Vitexin as a Potential Antidysmenorrheic Agent: Development of a ZIF-8-Based Immediate-Release System and Evaluation via In Vivo and In Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Planning and Synthesis of Vitexin@ZIF-8 System (VIT@ZIF-8)
2.2. Characterization Tests
2.2.1. Fourier Transform Infrared Absorption Spectroscopy (FT-IR)
2.2.2. Thermal Analysis: Differential Scanning Calorimetry (DSC) and Thermogravimetry (TG)
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. X-Ray Diffractometry (XRD)
2.2.5. In Vitro Release Test
2.3. Evaluation of the Effect of Vitexin and the VIT@ZIF-8 on Abdominal Contortions in Mice in an Experimental Model of Primary Dysmenorrhea
2.4. Molecular Docking and Molecular Dynamics Calculations
2.5. In Silico ADME Predictions
3. Results and Discussion
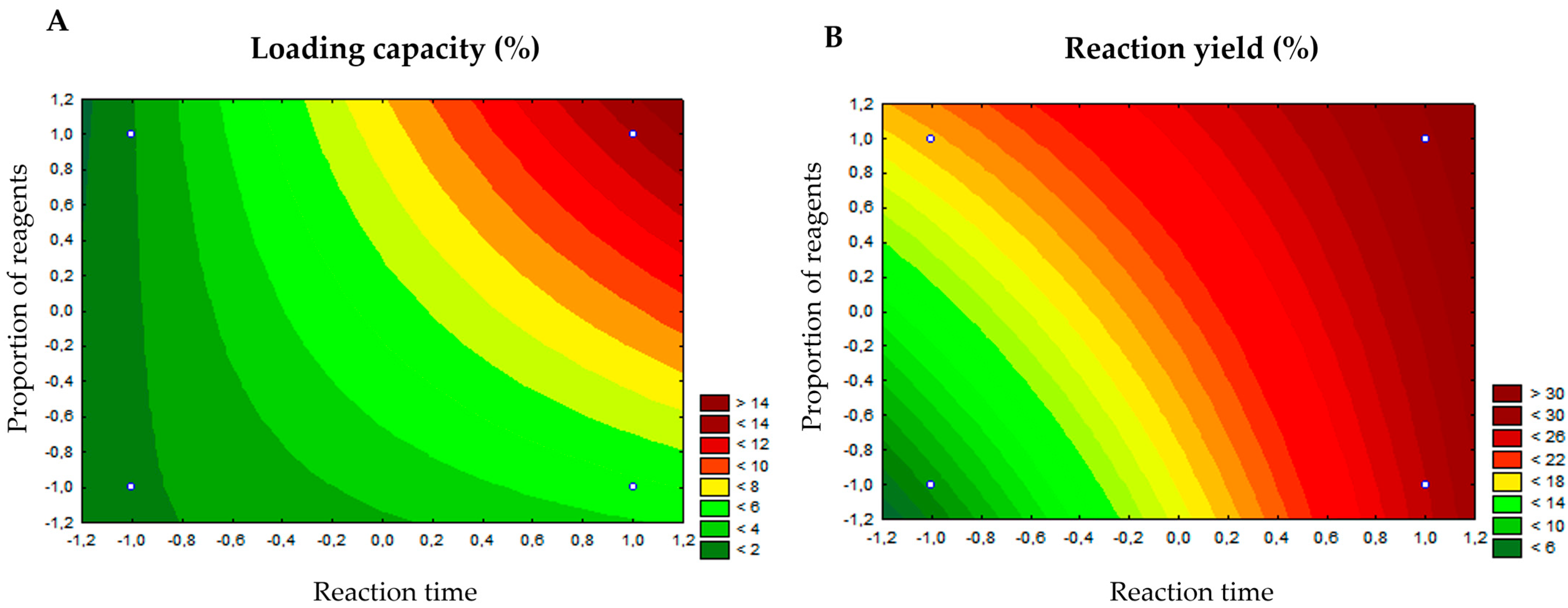
3.1. Synthesis of the VIT@ZIF-8 System and Determination of the Loading Capacity
3.2. Characterization Tests
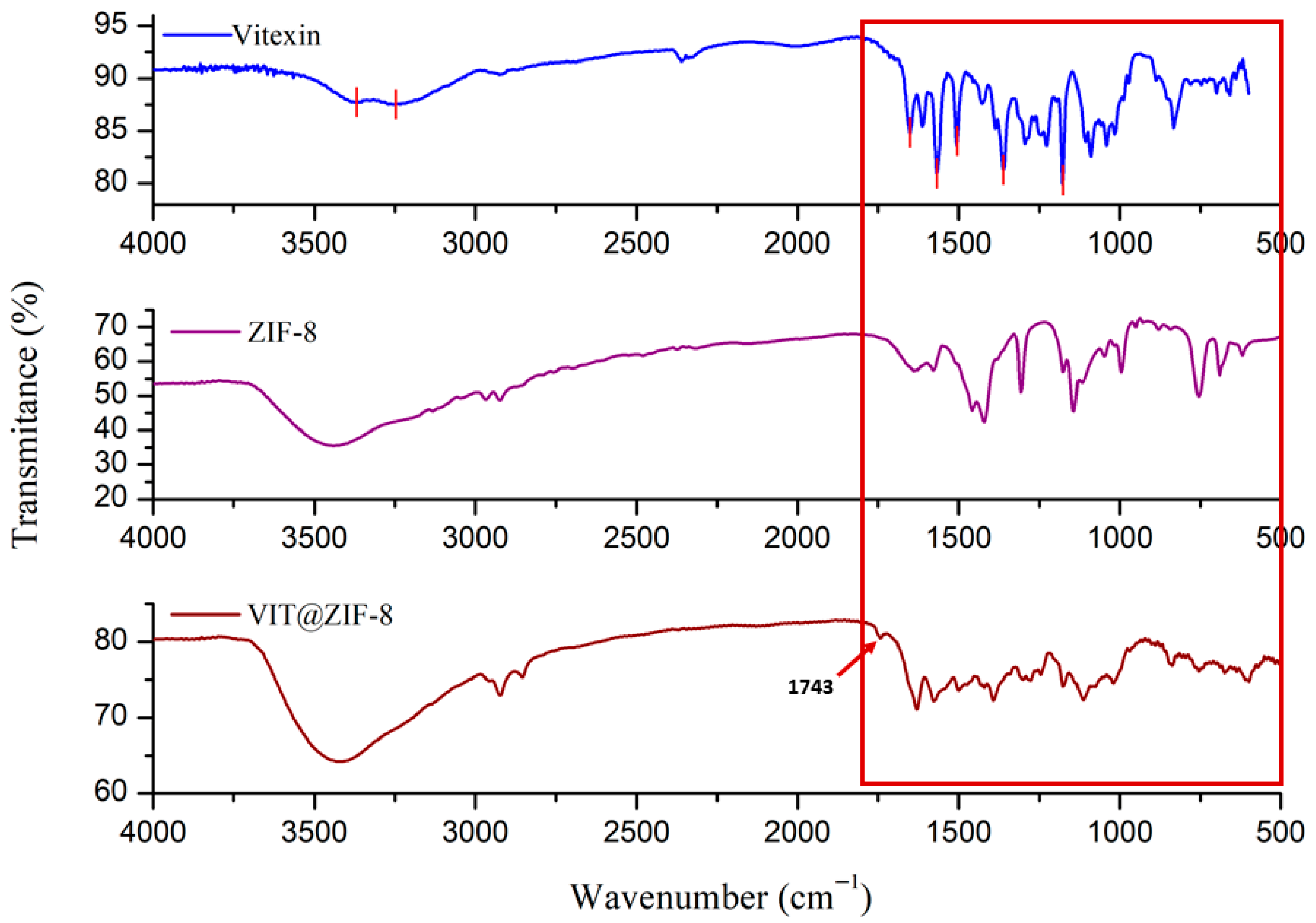
3.2.1. FT-IR Analysis
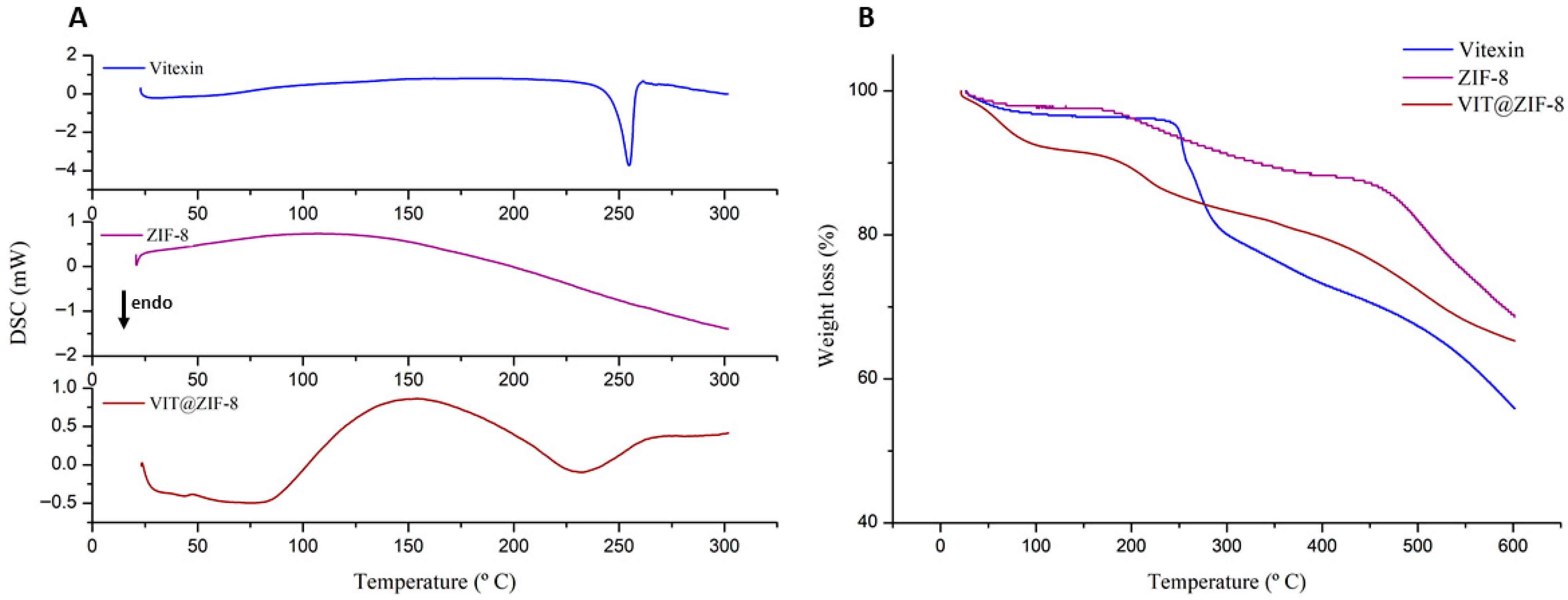
3.2.2. Thermal Analysis
3.2.3. SEM Analysis
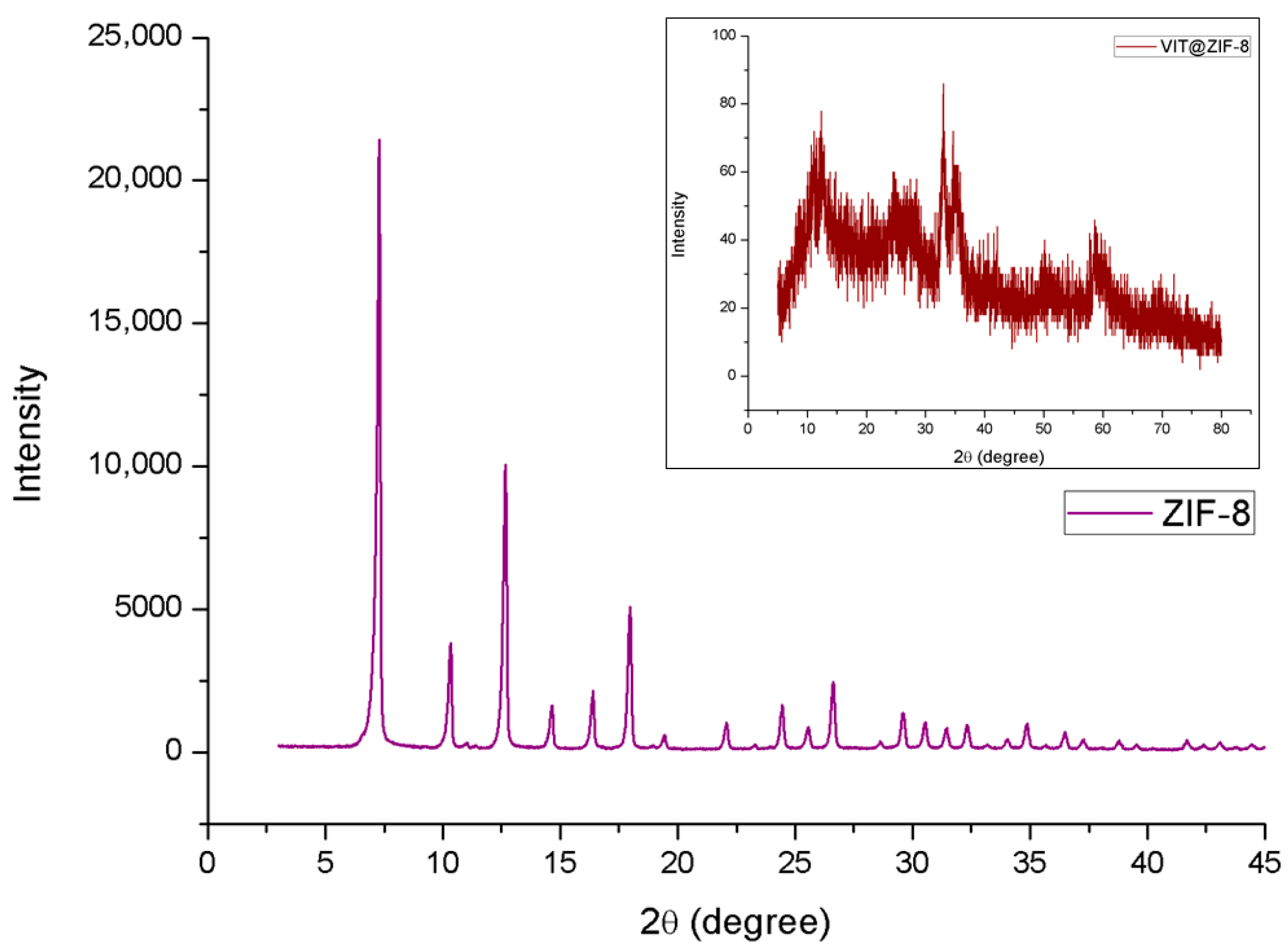
3.2.4. XDR Analysis
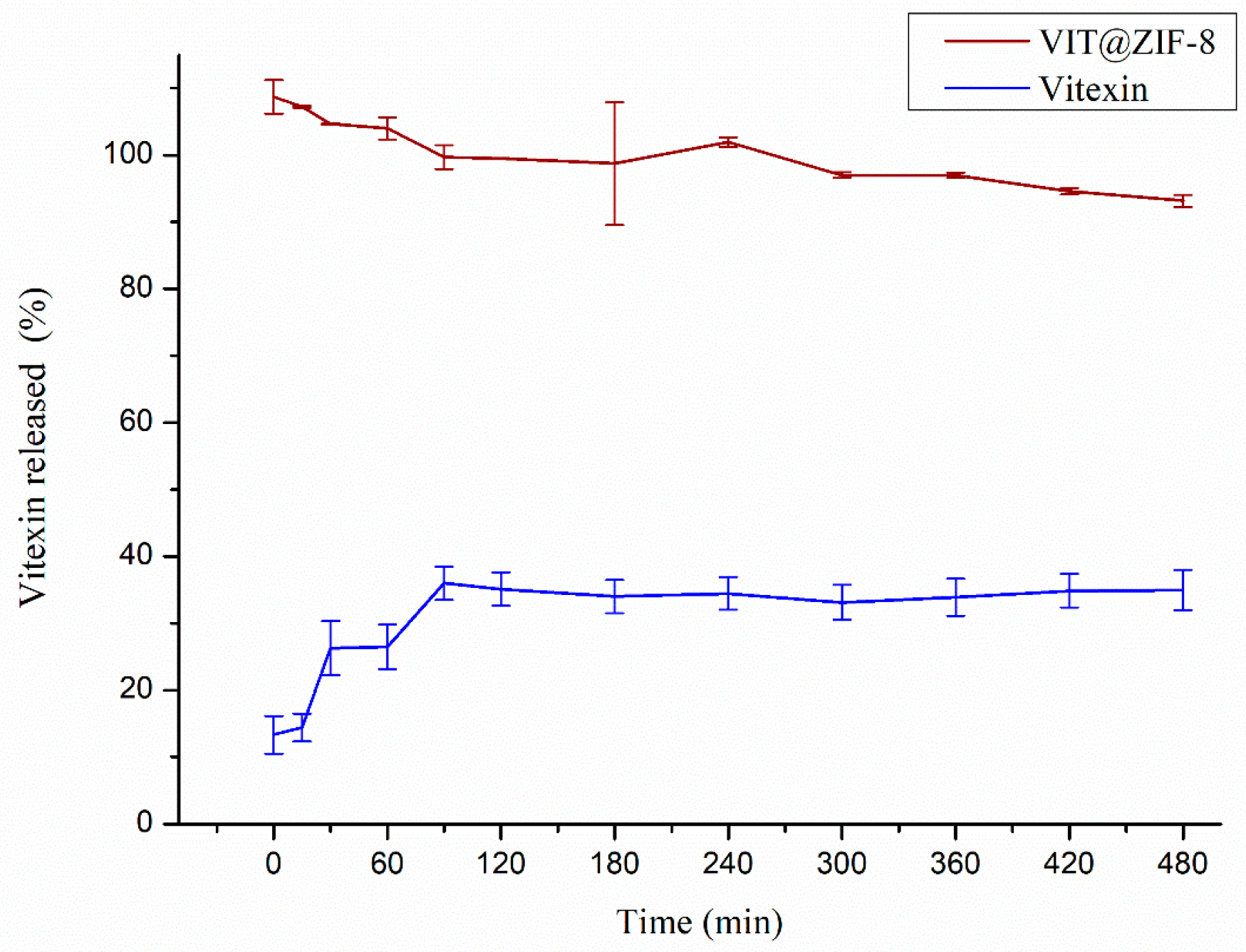
3.3. In Vitro Release
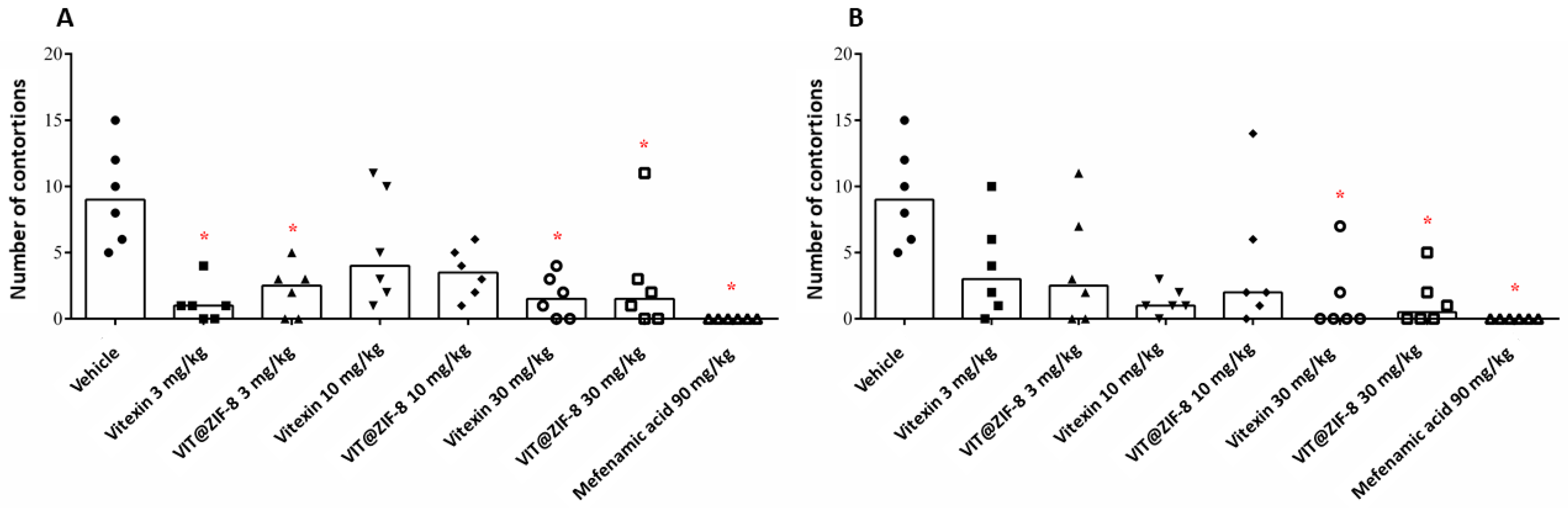
3.4. Antidysmenorrheic Activity
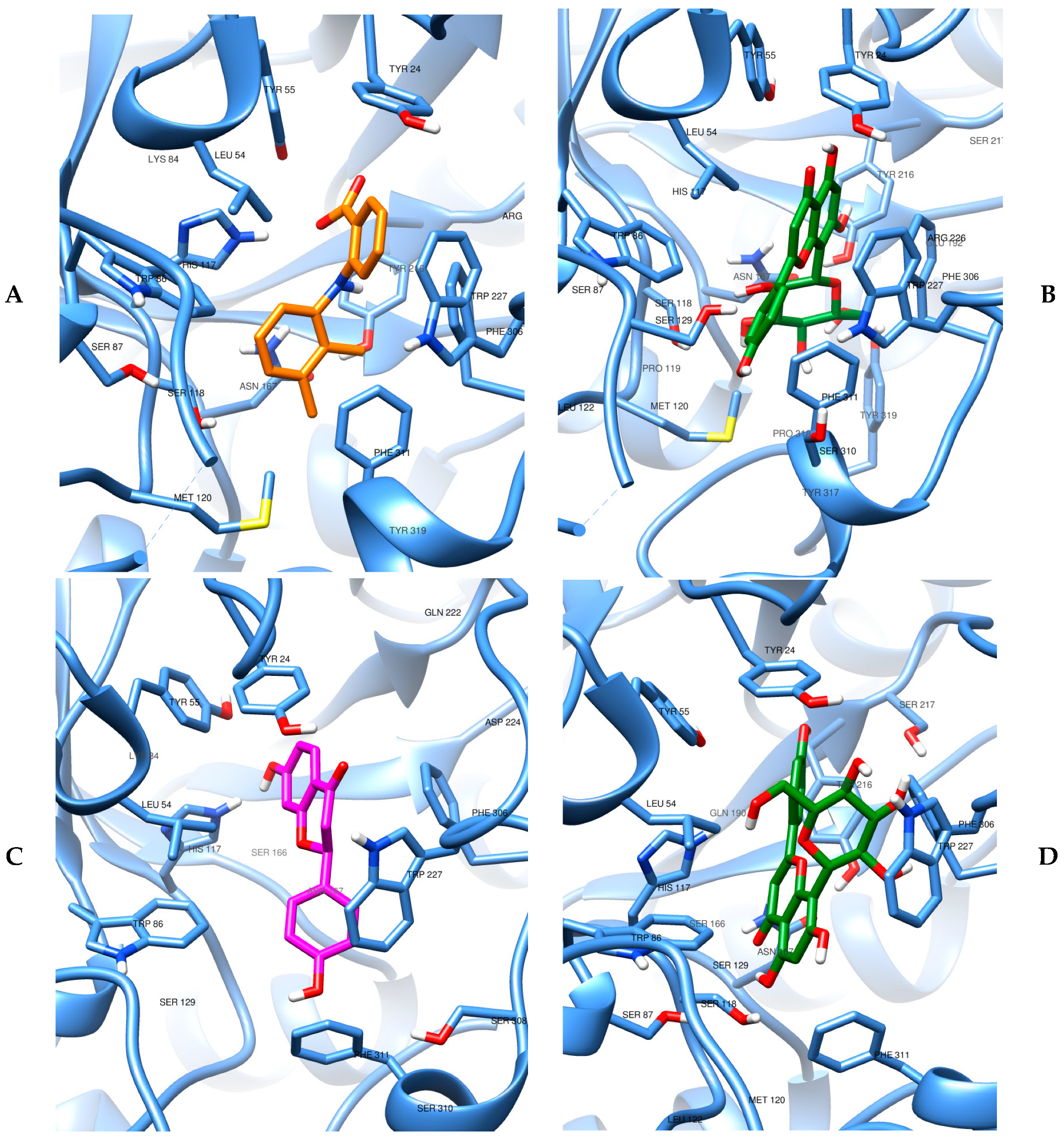
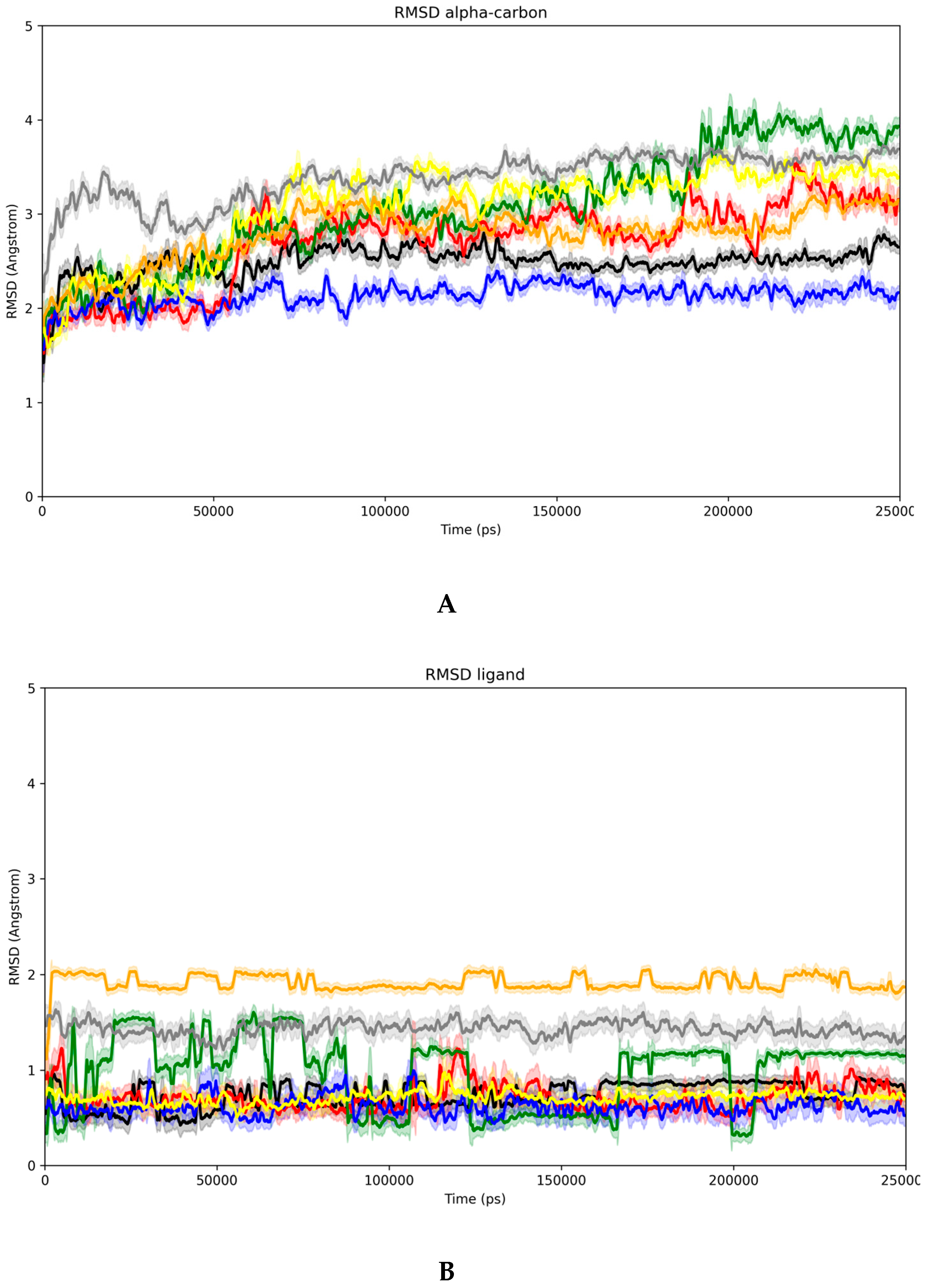
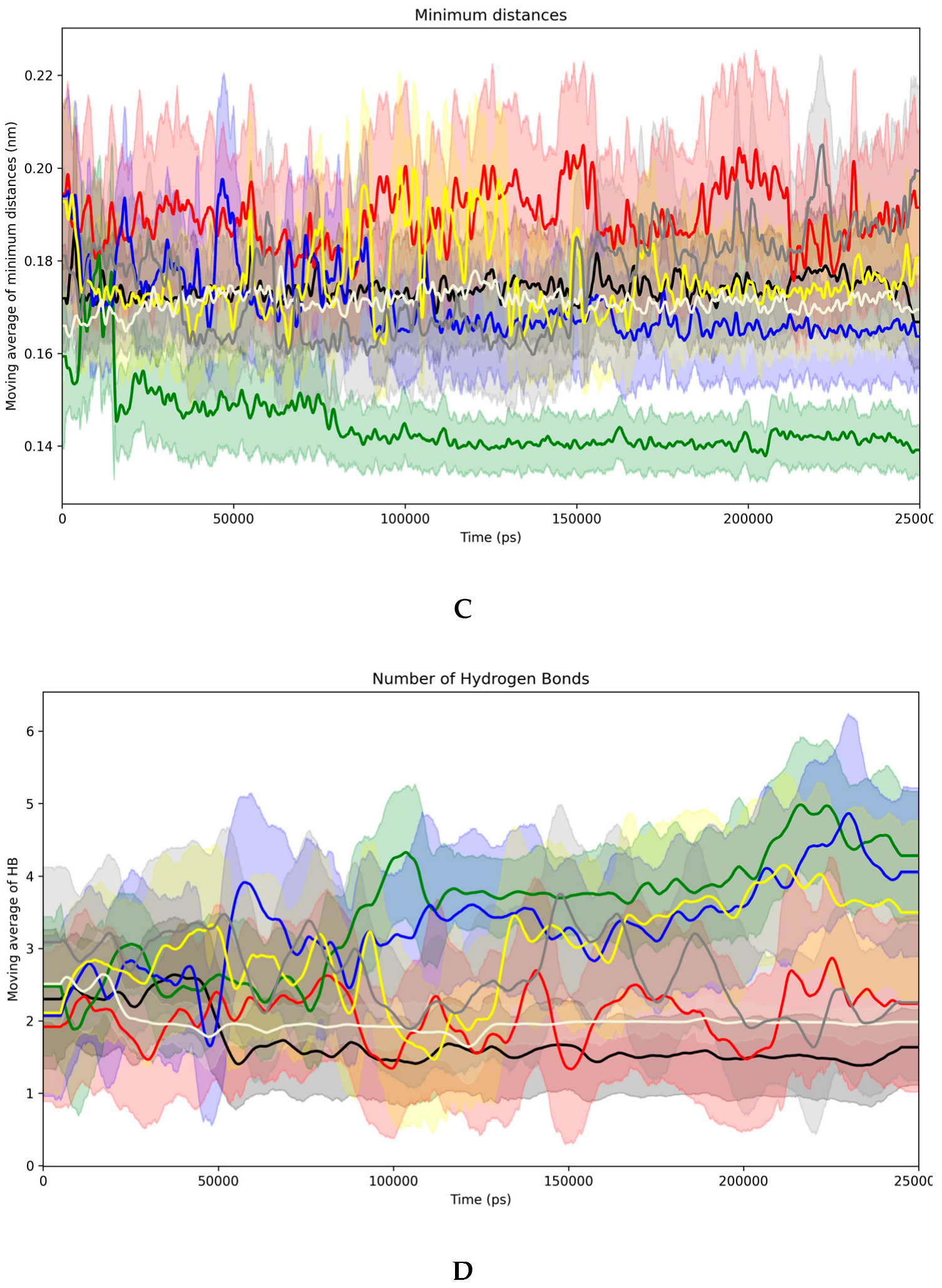
3.5. Evidence of the Mechanism of Action by Molecular Docking and Dynamics
3.6. In Silico ADME Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Zhao, W.; Li, T.; Bu, H.; Zhao, Z.; Zhao, Y.; Song, S. Effects of Acupoint-Stimulation for the Treatment of Primary Dysmenorrhoea Compared with NSAIDs: A Systematic Review and Meta-Analysis of 19 RCTs. BMC Complement. Altern. Med. 2017, 17, 436. [Google Scholar] [CrossRef]
- Kim, K.-I.; Nam, H.J.; Kim, M.; Lee, J.; Kim, K. Effects of Herbal Medicine for Dysmenorrhea Treatment on Accompanied Acne Vulgaris: A Study Protocol for a Randomized Controlled Trial. BMC Complement. Altern. Med. 2017, 17, 318. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.-Y.; Myung, C.-S.; Lee, M.S. Herbal Medicine Shaofu Zhuyu Decoction for Primary Dysmenorrhea: A Systematic Review Protocol. Syst. Rev. 2016, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.; Bernasconi, S.; Bianchin, L.; Bona, G.; Bozzola, M.; Buzi, F.; De Sanctis, C.; Tonini, G.; Rigon, F.; et al. Primary Dysmenorrhea in Adolescents: Prevalence, Impact and Recent Knowledge. Pediatr. Endocrinol. Rev. 2015, 13, 512–520. [Google Scholar] [PubMed]
