Urinary Vitamin D Binding Protein and Kidney Injury Molecule-1 Are Potent Predictors of Acute Kidney Injury After Left Ventricular Assist Device Implantation
Abstract
1. Introduction
2. Materials and Methods
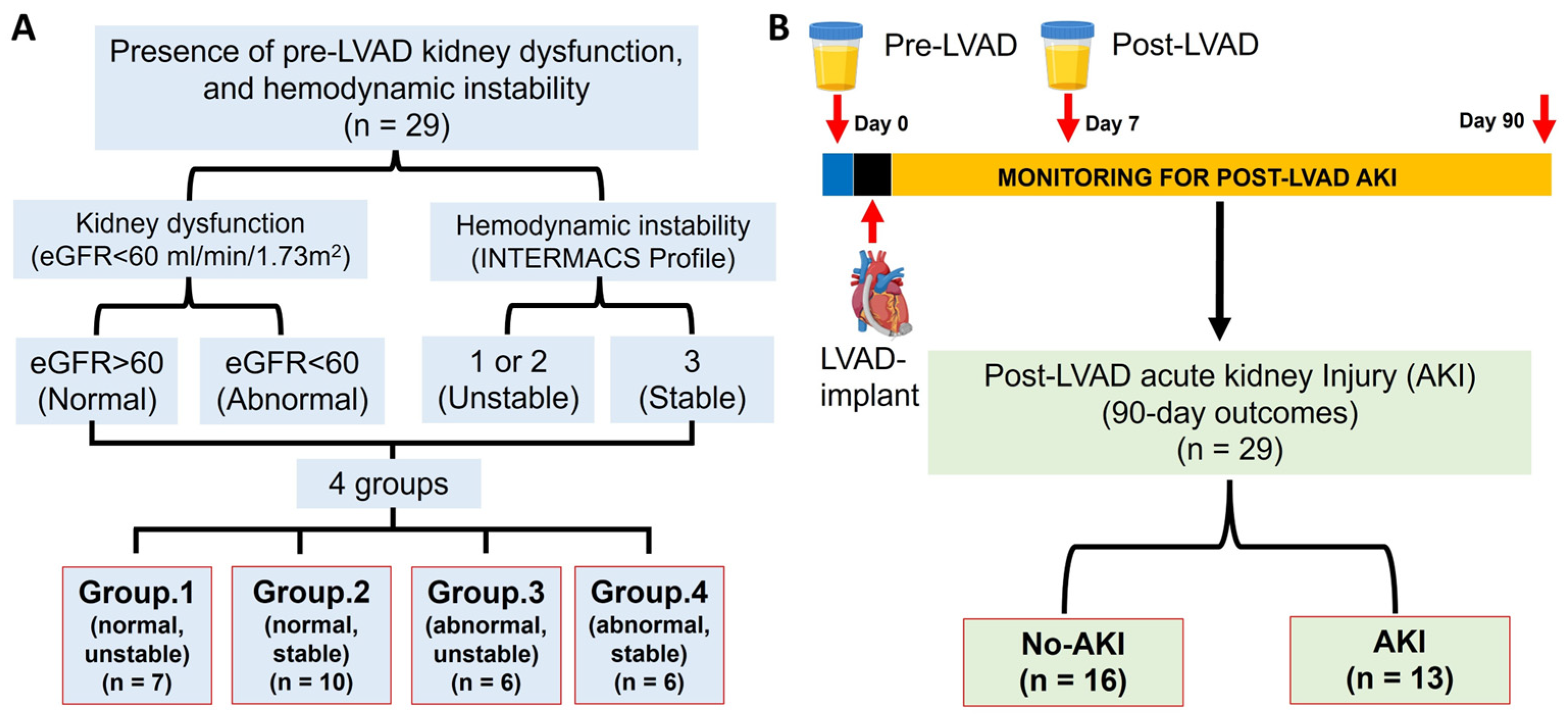
2.1. Study Design and Groups
- Group 1: normal kidney function (eGFR > 60) with unstable (INTERMACS 1 & 2) hemodynamics (N = 7).
- Group 2: normal kidney function (eGFR > 60) with stable (INTERMACS 3) hemodynamics (N = 10).
- Group 3: impaired kidney function (eGFR < 60) with unstable (INTERMACS 1 & 2) hemodynamics (N = 6).
- Group 4: impaired kidney function (eGFR < 60) with stable (INTERMACS 3) hemodynamics (N = 6).
