From Biomechanics to Bioinnovation: Emerging Applications of Piezoelectric Materials and Phenomena in Dentistry
Abstract
1. Introduction
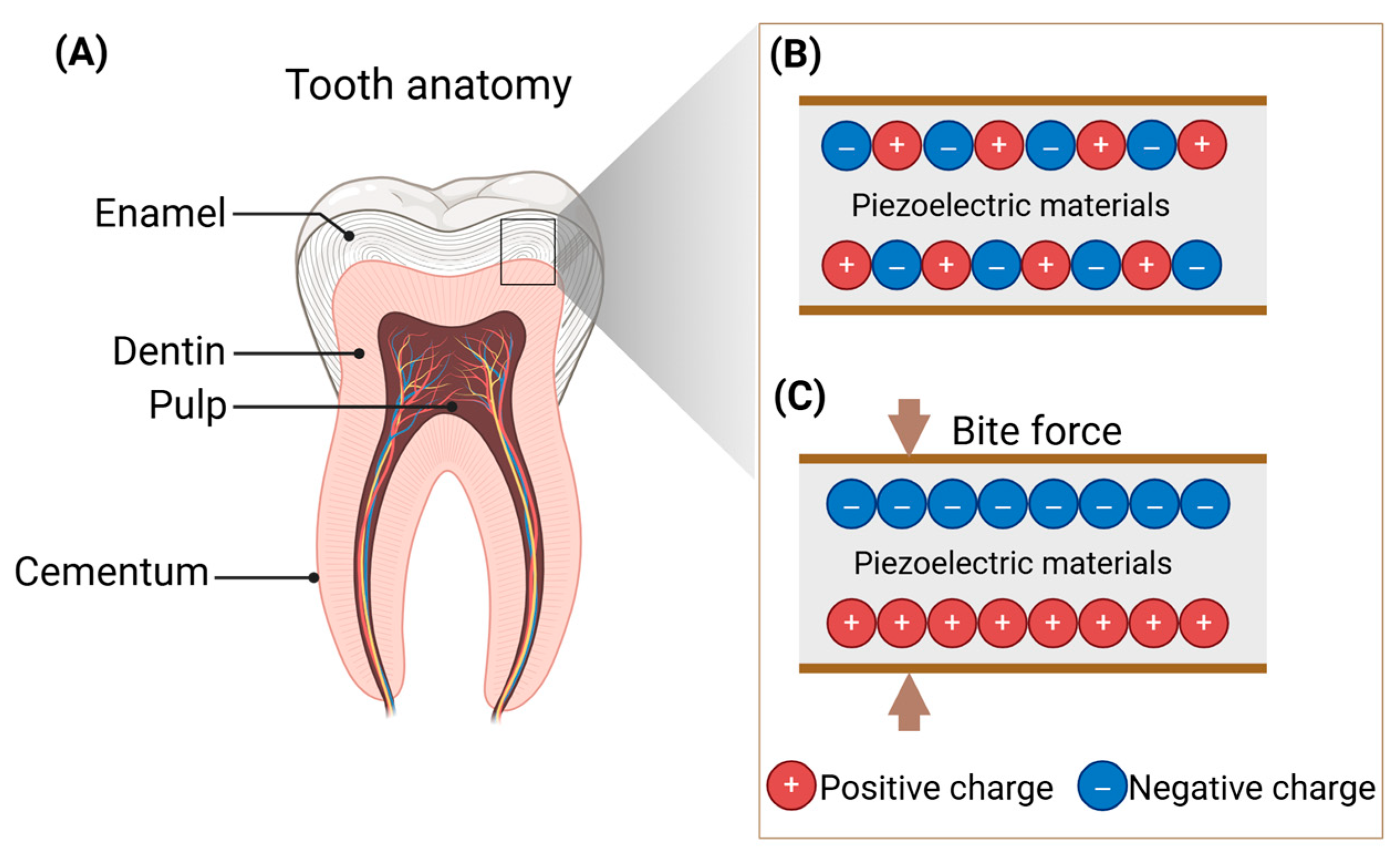
2. Piezoelectricity in Teeth
2.1. Fundamental Principles of the Piezoelectric Effect
2.2. The Piezoelectric Properties of Teeth
2.3. Role of Piezoelectricity in Teeth
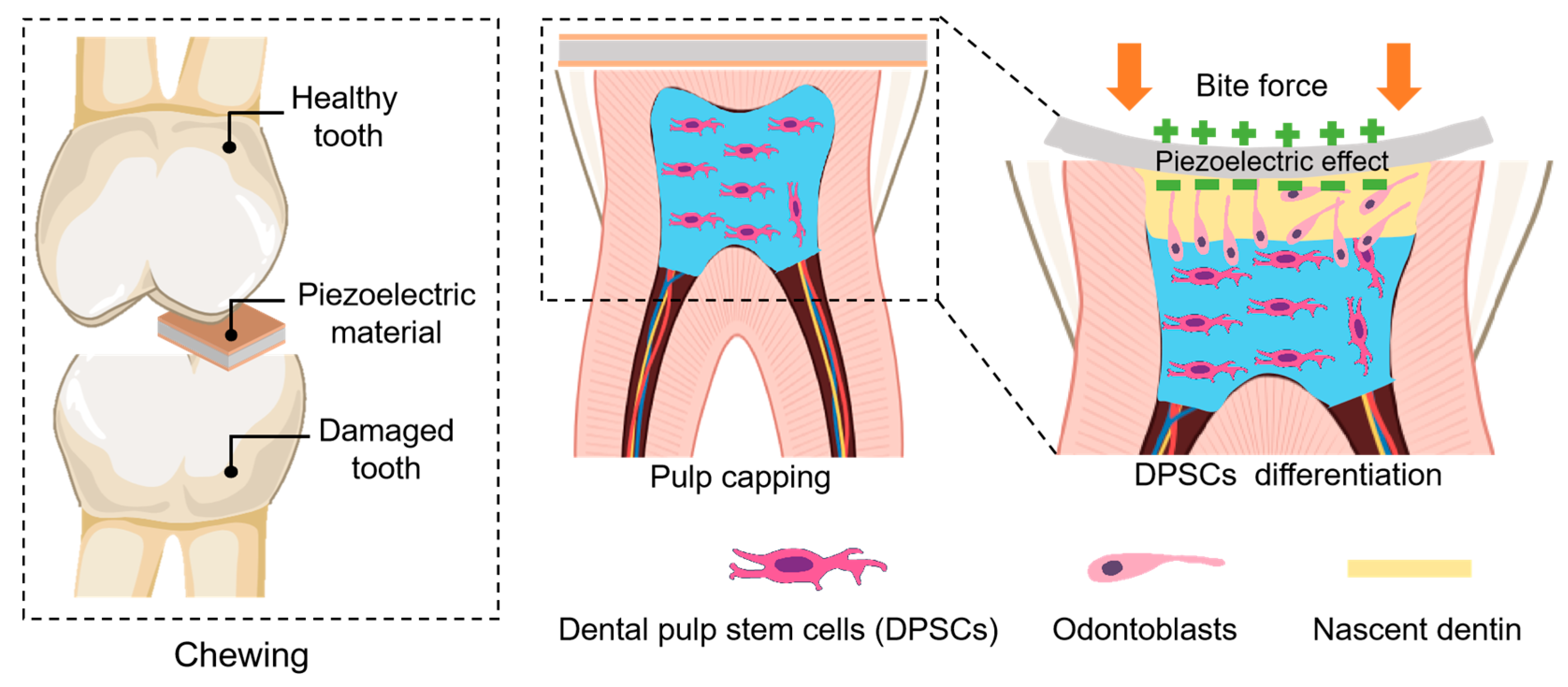
2.3.1. Effects on Cell Proliferation and Differentiation
2.3.2. Effects on Angiogenesis in Dental Pulp
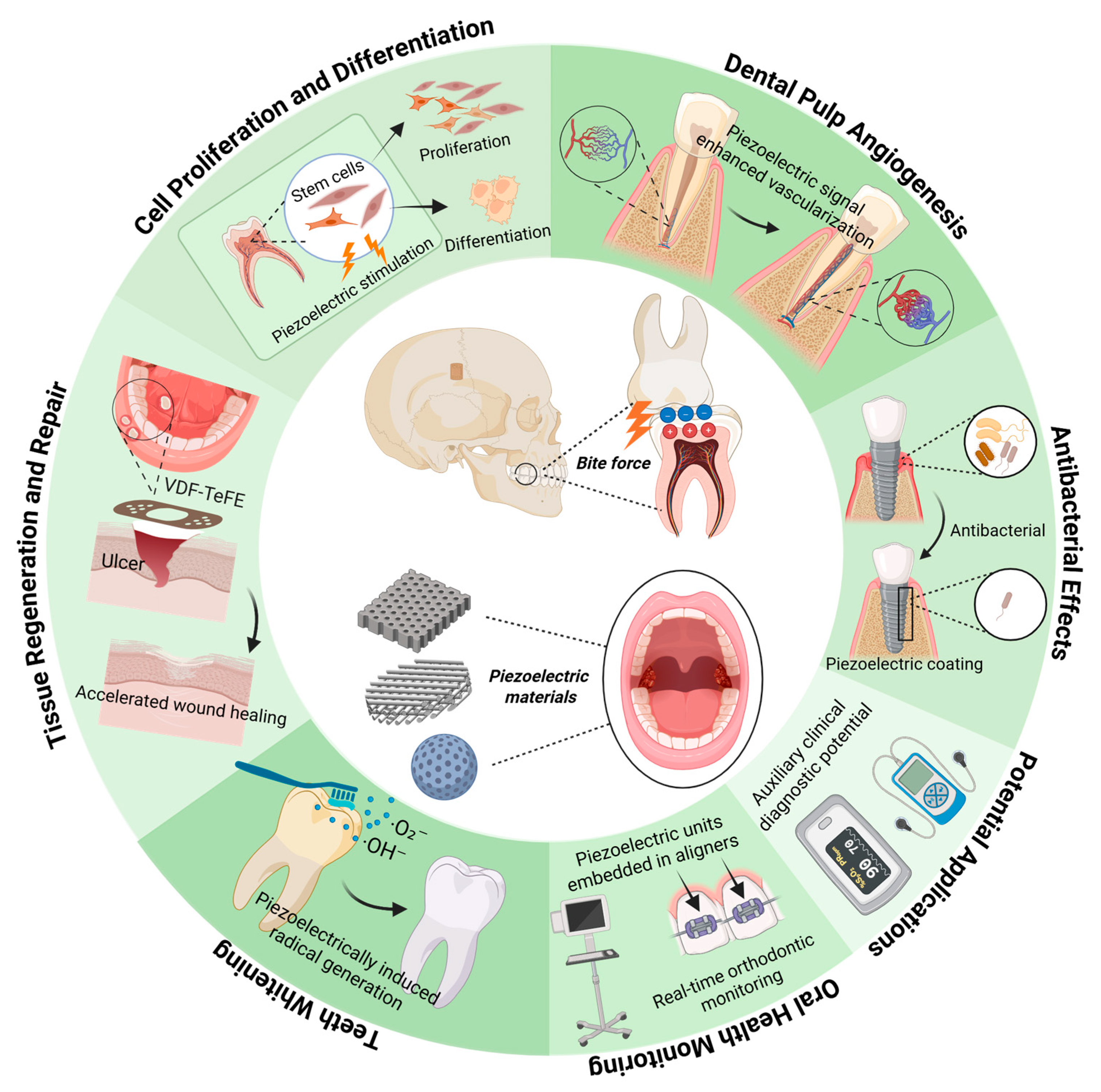
3. Application of Piezoelectric Materials in Dentistry
3.1. Tissue Regeneration and Repair
3.2. Antibacterial Effects
3.3. Teeth Whitening
3.4. Oral Health Monitoring
3.5. Potential for Diagnosis and Treatment of Oral Diseases
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERK | Extracellular signal-regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| cAMP | Cyclic adenosine monophosphate |
| PKA | Protein kinase A |
| VEGF | Vascular endothelial growth factor |
| FGF | Fibroblast growth factor |
| PI3K | Phosphatidylinositol 3-kinase |
| Akt | Protein kinase B |
| Runx2 | Runt-related transcription factor 2 |
| DSPP | Dentin sialophosphoprotein |
| DMP-1 | Dentin matrix protein 1 |
| ATP | Adenosine triphosphate |
| VDF-TeFE | Vinylidene fluoride and tetrafluoroethylene |
| PVDF | Polyvinylidene fluoride |
| ROS | Reactive oxygen species |
| Zn-MSNs | Zinc-doped mesoporous silica nanoparticles |
| K18-MMA | K18-methyl methacrylate |
| PiezoGEL | Piezoelectric hydrogel |
| GelMA | Gelatin methacryloyl |
| DA | Dopamine |
| PVA | Polyvinyl alcohol |
| PFM | Piezoresponse force microscopy |
| DPSCs | Dental pulp stem cells |
| SBF | Simulated Body Fluid |
References
- Wang, Y.; Liu, S.; Li, L.; Li, H.; Yin, Y.; Rencus-Lazar, S.; Guerin, S.; Ouyang, W.; Thompson, D.; Yang, R.; et al. Manipulating the piezoelectric response of amino acid-based assemblies by supramolecular engineering. J. Am. Chem. Soc. 2023, 145, 15331–15342. [Google Scholar] [CrossRef]
- Wang, Y.; Rencus-Lazar, S.; Zhou, H.; Yin, Y.; Jiang, X.; Cai, K.; Gazit, E.; Ji, W. Bioinspired amino acid based materials in bionanotechnology: From minimalistic building blocks and assembly mechanism to applications. ACS Nano 2023, 18, 1257–1288. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, Z.; Xu, J.; Li, J.; Yan, K.; Cheng, W.; Xin, M.; Zhu, T.; Du, J.; Chen, S.; et al. Versatile self-assembled electrospun micro-pyramid arrays for high-performance on-skin devices with minimal sensory interference. Nat. Commun. 2022, 13, 5839. [Google Scholar] [CrossRef]
- Xue, H.; Jin, J.; Huang, X.; Tan, Z.; Zeng, Y.; Lu, G.; Hu, X.; Chen, K.; Su, Y.; Hu, X.; et al. Wearable flexible ultrasound microneedle patch for cancer immunotherapy. Nat. Commun. 2025, 16, 2650. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lyu, H.; Jiang, H.; Wang, Y.; Yang, R.; Tofail, S.A.M.; Xu, H.; Guo, C.; Mei, D.; Gazit, E.; et al. Bioinspired supramolecular fibrillization enables stretchable and biodegradable piezoelectric bioelectronics. Sci. Adv. 2025, 11, eadu6759. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Gong, C.; Lu, G.; Wu, J.; Wan, X.; Yang, Y.; Ji, J.; Zhang, J.; Li, R.; Sun, Y.; et al. A programmable and self-adaptive ultrasonic wireless implant for personalized chronic pain management. Nat. Electron. 2025, 8, 437–449. [Google Scholar] [CrossRef]
- Li, W.; Mo, Y.; Xie, S.; Fu, L.; Ye, Y.; Zhou, Y.; Lai, J.; Wang, W.; Lu, Y.; Li, G.; et al. Self-reinforced piezoelectric chip for scaffold-free repair of critical-sized bone defects. Nat. Commun. 2025, 16, 5800. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.; Huo, Y.; Liu, S.; Yin, Y.; Tan, M.L.; Cai, K.; Ji, W. Piezoelectric biomaterials inspired by nature for applications in biomedicine and nanotechnology. Adv. Mater. 2024, 36, e2406192. [Google Scholar] [CrossRef] [PubMed]
