Under a Spell: Neurologic Evaluation of Presyncope as a Feature of Dysautonomia
Abstract
1. Introduction
2. Illustrative Case
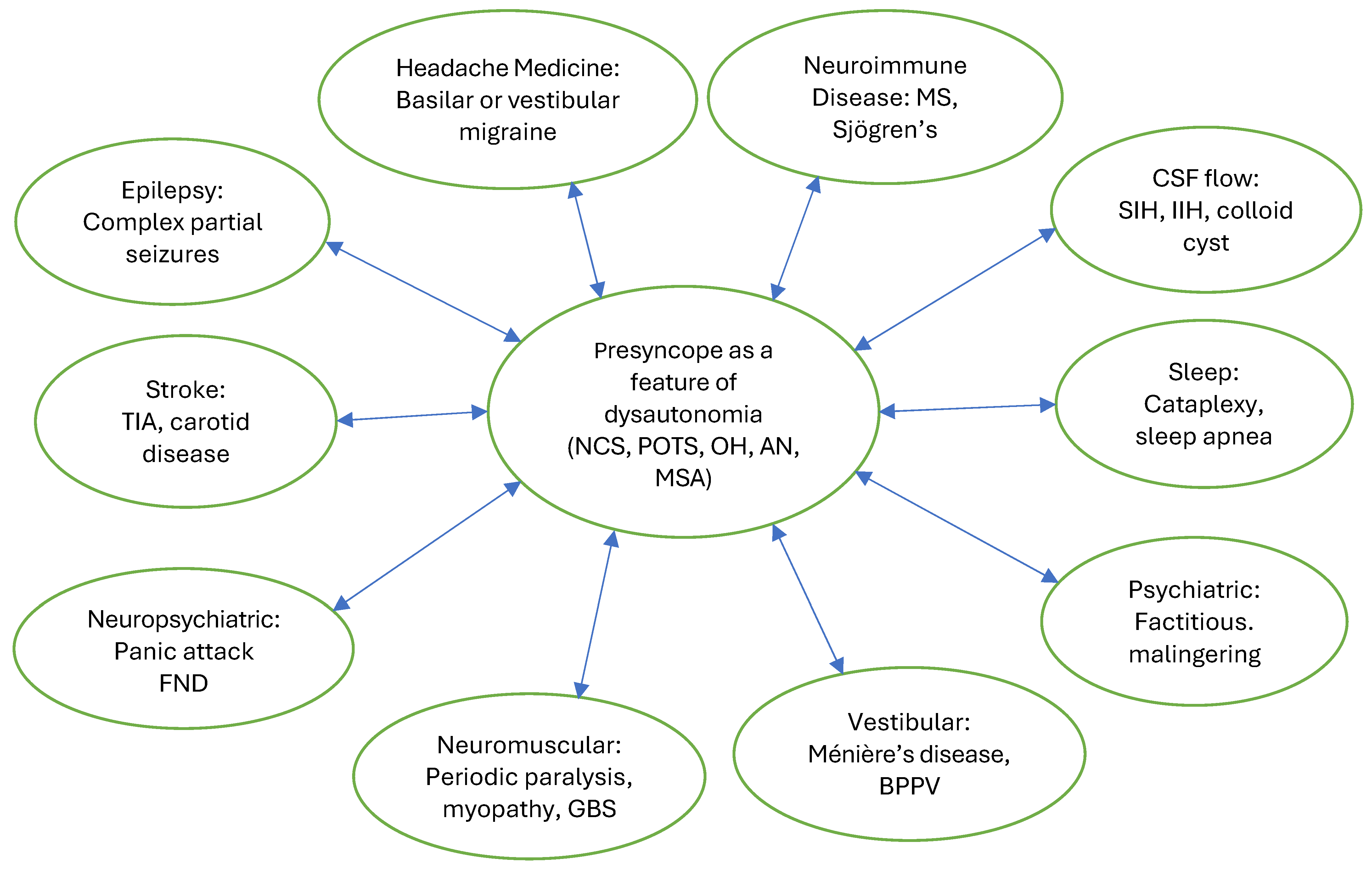
3. Presyncope and Autonomic Dysfunction
4. Neurologic Evaluation
4.1. History
4.2. Examination
- The patient lies down quietly for 5 min; blood pressure and heart rate are obtained at the upper arm using a sphygmomanometer.