2.2. Biomarkers’ Measurements
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics Among Those with or Without Pre-LVAD Kidney Dysfunction and/or Hemodynamic Instability
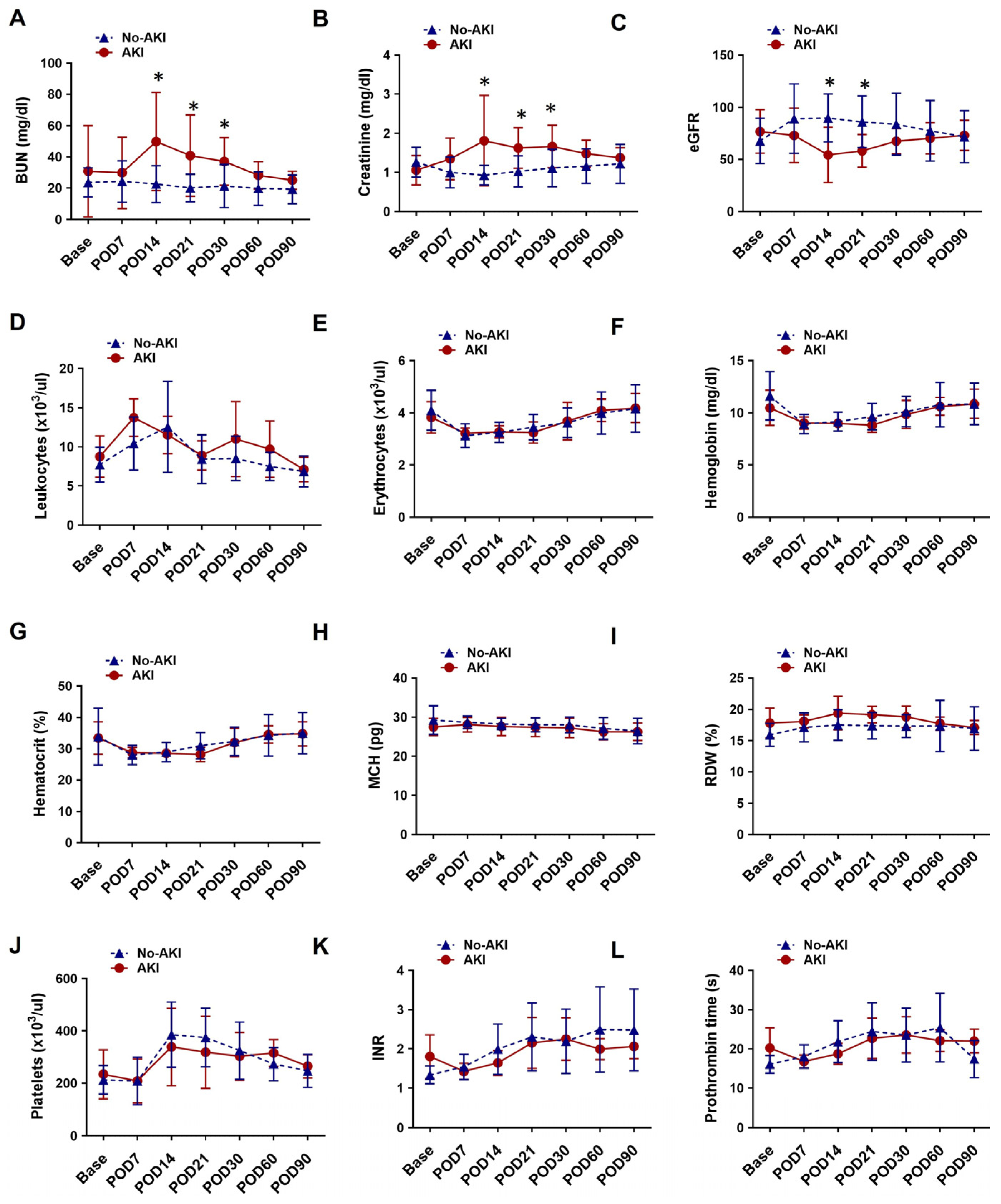
3.2. Patient Characteristics by Development of Post-LVAD AKI
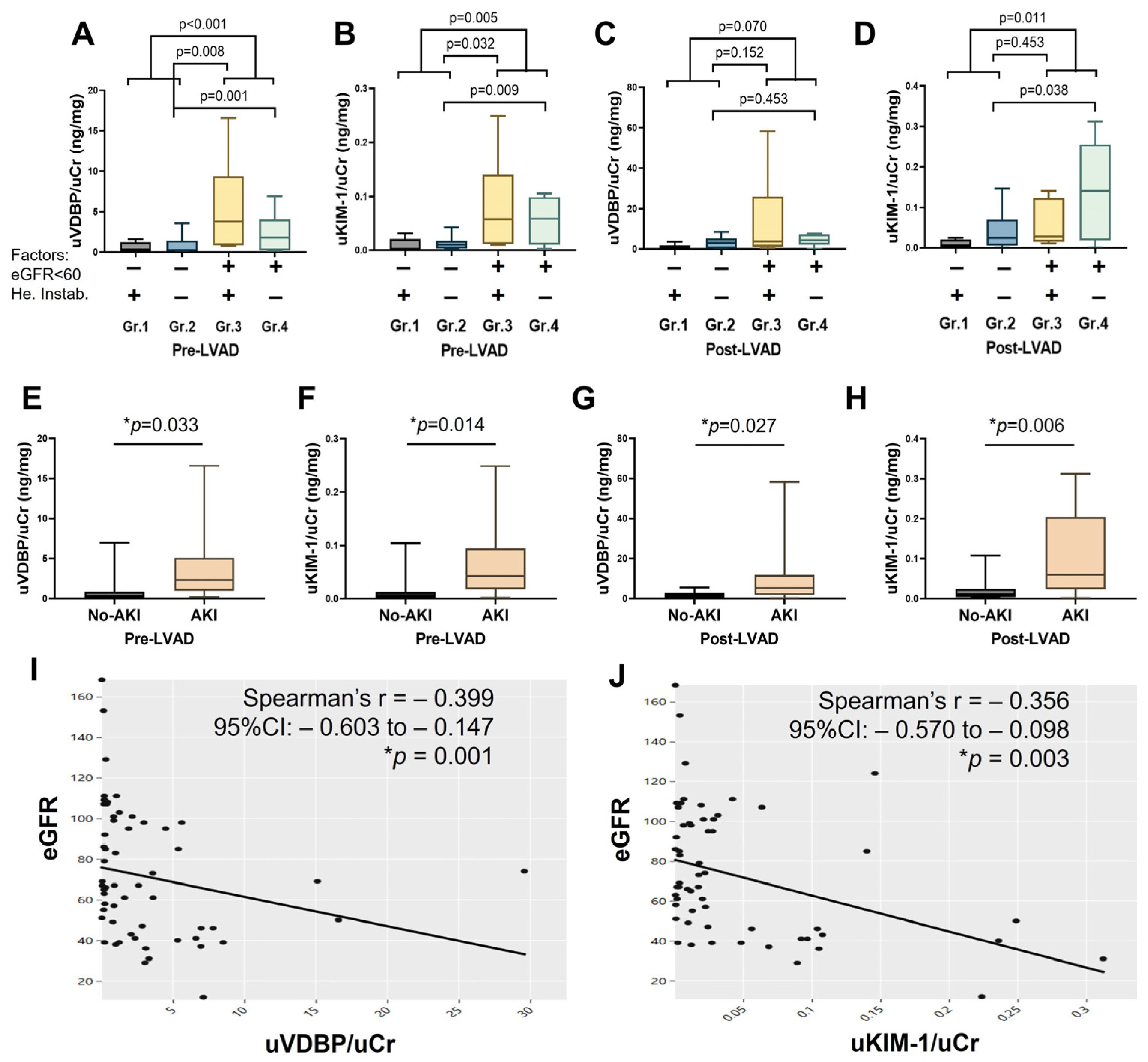
3.3. Biochemistry for Patients with or Without Pre-LVAD Kidney Dysfunction and/or Hemodynamic Instability
3.4. Laboratory Hematology and Blood Chemistry for Patients with or Without Post-LVAD AKI
3.5. Change in Urinary VDBP, KIM-1, and Their Correlation with Creatinine-Based Estimated Glomerular Filtration Rate
3.6. Association of Urinary VDBP and KIM-1 with Post-LVAD AKI
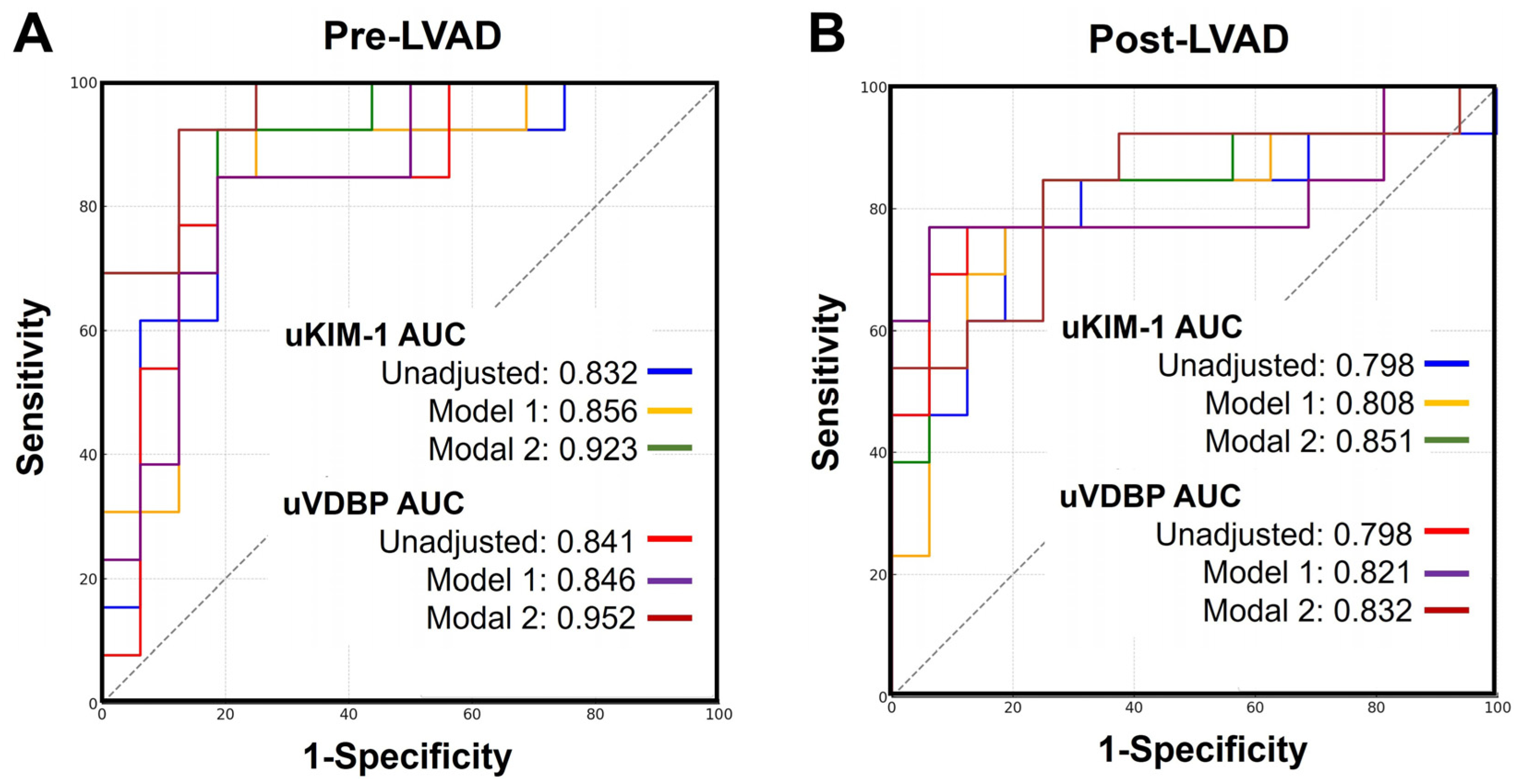
3.7. Urinary VDBP and KIM-1 for Post-LVAD AKI Prediction
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondal, N.K.; Sorensen, E.N.; Hiivala, N.J.; Feller, E.D.; Pham, S.M.; Griffith, B.P.; Wu, Z.J. Intraplatelet reactive oxygen species, mitochondrial damage and platelet apoptosis augment non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist device. Platelets 2015, 26, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Sorensen, E.N.; Feller, E.D.; Pham, S.M.; Griffith, B.P.; Wu, Z.J. Systemic Inflammatory Response Syndrome After Contentious-Flow Left Ventricular Assist Device Implantation and Change in Platelet Mitochondrial Membrane Potential. J. Card. Fail. 2015, 21, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, J.P.; Murrieta-Alvarez, I.; Li, S.; Xu, Z.; Zheng, G.; Uwaeze, J.; Walther, C.P.; Gray, Z.; Nordick, K.V.; Braverman, V.; et al. Machine Learning Assisted Stroke Prediction in Mechanical Circulatory Support: Predictive Role of Systemic Mitochondrial Dysfunction. ASAIO J. 2025, 71, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nordick, K.V.; Murrieta-Alvarez, I.; Garcia, I.; Kirby, R.P.; Bhattacharya, R.; Shafii, A.E.; Ghosh, S.; Hochman-Mendez, C.; Rosengart, T.K.; et al. Endostatin and Cystatin C as Predictors of 1 Month Renal Function Change in Patients With Left Ventricular Assist Device Support. ASAIO J. 2025, 71, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Brisco, M.A.; Kimmel, S.E.; Coca, S.G.; Putt, M.E.; Jessup, M.; Tang, W.W.; Parikh, C.R.; Testani, J.M. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ. Heart Fail. 2014, 7, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.W.; Stevens, G.R.; Wanchoo, R.; Majure, D.T.; Jauhar, S.; Fernandez, H.A.; Merzkani, M.; Jhaveri, K.D. Left Ventricular Assist Devices and the Kidney. Clin. J. Am. Soc. Nephrol. 2018, 13, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.P.; Civitello, A.B.; Lamba, H.K.; Mondal, N.K.; Navaneethan, S.D. Kidney Function Trajectories and Right Heart Failure Following LVAD Implantation. J. Am. Heart Assoc. 2024, 13, e031305. [Google Scholar] [CrossRef] [PubMed]