- Grandi, G.; Ferrari, S.; Xholli, A.; Cannoletta, M.; Palma, F.; Volpe, A.; Romani, C.; Cagnacci, A. Prevalence of Menstrual Pain in Young Women: What Is Dysmenorrhea? J. Pain. Res. 2012, 5, 169. [Google Scholar] [CrossRef]
- Coco, A.S. Primary Dysmenorrhea. Am. Fam. Physician 1999, 60, 489–496. [Google Scholar]
- Dawood, M.Y. Dysmenorrhoea and Prostaglandins: Pharmacological and Therapeutic Considerations. Drugs 1981, 22, 42–56. [Google Scholar] [CrossRef]
- Bresson, E.; Boucher-Kovalik, S.; Chapdelaine, P.; Madore, E.; Harvey, N.; Laberge, P.Y.; Leboeuf, M.; Fortier, M.A. The Human Aldose Reductase AKR1B1 Qualifies as the Primary Prostaglandin F Synthase in the Endometrium. J. Clin. Endocrinol. Metab. 2011, 96, 210–219. [Google Scholar] [CrossRef]
- Lacroix Pepin, N.; Chapdelaine, P.; Rodriguez, Y.; Tremblay, J.-P.; Fortier, M.A. Generation of Human Endometrial Knockout Cell Lines with the CRISPR/Cas9 System Confirms the Prostaglandin F2 Synthase Activity of Aldo-Ketoreductase 1B1. Mol. Hum. Reprod. 2014, 20, 650–663. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–Ligand Molecular Docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Sharghi, M.; Mansurkhani, S.M.; Ashtary-Larky, D.; Kooti, W.; Niksefat, M.; Firoozbakht, M.; Behzadifar, M.; Azami, M.; Servatyari, K.; Jouybari, L. An Update and Systematic Review on the Treatment of Primary Dysmenorrhea. JBRA Assist. Reprod. 2019, 23, 51–57. [Google Scholar] [CrossRef]
- Song, J.-A.; Lee, M.; Min, E.; Kim, M.-E.; Fike, G.; Hur, M.-H. Effects of Aromatherapy on Dysmenorrhea: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Jo, J.; Lee, S.H. Heat Therapy for Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Its Effects on Pain Relief and Quality of Life. Sci. Rep. 2018, 8, 16252. [Google Scholar] [CrossRef]
- Bajalan, Z.; Alimoradi, Z.; Moafi, F. Nutrition as a Potential Factor of Primary Dysmenorrhea: A Systematic Review of Observational Studies. Gynecol. Obs. Investig. 2019, 84, 209–224. [Google Scholar] [CrossRef]
- Kim, S.-D. Yoga for Menstrual Pain in Primary Dysmenorrhea: A Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Clin. Pract. 2019, 36, 94–99. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.O.; Farquhar, C.; Proctor, M. Nonsteroidal Anti-Inflammatory Drugs for Dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD001751. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What We Know about Primary Dysmenorrhea Today: A Critical Review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Zhang, X.; Kim, D.S.; Park, S. Efficacy of Ginger for Alleviating the Symptoms of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Pain. Med. 2015, 16, 2243–2255. [Google Scholar] [CrossRef]