- The patient stands up to assume an upright position without moving or talking; blood pressure and heart rate are obtained at 3, 5, 7 and 10 min of standing.
- Patient-reported symptoms are recorded throughout the test.
- Caution should be exercised for highly symptomatic patients who are unable to safely stand for 10 min due to orthostatic intolerance or neuromuscular disorders with impaired mobility.
- The test can be aborted earlier than 10 min if the patient is highly symptomatic and at risk for loss of consciousness or falling.
4.3. Diagnostic Tests
4.4. Etiology
4.5. Treatment
5. Considerations in Psychiatry, Psychology and Physician–Patient Communication
6. Future Directions
7. Key Points
- Unlike syncope where loss of consciousness leads to an established diagnostic and therapeutic approach, presyncope is harder to recognize and is less studied. Because there is no loss of consciousness, its prevalence and incidence in the general population or patients presenting to neurology clinics are unknown; the scope of presyncope symptoms and signs is broad, presenting a diagnostic challenge.
- New-onset presyncope may warrant an urgent diagnostic workup in the emergency department to rule out medical emergencies, including cardiac, pulmonary, neurologic and metabolic disorders.
- After medical emergencies have been ruled out, recurrent presyncope has a broad differential neurologic diagnosis: neurologists should consider presyncope as a feature of dysautonomia after cardiac arrhythmia, other structural cardiac abnormalities and complex partial seizures have been ruled out.
- Detailed history may include orthostatic dizziness, fatigue and exercise intolerance in addition to presyncope triggered by standing, prolonged sitting, mild exertion, heavy meal, heat, dehydration or menstrual symptoms.
- Neurologic exam with a 10 min stand test should be performed at the initial neurologic evaluation to avoid misdiagnosis and diagnostic delay and optimize appropriate therapies for autonomic dysfunction and treatment outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitledge, J.D.; Ali, N.; Basit, H.; Grossman, S.A. Presyncope [Updated 2023 Jul 17]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK459383/ (accessed on 18 September 2025).
- Blitshteyn, S.; Treisman, G.J.; Ruhoy, I.S.; Saperstein, D.S.; Schofield, J.R.; Goodman, B.P.; Davenport, T.E.; Cutchins, A.C.; Grubb, B.P. Postural orthostatic tachycardia syndrome and other common autonomic disorders are not functional neurologic disorders. Front. Neurol. 2024, 15, 1490744. [Google Scholar] [CrossRef]
- Benditt, D.G.; Fedorowski, A.; Sutton, R.; van Dijk, J.G. Pathophysiology of syncope: Current concepts and their development. Physiol. Rev. 2025, 105, 209–266. [Google Scholar] [CrossRef]
- Ganzeboom, K.S.; Mairuhu, G.; Reitsma, J.B.; Linzer, M.; Wieling, W.; van Dijk, N. Lifetime cumulative incidence of syncope in the general population: A study of 549 Dutch subjects aged 35–60 years. J. Cardiovasc. Electrophysiol. 2006, 17, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Shen, W.K.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Prevalence of syncope in a population aged more than 45 years. Am. J. Med. 2006, 119, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, W.N. Evaluation and outcome of patients with syncope. Medicine 1990, 69, 160–175. [Google Scholar] [CrossRef]
- Dulal, D.; Maraey, A.; Elsharnoby, H.; Chacko, P.; Grubb, B. Impact of COVID-19 pandemic on the incidence and prevalence of postural orthostatic tachycardia syndrome. Eur. Heart J. Qual. Care Clin. Outcomes 2025, 11, 698–704. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Grubb, B.P., II; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef]