- Muslem, R.; Caliskan, K.; Akin, S.; Sharma, K.; Gilotra, N.A.; Constantinescu, A.A.; Houston, B.; Whitman, G.; Tedford, R.J.; Hesselink, D.A.; et al. Acute kidney injury and 1-year mortality after left ventricular assist device implantation. J. Heart Lung Transplant. 2018, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, Y.C.; Bunge, J.J.H.; Guven, G.; Muslem, R.; Szymanski, M.; den Uil, C.A.; Hesselink, D.A.; Topkara, V.K.; Manintveld, O.C.; Colombo, P.C.; et al. Acute kidney injury following left ventricular assist device implantation: Contemporary insights and future perspectives. J. Heart Lung Transplant. 2019, 38, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M.; Doi, K.; Kinoshita, O.; Kimura, M.; Ono, M.; Hamasaki, Y.; Matsubara, T.; Ishii, T.; Yahagi, N.; Nangaku, M.; et al. Perioperative plasma neutrophil gelatinase-associated lipocalin measurement in patients who undergo left ventricular assist device implantation surgery. Circ. J. 2014, 78, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.; Papp, M.; Turan, C.; Koi, T.; Madach, K.; Hegyi, P.; Zubek, L.; Molnar, Z. Combination of urinary biomarkers can predict cardiac surgery-associated acute kidney injury: A systematic review and meta-analysis. Ann. Intensive Care 2025, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Bufkin, K.B.; Karim, Z.A.; Silva, J. Review of the limitations of current biomarkers in acute kidney injury clinical practices. SAGE Open Med. 2024, 12, 20503121241228446. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Riera, E.; Garcia-Arguinzonis, M.; Lopez, L.; Garcia-Moll, X.; Badimon, L.; Padro, T. Vitamin D Binding Protein and Renal Injury in Acute Decompensated Heart Failure. Front. Cardiovasc. Med. 2022, 9, 829490. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, J.H.; Alge, J.L.; Arthur, J.M.; Ayub, F.; Bin Homam, W.; Janech, M.G.; Ravula, S.; Karakala, N. Urinary Complement C3 and Vitamin D-Binding Protein Predict Adverse Outcomes in Patients with Acute Kidney Injury after Cardiac Surgery. Nephron 2025, 149, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.A.; Engstrom, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol. Dial. Transplant. 2020, 35, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.M.; Hill, E.G.; Alge, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D.; Investigators, S.A. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Krawczeski, C.D.; Goldstein, S.L.; Woo, J.G.; Wang, Y.; Piyaphanee, N.; Ma, Q.; Bennett, M.; Devarajan, P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J. Am. Coll. Cardiol. 2011, 58, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Ornellas, F.M.; Ornellas, D.S.; Martini, S.V.; Castiglione, R.C.; Ventura, G.M.; Rocco, P.R.; Gutfilen, B.; de Souza, S.A.; Takiya, C.M.; Morales, M.M. Bone Marrow-Derived Mononuclear Cell Therapy Accelerates Renal Ischemia-Reperfusion Injury Recovery by Modulating Inflammatory, Antioxidant and Apoptotic Related Molecules. Cell Physiol. Biochem. 2017, 41, 1736–1752. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Zhang, K.; Nie, Z.; Li, Q.; Jin, F. Kidney injury molecule-1 (KIM-1): A novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant. Rev. 2010, 24, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.S.; Parikh, U.M.; Lamba, H.; Walther, C. Chronic Kidney Disease and Acute Kidney Injury Outcomes Post Left Ventricular Assist Device Implant. Cureus 2020, 12, e7725. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nordick, K.V.; Elsenousi, A.E.; Bhattacharya, R.; Kirby, R.P.; Hassan, A.M.; Hochman-Mendez, C.; Rosengart, T.K.; Liao, K.K.; Mondal, N.K. Warm-ischemia and cold storage induced modulation of ferroptosis observed in human hearts donated after circulatory death and brain death. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H923–H936. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nordick, K.V.; Elsenousi, A.E.; Hassan, A.M.; Shaju, R.A.; Bhattacharya, R.; Peer, S.B.; Rajkumar, A.; Banerjee, S.; Kirby, R.P.; et al. Effect of cold storage solution on warm ischemia-induced myocardial plasma membrane damage and pyroptosis in human hearts from circulatory death donors. J. Thorac. Cardiovasc. Surg. 2025, in press. [CrossRef] [PubMed]
- Mondal, N.K.; Li, S.; Elsenousi, A.E.; Mattar, A.; Nordick, K.V.; Lamba, H.K.; Hochman-Mendez, C.; Rosengart, T.K.; Liao, K.K. NADPH oxidase overexpression and mitochondrial OxPhos impairment are more profound in human hearts donated after circulatory death than brain death. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H548–H562. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Li, S.; Elsenousi, A.E.; Mattar, A.; Hochman-Mendez, C.; Rosengart, T.K.; Liao, K.K. Myocardial edema, inflammation, and injury in human heart donated after circulatory death are sensitive to warm ischemia and subsequent cold storage. J. Thorac. Cardiovasc. Surg. 2024, 167, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Lertjitbanjong, P.; Cheungpasitporn, W.; Hansrivijit, P.; Fulop, T.; Kovvuru, K.; Kanduri, S.R.; Davis, P.W.; Vallabhajosyula, S.; Bathini, T.; et al. Incidence and impact of acute kidney injury on patients with implantable left ventricular assist devices: A Meta-analysis. Ren. Fail. 2020, 42, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, K.; Doorenbos, C.R.; Dam, W.A.; Lambers Heerspink, H.J.; Slagman, M.C.; Nauta, F.L.; Kramer, A.B.; Gansevoort, R.T.; van den Born, J.; Navis, G.; et al. Urinary vitamin D binding protein: A potential novel marker of renal interstitial inflammation and fibrosis. PLoS ONE 2013, 8, e55887. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.; Williams, N.J.; Cleeve, H.J. Studies on vitamin D binding protein in the nephrotic syndrome. Clin. Chem. 1985, 31, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Vilasi, A.; Cutillas, P.R.; Maher, A.D.; Zirah, S.F.; Capasso, G.; Norden, A.W.; Holmes, E.; Nicholson, J.K.; Unwin, R.J. Combined proteomic and metabonomic studies in three genetic forms of the renal Fanconi syndrome. Am. J. Physiol. Renal Physiol. 