- McGovern, C.E.; Cheung, C. Yoga and Quality of Life in Women with Primary Dysmenorrhea: A Systematic Review. J. Midwifery Womens Health 2018, 63, 470–482. [Google Scholar] [CrossRef]
- Li, M.; Bi, J.; Lv, B.; Zheng, W.; Wang, Z.; Xiao, W.; Sun, Y.; Li, E. An Experimental Study of the Anti-Dysmenorrhea Effect of Chinese Herbal Medicines Used in Jin Gui Yao Lue. J. Ethnopharmacol. 2019, 245, 112181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Proctor, M.; Bensoussan, A.; Wu, E.; Smith, C.A. Chinese Herbal Medicine for Primary Dysmenorrhoea. Cochrane Database Syst. Rev. 2008, 2, CD005288. [Google Scholar] [CrossRef]
- Costa, E.C.; Menezes, P.M.N.; Silva, F.S.; de Ribeiro, L.A.A.; Rolim, L.A.; Araújo, E.C.d.C.; Nunes, X.P. Jatropha Mutabilis, a New Source of Vitexin: HPLC Quantification and Pharmacological Evaluation. Nat. Prod. Res. 2020, 35, 6200–6203. [Google Scholar] [CrossRef]
- Costa, E.C.; Menezes, P.M.N.; de Almeida, R.L.; Silva, F.S.; de Araújo Ribeiro, L.A.; de Silva, J.A.; de Oliveira, A.P.; da Cruz Araújo, E.C.; Rolim, L.A.; Nunes, X.P. Inclusion of Vitexin in β-Cyclodextrin: Preparation, Characterization and Expectorant/Antitussive Activities. Heliyon 2020, 6, e05461. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A Review on the Pharmacological Effects of Vitexin and Isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the Effects of Vitexin in Oxidative Stress-related Diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.N.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A. Vitexin Inhibits Inflammatory Pain in Mice by Targeting TRPV1, Oxidative Stress, and Cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- García-Arieta, A. Interactions between Active Pharmaceutical Ingredients and Excipients Affecting Bioavailability: Impact on Bioequivalence. Eur. J. Pharm. Sci. 2014, 65, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Aulton, M. Aulton Delineamento de Formas Farmacêuticas, 4th ed.; GEN Guanabara Koogan: Rio de Janeiro, Brasil, 2016. [Google Scholar]
- Cheng, C.; Li, C.; Zhu, X.; Han, W.; Li, J.; Lv, Y. Doxorubicin-Loaded Fe3O4-ZIF-8 Nano-Composites for Hepatocellular Carcinoma Therapy. J. Biomater. Appl. 2019, 33, 1373–1381. [Google Scholar] [CrossRef]