- Balic, A. Biology explaining tooth repair and regeneration: A mini-review. Gerontology 2018, 64, 382–388. [Google Scholar] [CrossRef]
- Shoji, Y.; Yusof, M.; Idris, R.I.B.; Mitrirattanakul, S. Bite force of patients with tooth pain. Clin. Exp. Dent. Res. 2022, 8, 1213–1217. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Shekarian, M.; Samadi, F.; Briss, D.S.; Napoli, S.; Conte, M. The impact of ph on the piezoelectric properties of dentin in root canal treated teeth: Implications for dental materials and oral health. J. Endod. 2025, 51, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, S.; Wang, Y.; Yang, S.; Ding, M.; Xu, X.; Ji, W.; Song, J. Recycling of human teeth for piezoelectric energy harvesting. Giant 2024, 20, 100333. [Google Scholar] [CrossRef]
- Ouyang, X.; Yao, M.; Yang, T. Comparative investigation and mechanistic analysis of textured PMN-30PT ceramics using BaTiO3 and PbTiO3 templates. Dalton Trans. 2025, 54, 13672–13688. [Google Scholar] [CrossRef]
- Esmaeili, S.; Ehsani, M.H.; Toghraie, D.; Saber-Samandari, S. Investigation of the effect of crystal size on the piezoelectric features of lead-free barium titanate ceramic using molecular dynamics simulation. Sci. Rep. 2024, 14, 28353. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, X.; Zheng, M.; Gao, X.; Zhao, K.; Li, L.; Zhu, M.; Hou, Y. Boosting transduction coefficient in BaTiO3-based piezoceramic through phase boundary engineering. J. Eur. Ceram. Soc. 2022, 42, 2180–2187. [Google Scholar] [CrossRef]
- Lay, R.; Deijs, G.S.; Malmström, J. The intrinsic piezoelectric properties of materials—A review with a focus on biological materials. RSC Adv. 2021, 11, 30657–30673. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, Z.; Wang, J.; Chang, C.; Xu, B.; Chen, H.; Zheng, Y. Occlusion-activated piezoelectric synergistic antibacterial clear aligner coating. Mater. Today Commun. 2025, 46, 112860. [Google Scholar] [CrossRef]
- Aoki, R.; Takaba, M.; Abe, Y.; Nakazato, Y.; Ohara, H.; Maejima, K.; Baba, K. A pilot study to test the validity of a piezoelectric intra-splint force detector for monitoring of sleep bruxism in comparison to portable polysomnography. J. Oral Sci. 2022, 64, 63–68. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Yao, G.; Lou, W.; Zhang, T.; Zhang, Z.; Xie, M.; Gan, X.; Pan, T.; Gao, M.; et al. Effective orthodontic tooth movement via an occlusion-activated electromechanical synergistic dental aligner. ACS Nano 2023, 17, 16757–16769. [Google Scholar] [CrossRef]
- Subramanian, A.K.; Thayalan, D.; Edwards, A.I.; Almalki, A.; Venugopal, A. Biomedical waste management in dental practice and its significant environmental impact: A perspective. Environ. Technol. Inno. 2021, 24, 101807. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Z.; Zhang, Y.; Zhang, X.; Huang, X.; Xie, Z.; Chen, Z. KLF6-mediated glutamine metabolism governs odontogenic differentiation and matrix mineralization of dental pulp stem cells. Stem Cell Res. Ther. 2025, 16, 179. [Google Scholar] [CrossRef]
- Jiang, Z.; Tan, X.; Huang, Y. Piezoelectric effect enhanced photocatalysis in environmental remediation: State-of-the-art techniques and future scenarios. Sci. Total Environ. 2022, 806, 150924. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Tang, Y.-Y.; Gu, Z.-X.; Wang, P.; Chen, X.-G.; Lv, H.-P.; Li, P.-F.; Jiang, Q.; Gu, N.; Ren, S.; et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science 2024, 383, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Field, E.H.; Wang, Y.; Han, K.; Kumar, R.; Shimon, L.J.W.; Liu, S.; Guerin, S.; Berryman, J.T.; Ji, W.; et al. Fluorination induced inversion of helicity and self-assembly into cross-α like piezoelectric amyloids by minimalistic designer peptide. Small 2025, 21, 2500288. [Google Scholar] [CrossRef]