- Nwazue, V.C.; Raj, S.R. Confounders of vasovagal syncope: Postural tachycardia syndrome. Cardiol. Clin. 2013, 31, 101–109. [Google Scholar] [CrossRef]
- Atkins, D.; Hanusa, B.; Sefcik, T.; Kapoor, W. Syncope and orthostatic hypotension. Am. J. Med. 1991, 91, 179–185. [Google Scholar] [CrossRef]
- Ahmed, A.; Pothineni, N.V.K.; Charate, R.; Garg, J.; Elbey, M.; de Asmundis, C.; LaMeir, M.; Romeya, A.; Shivamurthy, P.; Olshansky, B.; et al. Inappropriate Sinus Tachycardia: Etiology, Pathophysiology, and Management: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Ramwell, C.B.; Gordish-Dressman, H.; Sule, S.D.; Bettini, E. Small fiber neuropathy in children, adolescents, and young adults with chronic orthostatic intolerance and postural orthostatic tachycardia syndrome: A retrospective study. Auton. Neurosci. 2024, 253, 103163. [Google Scholar] [CrossRef]
- Oaklander, A.L. Increasing associations of long-COVID with small-fiber neuropathy. Pain 2024, 165, e93–e95. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S. Dysautonomia: A common comorbidity of systemic disease. Immunol. Res. 2025, 73, 105. [Google Scholar] [CrossRef]
- Cheshire, W.P., Jr.; Goldstein, D.S. The physical examination as a window into autonomic disorders. Clin. Auton. Res. 2018, 28, 23–33. [Google Scholar] [CrossRef]
- Bačkorová, B.; Lazúrová, Z.; Lewaskiewicz, P.; Mitro, P.; Lazúrová, I. Increased adrenocortical activity in patients with vasovagal syncope. Auton. Neurosci. 2024, 254, 103196. [Google Scholar] [CrossRef]
- Lovelace, J.W.; Ma, J.; Yadav, S.; Chhabria, K.; Shen, H.; Pang, Z.; Qi, T.; Sehgal, R.; Zhang, Y.; Bali, T.; et al. Vagal sensory neurons mediate the Bezold–Jarisch reflex and induce syncope. Nature 2023, 623, 387–396. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whiteson, J.; Abramoff, B.; Azola, A.; Bartels, M.N.; Bhavaraju-Sanka, R.; Chung, T.; Fleming, T.K.; Henning, E.; Miglis, M.G.; et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R 2022, 14, 1270–1291. [Google Scholar] [CrossRef]
- Bayard, M.; Gerayli, F.; Holt, J. Syncope: Evaluation and Differential Diagnosis. Am. Fam. Physician 2023, 108, 454–463. [Google Scholar] [PubMed]
- Young, W.; Maddox, D.E. Spells: In Search of a Cause. Mayo Clin. Proc. 1995, 70, 757–765. [Google Scholar] [CrossRef]
- Peltier, A.C.; Garland, A.; Raj, S.R. Distal sudomotor findings in postural tachycardia syndrome. Clin. Auton. Res. 2010, 20, 93–99. [Google Scholar] [CrossRef]
- Goodman, B.P. Evaluation of postural tachycardia syndrome (POTS). Auton. Neurosci. 2018, 215, 12–19. [Google Scholar] [CrossRef]
- Kurklinsky, A.K.; Miller, V.M.; Rooke, T.W. Acrocyanosis: The Flying Dutchman. Vasc. Med. 2011, 16, 288–301, Erratum in Vasc. Med. 2011, 16, 409. [Google Scholar] [CrossRef]
- Blitshteyn, S. Dysautonomia, Hypermobility Spectrum Disorders and Mast Cell Activation Syndrome as Migraine Comorbidities. Curr. Neurol. Neurosci. Rep. 2023, 23, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Twahir, A.; Kempuraj, D. Mast cells in the autonomic nervous system and potential role in disorders with dysautonomia and neuroinflammation. Ann. Allergy Asthma Immunol. 2024, 132, 440–454. [Google Scholar] [CrossRef]
- Khan, M.; Gardezi, S.A.; Nangia, V.; Jahangir, A.; Tajik, A.J. Giant colloid cyst of the brain masquerading as vasovagal syncope. HeartRhythm Case Rep. 2019, 5, 314–316. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Bower, J.H. Evaluation of the Patient With Paroxysmal Spells Mimicking Epileptic Seizures. Neurologist 2023, 28, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Krahn, L.E. Reevaluating spells initially identified as cataplexy. Sleep Med. 2005, 6, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Legramante, J.M.; Rizza, S.; Federici, M. Vasovagal syncope: An overview of pathophysiological mechanisms. Eur. J. Intern. Med. 2023, 112, 6–14. [Google Scholar] [CrossRef]