2007, 293, F456–F467. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Pordal, A.; Haffner, C.; Pleasant, L.; Ma, Q.; Devarajan, P. Urinary Vitamin D-Binding Protein as a Biomarker of Steroid-Resistant Nephrotic Syndrome. Biomark. Insights 2016, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Semnani-Azad, Z.; Wang, W.Z.N.; Cole, D.E.C.; Johnston, L.W.; Wong, B.Y.L.; Fu, L.; Retnakaran, R.; Harris, S.B.; Hanley, A.J. Urinary Vitamin D Binding Protein: A Marker of Kidney Tubular Dysfunction in Patients at Risk for Type 2 Diabetes. J. Endocr. Soc. 2024, 8, bvae014. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Hung, C.C.; Yang, S.A.; Stevens, J.L.; Bonventre, J.V. Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal Physiol. 2004, 286, F552–F563. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Brilland, B.; Boud’hors, C.; Wacrenier, S.; Blanchard, S.; Cayon, J.; Blanchet, O.; Piccoli, G.B.; Henry, N.; Djema, A.; Coindre, J.P.; et al. Kidney injury molecule 1 (KIM-1): A potential biomarker of acute kidney injury and tubulointerstitial injury in patients with ANCA-glomerulonephritis. Clin. Kidney J. 2023, 16, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Qiu, Y.; Qin, Z.; Su, B. The value of kidney injury molecule 1 in predicting acute kidney injury in adult patients: A systematic review and Bayesian meta-analysis. J. Transl. Med. 2021, 19, 105. [Google Scholar] [CrossRef]
- Grosman-Rimon, L.; Hui, S.G.; Freedman, D.; Elbaz-Greener, G.; Cherney, D.; Rao, V. Biomarkers of Inflammation, Fibrosis, and Acute Kidney Injury in Patients with Heart Failure with and without Left Ventricular Assist Device Implantation. Cardiorenal Med. 2019, 9, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, S.; Bakaeen, F.; Tang, W.H.W.; Donaldson, C.; Taliercio, J.; Huml, A.; Gadegbeku, C.A.; Gillinov, A.M.; Insler, S. Hemodynamic Determinants of Cardiac Surgery-Associated Acute Kidney Injury. Crit. Care Explor. 2024, 6, e1063. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Bakitas, M.A.; Cheng, X.S.; Fang, J.C.; Fedson, S.E.; Fiedler, A.G.; Martens, P.; McCallum, W.I.; Ogunniyi, M.O.; Rangaswami, J.; et al. Evaluation and Management of Kidney Dysfunction in Advanced Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e280–e295. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Jamil, A.K.; Van Zyl, J.S.; Medel-Martinez, H.; Bottiglieri, T.; Wasek, B.; Felius, J.; Lima, B.; Hall, S.A.; Joseph, S.M. Urinary Cell-Cycle Arrest Biomarkers as Early Predictors of Acute Kidney Injury After Ventricular Assist Device Implantation or Cardiac Transplantation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Macedo, E. Biomarkers in acute kidney injury and cirrhosis. J. Transl. Crit. Care Med. 2024, 6, e23-00014. [Google Scholar] [CrossRef]