- Su, X.; Liu, S.; Gong, X.; Tong, X.; Li, L.; Huo, Y.; Liu, Q.; Wang, Y.; Tan, M.-L.; Li, Q.; et al. Regulating the piezoelectricity of cyclic dipeptide-based supramolecular materials through co-assembly strategy. J. Am. Chem. Soc. 2025, 147, 16255–16269. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yan, C.-F.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B Environ. Energy 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Jin, H.; Gao, X.; Ren, K.; Liu, J.; Qiao, L.; Liu, M.; Chen, W.; He, Y.; Dong, S.; Xu, Z.; et al. Review on piezoelectric actuators based on high-performance piezoelectric materials. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 3057–3069. [Google Scholar] [CrossRef]
- Ali, M.; Bathaei, M.J.; Istif, E.; Karimi, S.N.H.; Beker, L. Biodegradable piezoelectric polymers: Recent advancements in materials and applications. Adv. Healthc. Mater. 2023, 12, e2300318. [Google Scholar] [CrossRef]
- Qian, W.; Yang, W.; Zhang, Y.; Bowen, C.R.; Yang, Y. Piezoelectric materials for controlling electro-chemical processes. Nano-Micro Lett. 2020, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Máthé, M.T.; Salamon, P.; Gleeson, J.T.; Jákli, A. From solid to liquid piezoelectric materials. Mater. Horiz. 2025, 12, 8920–8942. [Google Scholar] [CrossRef]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Braden, M.; Bairstow, A.G.; Beider, I.; Ritter, B.G. Electrical and piezo-electrical properties of dental hard tissues. Nature 1966, 212, 1565–1566. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Bidlack, F.B. Tooth enamel and its dynamic protein matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized dentin matrix for dental and alveolar bone tissues regeneration: An innovative scope review. Tissue Eng. Regen. Med. 2022, 19, 687–701, Erratum in Tissue Eng. Regen. Med. 2022, 19, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.A.; Gross, B.D. Piezoelectricity in cementum, dentine and bone. Arch. Oral Biol. 1989, 34, 507–509. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomedicine 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Rodriguez, B.J.; Jesse, S.; Thundat, T.; Gruverman, A. Electromechanical imaging of biological systems with sub-10nm resolution. Appl. Phys. Lett. 2005, 87, 053901. [Google Scholar] [CrossRef]
- Reyes-Gasga, J.; Galindo-Mentle, M.; Brès, E.; Vargas-Becerril, N.; Orozco, E.; Rodríguez-Gómez, A.; García-García, R. Detection of the piezoelectricity effect in nanocrystals from human teeth. J. Phys. Chem. Solids 2020, 136, 109140. [Google Scholar] [CrossRef]
- Brès, E.F.; Reyes-Gasga, J.; Hemmerlé, J. Human tooth enamel, a sophisticated material. In Extracellular Matrix Biomineralization of Dental Tissue Structures; Goldberg, M., Den Besten, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Chapter 9; Volume 10, pp. 243–259. [Google Scholar] [CrossRef]
- Min, Q.; Gao, Y.; Wang, Y. Bioelectricity in dental medicine: A narrative review. Biomed. Eng. Online 2024, 23, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Zhang, Z.; Liu, H.; Li, L.; Wang, A.; Ouyang, J.; Xie, T.; Zhang, L.; Xue, J.; et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today 2023, 68, 177–203. [Google Scholar] [CrossRef]
- da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric phenomenon: A disregarded tool in tissue engineering and regenerative medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Gao, Q.; Cooper, P.R.; Walmsley, A.D.; Scheven, B.A. Role of piezo channels in ultrasound-stimulated dental stem cells. J. Endod. 2017, 43, 1130–1136. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z.; Liang, S.; Khan, Z.; Chen, Z.; Qian, A.; et al. Piezo channels: Awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar] [CrossRef]