- de Moura Ferraz, L.R.; Tabosa, A.É.G.A.; da Silva Nascimento, D.D.S.; Ferreira, A.S.; Silva, J.Y.R.; Junior, S.A.; Rolim, L.A.; Rolim-Neto, P.J. Benznidazole in Vitro Dissolution Release from a PH-Sensitive Drug Delivery System Using Zif-8 as a Carrier. J. Mater. Sci. Mater. Med. 2021, 32, 59. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Paul, A.K.; Bharadwaj, L.M.; Deep, A.; Kim, K.-H. Biological Applications of Zinc Imidazole Framework through Protein Encapsulation. Appl. Nanosci. 2016, 6, 951–957. [Google Scholar] [CrossRef]
- Liédana, N.; Galve, A.; Rubio, C.; Téllez, C.; Coronas, J. CAF@ZIF-8: One-Step Encapsulation of Caffeine in MOF. ACS Appl. Mater. Interfaces 2012, 4, 5016–5021. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and Characterization of ZIF-8 Nanoparticles for Controlled Release of 6-Mercaptopurine Drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Ho, P.H.; Salles, F.; Di Renzo, F.; Trens, P. One-Pot Synthesis of 5-FU@ZIF-8 and Ibuprofen@ZIF-8 Nanoparticles. Inorganica Chim. Acta 2020, 500, 119229. [Google Scholar] [CrossRef]
- Sampaio, P.A.; Serafim, S.C.; Nascimento Menezes, P.M.; Valença Pereira, E.C.; Sousa de Sá, P.G.; Texeira de Alencar Filho, J.M.; Gonçalves de Oliveira Júnior, R.; Rolim Neto, P.J.; da Silva, J.A.; Rolim, L.A. Development and Characterization of the Zeolite Imidazolate Framework for a Modified Release of the Drug Scopoletin. J. Drug Deliv. Sci. Technol. 2020, 61, 102131. [Google Scholar] [CrossRef]
- de Moura Ferraz, L.R.; Tabosa, A.É.G.A.; da Silva Nascimento, D.D.S.; Ferreira, A.S.; de Albuquerque Wanderley Sales, V.; Silva, J.Y.R.; Júnior, S.A.; Rolim, L.A.; de Souza Pereira, J.J.; Rolim-Neto, P.J. ZIF-8 as a Promising Drug Delivery System for Benznidazole: Development, Characterization, in Vitro Dialysis Release and Cytotoxicity. Sci. Rep. 2020, 10, 16815. [Google Scholar] [CrossRef]
- dos Santos, M.R.; Alcaraz-Espinoza, J.J.; da Costa, M.M.; de Oliveira, H.P. Usnic Acid-Loaded Polyaniline/Polyurethane Foam Wound Dressing: Preparation and Bactericidal Activity. Mater. Sci. Eng. C 2018, 89, 33–40. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Z.; Yu, B.; Chai, C. An in Vivo Mouse Model of Primary Dysmenorrhea. Exp. Anim. 2015, 64, 295–303. [Google Scholar] [CrossRef]
- Park, K.-S.; Park, K.-I.; Hwang, D.-S.; Lee, J.-M.; Jang, J.-B.; Lee, C.-H. A Review of In Vitro and In Vivo Studies on the Efficacy of Herbal Medicines for Primary Dysmenorrhea. Evid.-Based Complement. Altern. Med. 2014, 2014, 296860. [Google Scholar] [CrossRef]