- Graidis, S.; Papavramidis, T.S.; Papaioannou, M. Vitamin D and Acute Kidney Injury: A Two-Way Causality Relation and a Predictive, Prognostic, and Therapeutic Role of Vitamin D. Front. Nutr. 2020, 7, 630951. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Group 1 (Normal, Unstable) (N = 7) | Group 2 (Normal, Stable) (N = 10) | Group 3 (Abnormal, Unstable) (N = 6) | Group 4 (Abnormal, Stable) (N = 6) | p-Value |
|---|---|---|---|---|---|
| Demography | |||||
| Age in years, Median (IQR) | 59 (41–65) | 61 (56–68) | 62 (54–64) | 64 (49–72) | 0.576 |
| Sex, n (% male) | 6 (85.71%) | 7 (70.00%) | 5 (83.33%) | 6 (100%) | 0.639 |
| Race | 0.676 | ||||
| Caucasian white, n (%) | 5 (71.43%) | 5 (50.00%) | 5 (83.33%) | 3 (50.00%) | |
| African American, n (%) | 1 (14.29%) | 4 (40.00%) | 1 (16.67%) | 3 (50.00%) | |
| Other, n (%) | 1 (14.29%) | 1 (10.00%) | 0 | 0 | |
| Height in meters, Median (IQR) | 1.73 (1.68–1.78) | 1.69 (1.63–1.75) | 1.79 (1.73–1.83) | 1.79 (1.73–1.83) | 0.173 |
| Weight in kilograms, Median (IQR) | 79.9 (67.0–83.6) | 78.6 (69.4–95.3) | 86.4 (77.7–91.9) | 88.1 (75.1–101.5) | 0.584 |
| BMI, kg/m2, Median (IQR) | 25.62 (21.42–28.43) | 26.61 (23.56–30.75) | 26.58 (23.56–29.44) | 26.38 (26.10–29.50) | 0.798 |
| BSA, m2, Median (IQR) | 1.96 (1.75–2.06) | 1.95 (1.75–2.17) | 2.17 (1.97–2.24) | 2.06 (1.88–2.35) | 0.308 |
| History of smoking, n (%) | 4 (57.14%) | 3 (30.00%) | 4 (66.67%) | 2 (33.33%) | 0.459 |
| History of alcohol abuse, n (%) | 3 (42.86%) | 4 (40.00%) | 2 (33.33%) | 3 (50.00%) | 1.000 |
| History of drug abuse, n (%) | 1 (14.29%) | 0 | 1 (16.67%) | 0 | 0.421 |
| Hypertension, n (%) | 2 (28.57%) | 7 (70.00%) | 3 (50.00%) | 4 (66.67%) | 0.382 |
| Diabetes, n (%) | 4 (57.14%) | 4 (40.00%) | 1 (16.67%) | 2 (33.33%) | 0.615 |
| COPD, n (%) | 0 | 0 | 1 (16.67%) | 1 (16.67%) | 0.214 |
| Peripheral vascular disease, n (%) | 0 | 0 | 2 (33.33%) | 1 (16.67%) | 0.070 |
| Cerebral vascular accident, n (%) | 0 | 0 | 0 | 1 (16.67%) | 0.585 |
| SBP (mmHg), Median (IQR) | 102 (94–111) | 105 (99–115) | 103 (88–117) | 113 (110–116) | 0.547 |
| DBP (mmHg), Median (IQR) | 72 (64–72) | 72 (59–86) | 69 (63–72) | 71 (63–77) | 0.942 |
| Etiology of heart disease | 0.556 | ||||
| Ischemic cardiomyopathy, n (%) | 2 (28.57%) | 6 (60.00%) | 3(50.00%) | 4(66.67%) | |
| Non-ischemic cardiomyopathy, n (%) | 5 (71.43%) | 4 (40.00%) | 3(50.00%) | 2(33.33%) | |
| INTERMACS profile, median (IQR) | 2.00 (2.00–2.00) | 3 (3.00–3.00) | 1.83 (2.00–2.00) | 3.00 (3.00–3.00) | <0.001 * |
| NYHA classification, median (IQR) | 3.86 (4.00–4.00) | 3.80 (4.00–4.00) | 3.83 (4.00–4.00) | 4.00 (4.00–4.00) | 0.734 |
| Echocardiographic parameters | |||||
| LviDd in centimeters, n (%) | 6.10 (5.81–6.39) | 6.76 (6.33–7.01) | 7.09 (6.90–7.32) | 6.72 (6.34–6.85) | 0.0152 * |
| LVEF (%) | 18.81 (16.90–21.00) | 19.46 (17.30–21.90) | 20.98 (16.00–24.10) | 20.50 (13.40–23.70) | 0.811 |
| LVAD implantation goal | 0.414 | ||||
| BTT, n (%) | 0 | 0 | 0 | 1 (16.67%) | |