- Han, H.M.; Jeong, S.Y.; Cho, Y.S.; Choi, S.Y.; Bae, Y.C. Expression of piezo2 in the dental pulp, sensory root, and trigeminal ganglion and its coexpression with vesicular glutamate transporters. J. Endod. 2022, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Naruse, K.; Ozawa, S.; Kobayashi, Y.; Nakamura, N.; Kojima, N.; Omi, M.; Katanosaka, Y.; Nishikawa, T.; Naruse, K.; et al. Mechanical stretch increases the proliferation while inhibiting the osteogenic differentiation in dental pulp stem cells. Tissue Eng. Part A 2013, 19, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Aghazadeh, Z.; Abdolahinia, E.D.; Vahdati, A.; Daneshvar, S.; Noghani, A. The effect of electromagnetic fields on survival and proliferation rate of dental pulp stem cells. Acta Odontol. Scand. 2020, 78, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhou, Z.; Zhang, M.; Yao, S.; Yuan, L.; Xu, M.; Zhang, X.; Hu, X. Electrical stimulations generated by P(VDF-TrFE) films enhance adhesion forces and odontogenic differentiation of dental pulp stem cells (DPSCs). ACS Appl. Mater. Interfaces 2024, 16, 28029–28040. [Google Scholar] [CrossRef]
- Zheng, J.; Zuo, J.; Xiao, C.; Guo, Q.; Fu, X.; Ning, C.; Yu, P. Wireless electrical stimulation generated by piezoelectric nanomaterial promotes the dental pulp regeneration via regulating mitochondrial Ca2+/PKA signaling pathway. J. Mater. Sci. Technol. 2024, 168, 24–34. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef]
- Nouri, S.; Roghanian, R.; Emtiazi, G.; Gunduz, O.; Shafiei, R. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth by probiotic hydroxyapatite. Cell J. 2023, 25, 753–763. [Google Scholar]
- Mirzaei, A.; Saburi, E.; Enderami, S.E.; Barati Bagherabad, M.; Enderami, S.E.; Chokami, M.; Shapouri Moghadam, A.; Salarinia, R.; Ardeshirylajimi, A.; Mansouri, V.; et al. Synergistic effects of polyaniline and pulsed electromagnetic field to stem cells osteogenic differentiation on polyvinylidene fluoride scaffold. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3058–3066. [Google Scholar] [CrossRef]
- Deng, X.; Zhuang, Y.; Cui, J.; Wang, L.; Zhan, H.; Wang, X.; Lin, K.; Yuan, C. Open challenges and opportunities in piezoelectricity for tissue regeneration. Adv. Sci. 2025, 12, e10349. [Google Scholar] [CrossRef]
- Wang, X.; Stefanello, S.T.; Shahin, V.; Qian, Y. From mechanoelectric conversion to tissue regeneration: Translational progress in piezoelectric materials. Adv. Mater. 2025, 37, 2417564. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhou, J.Y.; Chen, K.; Yu, X.; Dong, Z.Y.; Liu, Y.S.; Meng, X.T. Electrical stimulation induced pre-vascularization of engineered dental pulp tissue. Regen. Ther. 2024, 26, 354–365. [Google Scholar] [CrossRef]
- Di, T.; Feng, C.; Wang, L.; Xu, J.; Du, Y.; Cheng, B.; Chen, Y.; Wu, L. Enhancing vasculogenesis in dental pulp development: DPSCs-ECs communication via FN1-ITGA5 signaling. Stem Cell Rev. Rep. 2024, 20, 1060–1077. [Google Scholar] [CrossRef]
- He, L.; Zhang, W.; Shao, L.; Cui, P. Efficacy of concentrated growth factor-assisted pulpal revascularization for chronic apical periodontitis in young permanent teeth. Asian J. Surg. 2025, 48, 3087–3089. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, C.; Gao, X.; Huang, H.; Xing, X.; Tang, Q.; Yang, J.; Wu, Y.; Li, M.; Li, H.; et al. Adipose tissue-derived microvascular fragments as vascularization units for dental pulp regeneration. J. Endod. 2021, 47, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Andreas, C.; Grigorios, P.; Evangelos, E.; Christina, P. The pivotal role of transient receptor potential channels in oral physiology. Curr. Med. Chem. 2022, 29, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, C.; Suzuki, A.; Shim, J.; Ambrose, C.G.; Iwata, J. Autophagy plays a crucial role in ameloblast differentiation. J. Dent. Res. 2023, 102, 1047–1057. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Fu, J.; Xue, Y.; Chen, Z.; Yang, G.; Chen, Y.; Chen, D.; Yuan, G. CHIP inhibits odontoblast differentiation through promoting DLX3 polyubiquitylation and degradation. Development 2023, 150, dev200848. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Xia, Y.; Qi, X.; Jiang, C.; Xiao, Y.; Jiang, F.; Jiang, X.; Yuan, G. Strontium-containing piezoelectric biofilm promotes dentin tissue regeneration. Adv. Mater. 2024, 36, e2313419. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional dental composite with piezoelectric nanofillers for combined antibacterial and mineralization effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wan, X.; Sui, B.; Hu, Q.; Liu, Z.; Ding, T.; Zhao, J.; Chen, Y.; Wang, Z.L.; Li, L. Piezoelectric hydrogel for treatment of periodontitis through bioenergetic activation. Bioact. Mater. 2024, 35, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, F.; Li, Z.; Duan, J.; Kong, Y.; Hao, M.; Ge, S.; Jiang, H.; Liu, H. Piezoelectric nylon-11 nanoparticles with ultrasound assistance for high-efficiency promotion of stem cell osteogenic differentiation. J. Mater. Chem. B 2019, 7, 1847–1854. [Google Scholar] [CrossRef]
- Dai, J.; Shao, J.; Zhang, Y.; Hang, R.; Yao, X.; Bai, L.; Hang, R. Piezoelectric dressings for advanced wound healing. J. Mater. Chem. B 2024, 12, 1973–1990. [Google Scholar] [CrossRef]
- Chernova, U.V.; Varakuta, E.Y.; Koniaeva, A.D.; Leyman, A.E.; Sagdullaeva, S.A.; Plotnikov, E.; Melnik, E.Y.; Tran, T.H.; Rutkowski, S.; Kudryavtseva, V.L.; et al. Piezoelectric and dielectric electrospun fluoropolymer membranes for oral mucosa regeneration: A comparative study. ACS Appl. Mater. Interfaces 2024, 16, 20245–20259. [Google Scholar] [CrossRef]
- Konyaeva, A.D.; Varakuta, E.Y.; Leiman, A.E.; Bolbasov, E.N.; Chernova, U.V. The specifics of neovascularization of wound defects in the oral mucosa during its regeneration under a piezoelectric polymer membrane. Bull. Exp. Biol. Med. 2023, 174, 801–805. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Z.; Wang, X.; Guo, Y.; Xie, Y.; Yao, W.; Kawasaki, H. Piezoelectric nanomaterials for antibacterial strategies. Appl. Mater. Today 2024, 40, 102419. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Liu, Y.; Jia, J.; Li, Y.; Dai, X.; Liu, O. Piezoelectric materials for anti-infective bioapplications. J. Mater. Chem. B 2024, 12, 11063–11075. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.S.; Abd Rahman, N.A.; Ming, L.C.; Dhaliwal, S.K.S.; Knights, J.; Albuquerque Junior, R.F. Microbial biofilm decontamination on dental implant surfaces: A mini review. Front. Cell. Infect. Microbiol. 2021, 11, 736186. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, X.; Ma, K.; Chen, R.; Mao, Y.; Chao, R.; Wang, H.; Yu, B.; Wang, J.; Zhang, S. Novel titanium implant: A 3D multifunction architecture with charge-trapping and piezoelectric self-stimulation. Adv. Healthc. Mater. 2023, 12, e2202620. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Li, K.; Shen, L.; Liu, X.; Chen, Y.; Feng, J.; Wang, W.; Zhao, W.; Shao, J.; et al. Surface-confined piezocatalysis inspired by ROS generation of mitochondria respiratory chain for ultrasound-driven noninvasive elimination of implant infection. ACS Nano 2023, 17, 9415–9428. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Liu, Y.; Zhang, Z.; Bao, Z.; Zhang, X.; Liang, Z.; Luan, S.; Shi, H.; Ma, P. Respiration electron of pathogenic bacteria activated ZnO@Viologen-COF heterojunction to boost piezo-phototronic effect for microbiota regulation. Sci. Bull. 2025, in press. [CrossRef]
- Chen, J.; Wang, Y.; Zeng, D.; Li, Z.; Pei, H.; Cao, X.; Wang, Y.; Dong, Z.; Zhang, Z.; Liu, Y. Piezoelectric effect-mediating reactive oxygen species in NiTiO3 nanorods for photocatalytic removal of U(VI). Inorg. Chem. 2024, 63, 16233–16242. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Dong, S.; Wang, P.; Chen, W.; Lu, Z.; Ye, D.; Pan, B.; Wu, D.; Vecitis, C.D.; et al. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat. Commun. 2021, 12, 3508. [Google Scholar] [CrossRef]