- Rižner, T.L. Enzymes of the AKR1B and AKR1C Subfamilies and Uterine Diseases. Front. Pharmacol. 2012, 3, 21348. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and Testing of the GROMOS Force-Field Versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef]
- Pullman, B. Intermolecular Forces; Pullman, B., Ed.; Springer: Dordrecht, The Netherlands, 1981; Volume 14, ISBN 978-90-481-8368-5. [Google Scholar]
- Kumari, R.; Kumar, R.; Lynn, A. G_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Carvalho, A., Jr.; Riger, C.; Castro, R.; Silva, T.; Carvalho, M. Constituintes Químicos e Atividade Antioxidante in Vivo de Flavonoides Isolados de Clusia Lanceolata (Clusiaceae). Quim Nova 2016. [Google Scholar] [CrossRef]
- Yu, X.-X.; Huang, J.-Y.; Xu, D.; Xie, Z.-Y.; Xie, Z.-S.; Xu, X.-J. Isolation and Purification of Orientin and Vitexin from Trollius Chinensis Bunge by High-Speed Counter-Current Chromatography. Nat. Prod. Res. 2014, 28, 674–676. [Google Scholar] [CrossRef]
- Choo, C.Y.; Sulong, N.Y.; Man, F.; Wong, T.W. Vitexin and Isovitexin from the Leaves of Ficus Deltoidea with In-Vivo α-Glucosidase Inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the Extraction Mode on the Yield of Hyperoside, Vitexin and Vitexin-2′′-O-rhamnoside from Crataegus Monogyna Jacq. (Hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Teixeira de Alencar Filho, J.M.; Sampaio, P.A.; Silva de Carvalho, I.; Rocha da Silva, A.; Pereira, E.C.V.; Araujo e Amariz, I.; Nishimura, R.H.V.; Cavalcante da Cruz Araújo, E.; Rolim-Neto, P.J.; Rolim, L.A. Metal Organic Frameworks (MOFs) with Therapeutic and Biomedical Applications: A Patent Review. Expert. Opin. Ther. Pat. 2021, 31, 937–949. [Google Scholar] [CrossRef]
- Bim-Júnior, O.; Gaglieri, C.; Bedran-Russo, A.K.; Bueno-Silva, B.; Bannach, G.; Frem, R.; Ximenes, V.F.; Lisboa-Filho, P.N. MOF-Based Erodible System for On-Demand Release of Bioactive Flavonoid at the Polymer–Tissue Interface. ACS Biomater. Sci. Eng. 2020, 6, 4539–4550. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, A.; Garg, N.; Randhawa, J.K. Curcumin Encapsulated Zeolitic Imidazolate Frameworks as Stimuli Responsive Drug Delivery System and Their Interaction with Biomimetic Environment. Sci. Rep. 2017, 7, 12598. [Google Scholar] [CrossRef]