- Bourne, K.M.; Sheldon, R.S.; Exner, D.V.; Runte, M.; Raj, S.R. One Size Does Not Fit All: An Exploration of Compression Garment Use in Patients With Postural Orthostatic Tachycardia Syndrome. CJC Open 2024, 6, 1324–1333. [Google Scholar] [CrossRef]
- Ballantyne, B.A.; Letourneau-Shesaf, S.; Raj, S.R. Management of vasovagal syncope. Auton. Neurosci. 2021, 236, 102904. [Google Scholar] [CrossRef]
- Trimble, K.Z.; Switzer, J.N.; Blitshteyn, S. Exercise in Postural Orthostatic Tachycardia Syndrome: Focus on Individualized Exercise Approach. J. Clin. Med. 2024, 13, 6747. [Google Scholar] [CrossRef]
- Zha, K.; Brook, J.; McLaughlin, A.; Blitshteyn, S. Gluten-free diet in postural orthostatic tachycardia syndrome (POTS). Chronic Illn. 2023, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Breier, N.C.; Paranjape, S.Y.; Scudder, S.; Mehr, S.E.; Diedrich, A.; Flynn, C.R.; Okamoto, L.E.; Hartmann, B.; Gasbjerg, L.S.; Shibao, C.A. Worsening Postural Tachycardia Syndrome Is Associated With Increased Glucose-Dependent Insulinotropic Polypeptide Secretion. Hypertension 2022, 79, e89–e99. [Google Scholar] [CrossRef]
- Ruzieh, M.; Baugh, A.; Dasa, O.; Parker, R.L.; Perrault, J.T.; Renno, A.; Karabin, B.L.; Grubb, B. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J. Interv. Card. Electrophysiol. 2017, 48, 255–260. [Google Scholar] [CrossRef]
- Shaw, B.H.; Stiles, L.E.; Bourne, K.; Green, E.A.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Diedrich, A.; Raj, V.; et al. The face of postural tachycardia syndrome—Insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019, 286, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S.; Funez-dePagnio, G.; Szombathy, A.; Hutchinson, M. Immunotherapies for postural orthostatic tachycardia syndrome, other common autonomic disorders, and Long COVID: Current state and future direction. Front. Cell. Infect. Microbiol. 2025, 15, 1647203. [Google Scholar] [CrossRef] [PubMed]
- Bourne, K.M.; Chew, D.S.; Stiles, L.E.; Shaw, B.H.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Peltier, A.; Diedrich, A.; et al. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. J. Intern. Med. 2021, 290, 203–212. [Google Scholar] [CrossRef]
- Smyth, N.J.; Blitshteyn, S. Language Matters: What Not to Say to Patients with Long COVID, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, and Other Complex Chronic Disorders. Int. J. Environ. Res. Public Health 2025, 22, 275. [Google Scholar] [CrossRef]
- Saklani, P.; Krahn, A.; Klein, G. Syncope. Circulation 2013, 127, 1330–1339. [Google Scholar] [CrossRef]
- Benrud-Larson, L.M.; Dewar, M.S.; Sandroni, P.; Rummans, T.A.; Haythornthwaite, J.A.; Low, P.A. Quality of life in patients with postural tachycardia syndrome. Mayo Clin. Proc. 2002, 77, 531–537. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.L.M.C.; Visser, F.C. Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients. Medicina 2022, 58, 98. [Google Scholar] [CrossRef] [PubMed]
- Alciati, A.; Shiffer, D.; Dipaola, F.; Barbic, F.; Furlan, R. Psychogenic Pseudosyncope: Clinical Features, Diagnosis and Management. J. Atr. Fibrillation 2020, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
| Subjective Symptoms and Description | Possible Objective Findings and Observations |
|---|---|
| Lightheadedness | Pallor or flushing |
| Dizziness | Slow gait |
| Near-fainting | Unsteady gait |
| About to fall over | Tremulousness |
| Imbalance | Perspiration |
| Unsteadiness | Rapid breathing |
| Weakness | Distressed appearance |
| Palpitations | Anxious appearance |
| Racing heart | Acrocyanosis |
| Shortness of breath | Blue lips |
| Headache | Tachycardia |
| Tunnel vision | Bradycardia |
| Blackout vision | Faint pulse |
