infusion 10.4–21.3 IU/kg/h to achieve anticoagulation targets aPTT 40–80 s | Reticuloendothelial system and the kidneys |
| Nafamostat mesylate | Serine protease inhibitor | ACT, aPTT | 5–8 min | 1.0–1.7 mg/kg/hr | Metabolism in the liver and bloodstream, eliminated via the kidneys and intestines |
| Bivalirudin | Direct thrombin inhibitor | ACT, aPPT, PTT, TT, dTT, ECT, Viscoelastic methods, CAA | Half-life: 25 min/ Onset of action 2–4 min | A loading dose ranging from 0.2 to 0.75 mg/kg; maintenance infusion rates ranging from 0.05 to 0.15 mg/kg/h | Metabolism: proteolytic degeneration and partial renal excretion |
| Argatroban | Direct thrombin inhibitor | ACT, aPTT, drug concentartion, TT, dTT, ECT, Viscoelastic methods, CAA | Half-life: 45 min/Onset of action 30 min | The initial infusion rate 0.2–2 mcg/kg/min; Maintenance dose 0.1–0.2 mcg/kg/min | Metabolism: Liver-dependent |
| Low-molecular-weight heparin | Factor Ila and Xa inhibition, predominantly inactivating factor Xa | Anti-FXa, aPTT | Half-life: 3–6 h | Enoxaparin, a bolus dose IV 0.5 mg/kg before ECMO cannulation, followed by continuous administration, with anti-Xa target levels of 0.4–0.6 IU/mL | Renal |
| Lepirudin | Direct thrombin inhibitor | ACT, aPTT, ECT | Half-life: 1–2 h | Bolus of 0.4 mg/kg followed by 0.15 mg/kg/h | Renal |
| Fondaparinux | Direct-Xa inhibitor | Anti-FXa | Half-life: 13–21 h | 1 × 2.5 mg/day | Renal |
| Danaparoid | Factor Xa and IIa inhibition | Anti-FXa | Half-life: 25 h | 400 IU/h for 4 h, then 300 IU/h (0.5–0.8 U/mL anti-Xa factor activity goal) | Renal |
| Agent | Binding Mode | Sites of Binding | Notes |
|---|---|---|---|
| Argatroban | Univalent DTI | Active (catalytic) site only | Simple active-site blockade |
| Bivalirudin | Bivalent DTI | Active site + Exosite 1 (fibrinogen-binding) | Initial dual-site blockade, then cleavage at active site, partial binding remains at exosite 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unic-Stojanovic, D.; Vukovic, P.; Ilic, I.; Stojicic, M.M.; Tanaskovic, S.; Kangrga, N.; Rajsic, S. Alternative Anticoagulation for Patients with Heparin-Induced Thrombocytopenia on ECMO: A Narrative Review. Biomedicines 2025, 13, 2705. https://doi.org/10.3390/biomedicines13112705
Unic-Stojanovic D, Vukovic P, Ilic I, Stojicic MM, Tanaskovic S, Kangrga N, Rajsic S. Alternative Anticoagulation for Patients with Heparin-Induced Thrombocytopenia on ECMO: A Narrative Review. Biomedicines. 2025; 13(11):2705. https://doi.org/10.3390/biomedicines13112705
Chicago/Turabian StyleUnic-Stojanovic, Dragana, Petar Vukovic, Ivan Ilic, Milica Miljkovic Stojicic, Slobodan Tanaskovic, Nikolina Kangrga, and Sasa Rajsic. 2025. "Alternative Anticoagulation for Patients with Heparin-Induced Thrombocytopenia on ECMO: A Narrative Review" Biomedicines 13, no. 11: 2705. https://doi.org/10.3390/biomedicines13112705
APA StyleUnic-Stojanovic, D., Vukovic, P., Ilic, I., Stojicic, M. M., Tanaskovic, S., Kangrga, N., & Rajsic, S. (2025). Alternative Anticoagulation for Patients with Heparin-Induced Thrombocytopenia on ECMO: A Narrative Review. Biomedicines, 13(11), 2705. https://doi.org/10.3390/biomedicines13112705