- Mokhtari, F.; Azimi, B.; Salehi, M.; Hashemikia, S.; Danti, S. Recent advances of polymer-based piezoelectric composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 122, 104669. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Childs, T.; Chu, L.; Barrera, L.; Ballard, C.; Fung, E.; Whang, K. Antimicrobial dental composites with K18-methyl methacrylate and K18-filler. Dent. Mater. 2024, 40, 59–65. [Google Scholar] [CrossRef]
- Taudte, N.; Linnert, M.; Rahfeld, J.U.; Piechotta, A.; Ramsbeck, D.; Buchholz, M.; Kolenko, P.; Parthier, C.; Houston, J.A.; Veillard, F.; et al. Mammalian-like type II glutaminyl cyclases in porphyromonas gingivalis and other oral pathogenic bacteria as targets for treatment of periodontitis. J. Biol. Chem. 2021, 296, 100263. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The roles of osteocytes in alveolar bone destruction in periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef]
- Roldan, L.; Montoya, C.; Solanki, V.; Cai, K.Q.; Yang, M.; Correa, S.; Orrego, S. A novel injectable piezoelectric hydrogel for periodontal disease treatment. ACS Appl. Mater. Interfaces 2023, 15, 43441–43454. [Google Scholar] [CrossRef]
- Tavangar, M.; Heidari, F.; Hayati, R.; Tabatabaei, F.; Vashaee, D.; Tayebi, L. Manufacturing and characterization of mechanical, biological and dielectric properties of hydroxyapatite-barium titanate nanocomposite scaffolds. Ceram. Int. 2020, 46, 9086–9095. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, K.; Shi, R.; Ma, Y.; Tang, Y.; Bian, T.; Wang, J. An investigation of the electrical, mechanical and biocompatibility properties of Barium Titanate / Hydroxyapatite bulk ceramics. Mater. Chem. Phys. 2020, 243, 122613. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Goettems, M.L.; Fernandez, M.D.S.; Donassollo, T.A.; Henn Donassollo, S.; Demarco, F.F. Impact of tooth bleaching on oral health-related quality of life in adults: A triple-blind randomised clinical trial. J. Dent. 2021, 105, 103564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, X.; Jia, Y.; Huang, M.; Wang, F.; Zhang, X.; Bai, Y.; Yuan, G.; Wang, Y. Piezo-catalysis for nondestructive tooth whitening. Nat. Commun. 2020, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Bae, J.; Heo, J.H.; Park, C.H.; Kim, E.B.; Lee, J.H. Nanoparticles as next-generation tooth-whitening agents: Progress and perspectives. ACS Nano 2022, 16, 10042–10065. [Google Scholar] [CrossRef]
- Ma, G.; Wu, A.; Zhou, S.; Wang, M.; Zhang, B.; Liu, Y.; Liang, J. Tooth whitening and caries prevention toothbrush based on ptfe electret. J. Mater. Sci. 2024, 59, 2522–2533. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, U.; Jain, D.; Kushwaha, H.S. NaNbO3/ZnO piezocatalyst for non-destructive tooth cleaning and antibacterial activity. iScience 2022, 25, 104915. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, S.; Hou, Y.; Liu, S.; Zhang, Z.; Zhao, M.; He, L.; Wang, R.; Liu, S. Bifunctional defect mediated direct Z-scheme g-C3N4-x/Bi2O3-y heterostructures with enhanced piezo-photocatalytic properties for efficient tooth whitening and biofilm eradication. J. Mater. Chem. B 2023, 11, 7103–7116. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Rencus-Lazar, S.; Cai, K.; Gazit, E.; Ji, W. Minimalistic metabolite-based building blocks for supramolecular functional materials. Chemsystemschem 2022, 4, e202200021. [Google Scholar] [CrossRef]
- Ji, W.; Xue, B.; Yin, Y.; Guerin, S.; Wang, Y.; Zhang, L.; Cheng, Y.; Shimon, L.J.W.; Chen, Y.; Thompson, D.; et al. Modulating the electromechanical response of bio-inspired amino acid-based architectures through supramolecular co-assembly. J. Am. Chem. Soc. 2022, 144, 18375–18386. [Google Scholar] [CrossRef]
- Xu, T.; Jin, L.; Ao, Y.; Zhang, J.; Sun, Y.; Wang, S.; Qu, Y.; Huang, L.; Yang, T.; Deng, W.; et al. All-polymer piezo-ionic-electric electronics. Nat. Commun. 2024, 15, 10876. [Google Scholar] [CrossRef]