| Difficulty concentrating | Hypotension |
| Dreamlike state | Hypertension |
| Derealization and depersonalization | Narrow pulse pressure |
| Spaced out feeling | Give-way weakness |
| Disconnected feeling | Fine postural tremor |
| Nausea | Hyperreflexia |
| Dyspnea or hyperventilation | Hypotonia |
| Numbness or tingling | Hypertonia |
| Heavy or cement legs | Shivering |
| Feeling like the legs cannot move | Piloerection |
| Sleepiness | Dilated pupils |
| Orthostatic vital signs |
| A 10 min stand test |
| Pulse oximetry |
| Neurologic exam |
| Neurologic tests: MRI of the brain, sleep-deprived EEG with hyperventilation, tilt table test and other autonomic function tests if available, polysomnography with mean sleep latency test |
| Cardiac tests: ECG, cardiac echo, 48-Holter monitor, 30-day cardiac event monitor |
| Possible other diagnostic tests: EMG, skin biopsy for small fiber neuropathy, MRA of the head and neck, MRV, exercise stress test, implantable loop recorder, cardiac MRI, supine and standing transcranial Dopplers, minor salivary gland biopsy to rule out Sjögren’s disease, audiogram to rule out Ménière’s disease |
| Labs: CBC, CMP, TFT, ferritin, morning cortisol, vitamin B12, methymalonic acid, homocysteine, vitamin B6, immunofixation serum and urine, CRP, ESR, RF, ANA |
| Possible other labs: Sjögren’s antibodies, thyroid antibodies, celiac panel, anti-parietal antibodies, ganglionic AchR antibodies, voltage-gated calcium and potassium channel antibodies, supine and standing serum catecholamines, plasma metanephrines and urine fractionated metanephrines, 24 h urine 5-HIAA and porphobilinogens, serum tryptase and histamine, LH, FSH, testosterone, estrogen, insulin, C-peptide, serum and urine mitochondrial biomarkers |
| Neurologic: |
| Seizure |
| Transient ischemic attack |
| Migraine with aura |
| Basilar migraine |
| Vestibular migraine |
| Transient migraine accompaniments |
| Cataplexy |
| Spontaneous intracranial hypotension |
| Idiopathic intracranial hypertension |
| Cranio-cervical instability |
| Hypokalemic periodic paralysis |
| Vestibular dysfunction |
| Meniere’s disease |
| Pain-induced |
| Cardiopulmonary: |
| Cardiac arrhythmia |
| Cardiac conduction disorder |
| Coronary arterial disease |
| Cardiomyopathy |
| Cardiac valvular disease |
| Pulmonary embolism |
| Pulmonary hypertension |
| Hyperventilation |
| Endocrine: |
| Hypoglycemia |
| Thyrotoxicosis |
| Pheochromocytoma |
| Adrenal insufficiency |
| Menopausal hot flashes |
| Pregnancy |
| Testosterone deficiency |
| General medical: |
| Medication side effect |
| Anemia |
| Dehydration |
| Overheating |
| Infection |
| Substance use |
| Allergic reaction |
| Labile hypertension |
| Orthostatic hypotension |
| Porphyria |
| Psychiatric: |
| Panic attack |
| Anxiety |
| PTSD |
| Functional Neurologic Disorder |
| Factitious disorder |
| Malingering |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blitshteyn, S.; Chémali, K.R.; Lau, D.H. Under a Spell: Neurologic Evaluation of Presyncope as a Feature of Dysautonomia. Biomedicines 2025, 13, 2698. https://doi.org/10.3390/biomedicines13112698
Blitshteyn S, Chémali KR, Lau DH. Under a Spell: Neurologic Evaluation of Presyncope as a Feature of Dysautonomia. Biomedicines. 2025; 13(11):2698. https://doi.org/10.3390/biomedicines13112698
Chicago/Turabian StyleBlitshteyn, Svetlana, Kamal R. Chémali, and Dennis H. Lau. 2025. "Under a Spell: Neurologic Evaluation of Presyncope as a Feature of Dysautonomia" Biomedicines 13, no. 11: 2698. https://doi.org/10.3390/biomedicines13112698
APA StyleBlitshteyn, S., Chémali, K. R., & Lau, D. H. (2025). Under a Spell: Neurologic Evaluation of Presyncope as a Feature of Dysautonomia. Biomedicines, 13(11), 2698. https://doi.org/10.3390/biomedicines13112698