- Freire, R.V.M.; de Dias, D.C.A.; Silva, J.Y.R.; Santos, D.K.D.d.N.; Jesus, L.T.; Freire, R.O.; Junior, S.A. Experimental and Theoretical Investigation of Phytochemical Euphol Incorporated in ZIF-8 as a Drug Delivery System for Cancer Treatment. Mater. Chem. Phys. 2024, 312, 128648. [Google Scholar] [CrossRef]
- Pavia, D.; Lampman, G.; Kriz, G.; Vyvyan, J. Introdução à Espectroscopia, 2nd ed.; Cengage Learning: São Paulo, Brasil, 2015. [Google Scholar]
- Silverstein, R.; Webster, F.; Kiemle, D.; Bryce, D. Identificação Espectrométrica de Compostos Orgânicos, 8th ed.; LTC: Rio de Janeiro, Brasil, 2019. [Google Scholar]
- Ta, D.N.; Nguyen, H.K.D.; Trinh, B.X.; Le, Q.T.N.; Ta, H.N.; Nguyen, H.T. Preparation of Nano-ZIF-8 in Methanol with High Yield. Can. J. Chem. Eng. 2018, 96, 1518–1531. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X.; et al. Drug Nanoclusters Formed in Confined Nano-Cages of CD-MOF: Dramatic Enhancement of Solubility and Bioavailability of Azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc−Adeninate Metal−Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, T.R.; dos Santos, T.V.; da Viana, R.S.; Plentz Meneghetti, S.M.; do Espírito Santo, C.D.A. Study of the Morphological, Structural and Photophysical Properties of Dual Emission Europium-Doped ZIF-8 Particles. Opt. Mater. 2021, 111, 110581. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A Combined Experimental-Computational Investigation on Water Adsorption in Various ZIFs with the SOD and RHO Topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef]
- Şahin, F.; Topuz, B.; Kalıpçılar, H. Synthesis of ZIF-7, ZIF-8, ZIF-67 and ZIF-L from Recycled Mother Liquors. Microporous Mesoporous Mater. 2018, 261, 259–267. [Google Scholar] [CrossRef]
- James, J.B.; Lin, Y.S. Kinetics of ZIF-8 Thermal Decomposition in Inert, Oxidizing, and Reducing Environments. J. Phys. Chem. C 2016, 120, 14015–14026. [Google Scholar] [CrossRef]
- Rostamkhani, N.; Salimi, M.; Adibifar, A.; Karami, Z.; Agh-Atabay, A.-H.; Rostamizadeh, K.; Abdi, Z. Enhanced Anti-Tumor and Anti-Metastatic Activity of Quercetin Using PH-Sensitive Alginate@ZIF-8 Nanocomposites: In Vitro and in Vivo Study. Nanotechnology 2024, 35, 475102. [Google Scholar] [CrossRef]
- Kan, T.; Tian, Z.; Sun, L.; Kong, W.; Yan, R.; Yu, Z.; Tian, Q.-W.; Liu, C. Quercetin-Loaded Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles Attenuate Osteoarthritis by Activating Autophagy via the Pi3k/Akt Signaling. ACS Appl. Mater. Interfaces 2024, 16, 40444–40454. [Google Scholar] [CrossRef]