- Deng, W.; Zhou, Y.; Libanori, A.; Chen, G.; Yang, W.; Chen, J. Piezoelectric nanogenerators for personalized healthcare. Chem. Soc. Rev. 2022, 51, 3380–3435. [Google Scholar] [CrossRef]
- Tan, X.; Wang, H.; Wang, K.; Zhang, X.; Ding, H.; Li, S.; Li, J.; Song, J. One-step construction of biocompatible glycine assemblies with exceptional piezoelectric performance for sustainable electronics. Adv. Funct. Mater. 2025, 35, 2506107. [Google Scholar] [CrossRef]
- Liu, J.-H.; Li, W.-D.; Jia, J.; Tang, C.-Y.; Wang, S.; Yu, P.; Zhang, Z.-M.; Ke, K.; Bao, R.-Y.; Liu, Z.-Y.; et al. Structure-regenerated silk fibroin with boosted piezoelectricity for disposable and biodegradable oral healthcare device. Nano Energy 2022, 103, 107787. [Google Scholar] [CrossRef]
- Matusz, K.; Maciejewska-Szaniec, Z.; Gredes, T.; Pobudek-Radzikowska, M.; Glapiński, M.; Górna, N.; Przystańska, A. Common therapeutic approaches in sleep and awake bruxism—An overview. Neurol. Neurochir. Pol. 2022, 56, 455–463. [Google Scholar] [CrossRef]
- Baba, K.; Clark, G.T.; Watanabe, T.; Ohyama, T. Bruxism force detection by a piezoelectric film-based recording device in sleeping humans. J. Orofac. Pain 2003, 17, 58–64. [Google Scholar] [PubMed]
- Yu, K.; Zhang, Q.; Dai, Z.; Zhu, M.; Xiao, L.; Zhao, Z.; Bai, Y.; Zhang, K. Smart dental materials intelligently responding to oral pH to combat caries: A literature review. Polymers 2023, 15, 2611. [Google Scholar] [CrossRef]
- Yap, R.C.; Alghanem, M.; Martin, N. A narrative review of cracks in teeth: Aetiology, microstructure and diagnostic challenges. J. Dent. 2023, 138, 104683. [Google Scholar] [CrossRef]
- El-Sharkawy, Y.H.; Badr, Y.; Gadallah, M.; Elsherif, A.F. Diagnostic of human teeth using photoacoustic response. Laser in Dentistry 2006, 6137, 613701. [Google Scholar]
- Yang, T.; Sa, R.; Wang, F.; Chen, C.; Zheng, L. Research progress of piezoelectric materials in protecting oral health and treating oral diseases: A mini-review. Front. Bioeng. Biotechnol. 2024, 12, 1473126. [Google Scholar] [CrossRef] [PubMed]
- Chorsi, M.T.; Le, T.T.; Lin, F.; Vinikoor, T.; Das, R.; Stevens, J.F.; Mundrane, C.; Park, J.; Tran, K.T.M.; Liu, Y.; et al. Highly piezoelectric, biodegradable, and flexible amino acid nanofibers for medical applications. Sci. Adv. 2023, 9, eadg6075. [Google Scholar] [CrossRef] [PubMed]
| Application Fields | Key Materials | Piezoelectricity | Development Stage | Ref. |
|---|---|---|---|---|
| Tissue regeneration and repair | Strontium-doped biofilm | d33 = 14 pC/N | Preclinical | [64] |
| BaTiO3 dental composite resins | d33 = 0.5–4.2 pC/N | Preclinical | [65] | |
| Piezoelectric hydrogels | Output voltage = 45.4 mV | Preclinical | [66] | |
| Nylon-11 nanoparticles | PFM amplitude ~10 mV | Preclinical | [67] | |
| VDF-TeFE copolymer | d33 = 4 pC/N | Preclinical | [68,69,70] | |
| Antibacterial effects | PVDF/TiO2 nanotubes coating | / | Preclinical | [74] |
| Metal/piezoelectric nanostructures | PFM amplitude with butterfly loop and phase hysteresis | Preclinical | [75] | |
| Zn-MSNs/K18-MMA/K18-Filler in dental resins | / | Preclinical | [81,82] | |
| Injectable PiezoGEL | Output voltage ≈ 10 mV | Preclinical | [85] | |
| Teeth whitening | BaTiO3-based systems | PFM amplitude with butterfly hysteresis loop and 180° phase switching | Preclinical | [90] |
| Z-scheme g-C3N4−x/Bi2O3−γ heterostructure | / | Preclinical | [94] | |
| Oral health monitoring | Piezoelectric units embedded in aligners | d33 = 200–400 pC/N | Preclinical | [19] |
| DA/PVA/glycine piezoelectric film | d33 = 10.8 pC/N | Clinical | [99] | |
| Piezoelectric film sensors | / | Clinical | [18,102] |
| Materials | Study Model | Trial Data (p < 0.05) | Functions | Ref. |
|---|---|---|---|---|