| DT, n (%) | 7 (100.00%) | 10 (100.00%) | 6 (100.00%) | 5 (83.33%) | |
| Post-LVAD mechanical ventilation (h), median (IQR) | 63 (40–68) | 59 (29–86) | 81 (21–69) | 77 (25–52) | 0.756 |
| Post-LVAD ICU stay (days), Median (IQR) | 15 (9–16) | 11 (7–15) | 29 (23–34) | 18 (10–27) | 0.056 |
| Length of total hospitalization (days), Median (IQR) | 41 (16–46) | 23 (18–23) | 48 (24–55) | 43 (31–52) | 0.043 * |
| Characteristics | No-AKI (N = 16) | AKI (N = 13) | p-Value |
|---|---|---|---|
| Demography | |||
| Age in years, Median (IQR) | 62 (49–66) | 61 (54–67) | 0.948 |
| Sex, n (% male) | 14 (87.50%) | 10 (76.92%) | 0.632 |
| Race | 0.168 | ||
| Caucasian white, n (%) | 11 (68.75%) | 7 (53.85%) | |
| African American, n (%) | 3 (18.75%) | 6 (46.15%) | |
| Other, n (%) | 2 (12.50%) | 0 | |
| Height in meters, Median (IQR) | 1.73 (1.65–1.79) | 1.74 (1.70–1.83) | 0.322 |
| Weight in kilograms, Median (IQR) | 80.5 (72.3–86.1) | 88.2 (73.5–96.6) | 0.273 |
| BMI, kg/m2, Median (IQR) | 25.9 (22.9–30.1) | 26.4 (25.5–29.4) | 0.776 |
| BSA, m2, Median (IQR) | 1.97 (1.77–2.09) | 2.11 (1.86–2.24) | 0.254 |
| History of smoking, n (%) | 8 (50.00%) | 5 (38.46%) | 0.711 |
| History of alcohol abuse, n (%) | 6 (37.50%) | 6 (46.15%) | 0.716 |
| History of drug abuse, n (%) | 1 (6.25%) | 1 (7.69%) | 1.000 |
| Hypertension, n (%) | 8 (50.00%) | 8 (61.54%) | 0.711 |
| Diabetes, n (%) | 7 (43.75%) | 4 (30.77%) | 0.702 |
| COPD, n (%) | 1 (6.25%) | 1 (7.69%) | 1.000 |
| CKD, n (%) | 11 (68.75%) | 10 (76.92%) | 0.697 |
| Peripheral vascular disease, n (%) | 2 (12.50%) | 1 (7.69%) | 1.000 |
| Cerebral vascular accident, n (%) | 2 (12.50%) | 1 (7.69%) | 1.000 |
| SBP (mmHg), Median (IQR) | 102 (93–112) | 109 (104–116) | 0.046 |
| DBP (mmHg), Median (IQR) | 67 (59–73) | 74 (67–82) | 0.865 |
| Etiology of heart disease | 0.715 | ||
| Ischemic cardiomyopathy, n (%) | 9 (56.25%) | 7 (43.75%) | |
| Non-ischemic cardiomyopathy, n (%) | 6 (46.15%) | 7 (53.85%) | |
| INTERMACS profile, median (IQR) | 2.38 (2.00–3.00) | 2.69 (3.00–3.00) | 0.071 |
| NYHA classification, median (IQR) | 4 (4–4) | 4 (4–4) | 0.826 |
| Echocardiographic parameters | |||
| LviDd in centimeters, n (%) | 6.54 (6.32–6.90) | 6.80 (6.34–7.01) | 0.469 |
| LVEF (%) | 20.71 (18.05–23.90) | 18.76 (16.90–21.90) | 0.148 |
| LVAD implantation goal | 0.448 | ||
| BTT, n (%) | 0 | 1 (7.69%) | |
| DT, n (%) | 16 (100.00%) | 12 (92.31%) | |
| Length of CPB (min), median (IQR) | 97 (69–105) | 113 (59–132) | 0.568 |
| Post-LVAD mechanical ventilation (h), median (IQR) | 46 (22–52) | 103 (40–140) | 0.064 |
| Post-LVAD ICU stay (days), Median (IQR) | 16 (8–20) | 20 (11–27) | 0.195 |
| Length of total hospitalization (days), Median (IQR) | 34 (16–39) | 47 (31–52) | 0.012 * |
| Odds Ratio (95% Confidence Intervals) | |||
|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |
| Pre-LVAD: uKIM1 | |||
| Per two-fold greater uKIM1 | 1.98 (1.28–3.71) | 4.39 (1.79–22.2) | 8.76 (2.03–311) |
| Pre-LVAD: uVDBP | |||