- Rahman, M.; Kabir, M.; Islam, T.; Wang, Y.; Meng, Q.; Liu, H.; Chen, S.; Wu, S. Curcumin-Loaded ZIF-8 Nanomaterials: Exploring Drug Loading Efficiency and Biomedical Performance. ACS Omega 2025, 10, 3067–3079. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Guan, J.; Lai, S.; Fang, L.; Su, J. PH-Responsive Curcumin-Based Nanoscale ZIF-8 Combining Chemophotodynamic Therapy for Excellent Antibacterial Activity. RSC Adv. 2022, 12, 10005–10013. [Google Scholar] [CrossRef]
- Zhang, H.; James, J.; Zhao, M.; Yao, Y.; Zhang, Y.; Zhang, B.; Lin, Y.S. Improving Hydrostability of ZIF-8 Membranes via Surface Ligand Exchange. J. Memb. Sci. 2017, 532, 1–8. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, H.; Yan, Y. Preparation and Characterization of a Novel ZIF-8 Membrane over High Voidage Paper-like Stainless Steel Fibers. J. Solid. State Chem. 2019, 269, 203–211. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Liu, Z.; Zhu, J.; Ma, Z.; Cui, Q.; Wang, H. Pore Structure Modification of ZIF-8 by Ligand Exchange for Separation Mono- and Di-Branched Isomers of Hexane via Thermodynamic and Kinetic Mechanism. Sep. Purif. Technol. 2023, 320, 124241. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Hu, C.; Xu, J.; Wang, Y.; Wei, M.; Lu, Z.; Cao, C. Core–Shell Crystalline ZIF-67@amorphous ZIF for High-Performance Supercapacitors. J. Mater. Sci. 2020, 55, 16360–16373. [Google Scholar] [CrossRef]
- Sá, P.; Souza, N.; Sampaio, P.; Silva, J.; Rolim, L. ZIF-8 as a Drug Delivery System (DDS) for Hesperidin: Synthesis, Characterization, and In Vitro Release Profile. Ceramics 2025, 8, 113. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, M.; Huang, Y.; Chen, L.; Fang, J.; Liu, J.; Wang, M.; Zhao, C. β-Cyclodextrin Metal-Organic Framework as a Green Carrier to Improve the Dissolution, Bioavailability, and Liver Protective Effect of Luteolin. Int. J. Pharm. X 2024, 7, 100250. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, P.; Zeinali, S.; Rastegari, B.; Najafipour, I. Functionalization of β-Cyclodextrin Metal-Organic Frameworks with Gelatin and Glutamine for Drug Delivery of Curcumin to Cancerous Cells. Heliyon 2024, 10, e30349. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid Dispersions as Strategy to Improve Oral Bioavailability of Poor Water Soluble Drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Cocrystals: Along the Path to Improved Medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Matzger, A.J. Enhanced Drug Delivery by Dissolution of Amorphous Drug Encapsulated in a Water Unstable Metal–Organic Framework (MOF). Angew. Chem. Int. Ed. 2019, 58, 16790–16794. [Google Scholar] [CrossRef]