| Piezoelectric units embedded in aligners | In vivo Rat model with Ni-Ti spring fixation between molar and incisor | Orthodontic efficiency increase: 34% (young) and 164% (aged) rats | Real-time orthodontic monitoring | [19] |
| Strontium-doped biofilm | In vivo Canine dentin defect | A 3-fold enhancement in dentin regeneration over conventional film. | Dentin mineralization | [64] |
| BaTiO3 dental composite resins | In vivo SBF solution, under cyclic mechanical loading | 11.6 ± 4.1 μm mineral layer (vs. ~5 μm in control) | Remineralization and self-repair | [65] |
| Piezoelectric hydrogels | In vivo Rat alveolar bone defect | 63.40 ± 5.58% alveolar bone regeneration (vs. 44.15 ± 10.01% in control) | Periodontal tissue regeneration | [66] |
| Nylon-11 nanoparticles | In vitro DPSCs co-culture with nylon-11 nanoparticles | ~3-fold upregulation of osteogenic markers compared to control | Alveolar bone repair | [67] |
| VDF-TeFE copolymer | In vivo Rat oral mucosa wound defect | Significantly smaller wound area (4.8 ± 2.1 mm2) than control group (8.2 ± 1.7 mm2) | Oral mucosal wound healing | [68,69,70] |
| PVDF/TiO2 nanotubes coating | In vitro Co-incubated with S. aureus and E. coli | Inhibition of bacterial adhesion: 30.4% (S. aureus) & ~61.9% (E. coli) | Prevents peri-implant infections | [74] |
| Metal/piezoelectric nanostructures | In vivo Rat subcutaneous S. aureus-piezoelectric implant infection model | With an in vivo antibacterial rate of 96.9% | Inhibits S. aureus adhesion | [75] |
| Zn-MSNs/K18-MMA/K18-Filler in dental resins | In vitro The resin surface was inoculated with S. mutans | Significantly fewer S. mutans (725,333 ± 162,578 CFUs) than control group (1,620,333 ± 577,037 CFUs) | Inhibits caries progression | [81,82] |
| Injectable PiezoGEL | In vivo Rat ligature-induced periodontitis | Higher bone volume (55%) vs. control (20%) | Reduces plaque-related periodontal diseases | [85] |
| BaTiO3-based systems | Ex vivo The extracted teeth | Whitening efficiency (ΔE): ~3-fold higher than control | Achieves efficient and non-destructive tooth whitening without using high-concentration peroxides | [90] |
| Z-scheme g-C3N4−x/Bi2O3−γ heterostructure | Ex vivo The extracted teeth | Pigment degradation efficiency: 97.6% (vs. 25.4% in control) | [94] | |
| DA/PVA/glycine piezoelectric film | Clinical trial Assembled into a waterproof force sensor and placed on teeth occlusal surfaces | Detecting four types of occlusal contact patterns | Monitoring of occlusal contact during orthodontic treatment | [99] |
| Piezoelectric film sensors | Clinical trial Nocturnal tooth-to-splint contact forces were recorded in home environment | Bruxism group exhibited a significantly longer event duration (27 s/h) compared to the control group (7.4 s/h) | Monitoring of bruxism and occlusal forces | [18,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Wang, Y.; Zhao, D.; Wang, H.; Xie, S.; Pan, L. From Biomechanics to Bioinnovation: Emerging Applications of Piezoelectric Materials and Phenomena in Dentistry. Biomedicines 2025, 13, 2683. https://doi.org/10.3390/biomedicines13112683
Kang W, Wang Y, Zhao D, Wang H, Xie S, Pan L. From Biomechanics to Bioinnovation: Emerging Applications of Piezoelectric Materials and Phenomena in Dentistry. Biomedicines. 2025; 13(11):2683. https://doi.org/10.3390/biomedicines13112683
Chicago/Turabian StyleKang, Wen, Yuehui Wang, Dan Zhao, Hongwei Wang, Sijing Xie, and Lijia Pan. 2025. "From Biomechanics to Bioinnovation: Emerging Applications of Piezoelectric Materials and Phenomena in Dentistry" Biomedicines 13, no. 11: 2683. https://doi.org/10.3390/biomedicines13112683
APA StyleKang, W., Wang, Y., Zhao, D., Wang, H., Xie, S., & Pan, L. (2025). From Biomechanics to Bioinnovation: Emerging Applications of Piezoelectric Materials and Phenomena in Dentistry. Biomedicines, 13(11), 2683. https://doi.org/10.3390/biomedicines13112683