| Per two-fold greater uVDBP | 1.99 (1.27–3.68) | 2.25 (1.23–4.90) | 7.74 (2.07–296) |
| Post-LVAD: uKIM1 | |||
| Per two-fold greater uKIM1 | 1.39 (0.84–2.30) | 1.59 (0.86–2.94) | 1.65 (0.87–3.16) |
| Post-LAVD: uVDBP | |||
| Per two-fold greater uVDBP | 1.26 (0.77–2.08) | 1.20 (0.71–2.03) | 1.01 (0.56–1.84) |
| Model 1 was adjusted for pre-LVAD kidney dysfunction. Model 2 was adjusted for pre-LVAD kidney dysfunction and baseline hemodynamic instability | |||
| Variables and Models | AUC (95%CI) | Sensitivity (%) | Specificity (%) | p Values | |
|---|---|---|---|---|---|
| Pre-LVAD | uKIM-1 | 0.832 (0.647–0.944) | 84.6% | 81.2% | <0.001 |
| uKIM-1/Model 1 | 0.856 (0.709–0.999) | 92.3% | 75.0% | <0.001 | |
| uKIM-1/Model 2 | 0.923 (0.814–0.999) | 92.3% | 81.2% | <0.001 | |
| uVDBP | 0.841 (0.658–0.950) | 84.6% | 81.2% | <0.001 | |
| uVDBP/Model 1 | 0.846 (0.695–0.997) | 84.6% | 81.2% | <0.001 | |
| uVDBP/Model 2 | 0.952 (0.865–0.999) | 92.3% | 87.5% | <0.001 | |
| Post-LVAD | uKIM-1 | 0.798 (0.608–0.923) | 76.9% | 81.2% | 0.001 |
| uKIM-1/Model 1 | 0.808 (0.641–0.974) | 84.6% | 75.0% | <0.001 | |
| uKIM-1/Model 2 | 0.851 (0.702–0.999) | 76.9% | 93.8% | <0.001 | |
| uVDBP | 0.798 (0.608–0.923) | 76.9% | 87.5% | 0.002 | |
| uVDBP/Model 1 | 0.812 (0.648–0.977) | 76.9% | 93.8% | <0.001 | |
| uVDBP/Model 2 | 0.832 (0.675–0.989) | 84.6% | 75.0% | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Murrieta-Álvarez, I.; Nordick, K.V.; Gray, Z.; Hochman-Mendez, C.; Shafii, A.E.; Liao, K.K.; Walther, C.P.; Mondal, N.K. Urinary Vitamin D Binding Protein and Kidney Injury Molecule-1 Are Potent Predictors of Acute Kidney Injury After Left Ventricular Assist Device Implantation. Biomedicines 2025, 13, 2682. https://doi.org/10.3390/biomedicines13112682
Li S, Murrieta-Álvarez I, Nordick KV, Gray Z, Hochman-Mendez C, Shafii AE, Liao KK, Walther CP, Mondal NK. Urinary Vitamin D Binding Protein and Kidney Injury Molecule-1 Are Potent Predictors of Acute Kidney Injury After Left Ventricular Assist Device Implantation. Biomedicines. 2025; 13(11):2682. https://doi.org/10.3390/biomedicines13112682
Chicago/Turabian StyleLi, Shiyi, Iván Murrieta-Álvarez, Katherine V. Nordick, Zachary Gray, Camila Hochman-Mendez, Alexis E. Shafii, Kenneth K. Liao, Carl P. Walther, and Nandan K. Mondal. 2025. "Urinary Vitamin D Binding Protein and Kidney Injury Molecule-1 Are Potent Predictors of Acute Kidney Injury After Left Ventricular Assist Device Implantation" Biomedicines 13, no. 11: 2682. https://doi.org/10.3390/biomedicines13112682
APA StyleLi, S., Murrieta-Álvarez, I., Nordick, K. V., Gray, Z., Hochman-Mendez, C., Shafii, A. E., Liao, K. K., Walther, C. P., & Mondal, N. K. (2025). Urinary Vitamin D Binding Protein and Kidney Injury Molecule-1 Are Potent Predictors of Acute Kidney Injury After Left Ventricular Assist Device Implantation. Biomedicines, 13(11), 2682. https://doi.org/10.3390/biomedicines13112682