- DELIGEOROGLOU, E. Dysmenorrhea. Ann. N. Y. Acad. Sci. 2006, 900, 237–244. [Google Scholar] [CrossRef]
- Dawood, M.Y. Primary Dysmenorrhea. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef]
- Åkerlund, M. Pathophysiology of Dysmenorrhea. Acta Obs. Gynecol. Scand. 1979, 58, 27–32. [Google Scholar] [CrossRef]
- Wu, C.-H.; Shieh, T.-M.; Wang, K.-L.; Huang, T.-C.; Hsia, S.-M. Quercetin, a Main Flavonoid in Onion, Inhibits the PGF2α-Induced Uterine Contraction in Vitro and in Vivo. J. Funct. Foods 2015, 19, 495–504. [Google Scholar] [CrossRef]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary Phytophenols Curcumin, Naringenin and Apigenin Reduce Infection-Induced Inflammatory and Contractile Pathways in Human Placenta, Foetal Membranes and Myometrium. MHR Basic Sci. Reprod. Med. 2013, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-C.; Yang, L.-L. Relaxant Effect Induced by Wogonin from Scutellaria Baicalensis on Rat Isolated Uterine Smooth Muscle. Pharm. Biol. 2012, 50, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.-R.; Ivell, R.; Ganz, N.; Fields, M.J.; Gimenez, T. Secretion of Oxytocin in Pregnant and Parturient Cows: Corpus Luteum May Contribute to Plasma Oxytocin at Term1. Biol. Reprod. 2001, 65, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Togashi, K.; Kido, A.; Nakai, A.; Fujiwara, T.; Koyama, T.; Fujii, S. Dysmenorrhea: Evaluation with Cine-Mode-Display MR Imaging—Initial Experience. Radiology 2005, 235, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.R.; Vidal, F.A.P. Transdução de Sinais: Uma Revisão Sobre Proteína G. Sci Med. 2011, 21, 31–36. [Google Scholar]
- Saei Ghare Naz, M.; Kiani, Z.; Rashidi Fakari, F.; Ghasemi, V.; Abed, M.; Ozgoli, G. The Effect of Micronutrients on Pain Management of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. J. Caring Sci. 2020, 9, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef]
- Dawood, M.Y.; Khan-Dawood, F.S. Differential Suppression of Menstrual Fluid Prostaglandin F2a, Prostaglandin E2, 6-Keto Prostaglandin F1a and Thromboxane B2 by Suprofen in Women with Primary Dysmenorrhea. Prostaglandins Other Lipid Mediat. 2007, 83, 146–153. [Google Scholar] [CrossRef]


| Independent Variables | Levels | |
|---|---|---|
| −1 | +1 | |
| Molar proportion between vitexin and reagents (Vitexin:Zn:2-MeIm) | 0.5:10:20 | 1:10:20 |
| Reaction time | 12 h | 24 h |
| Reaction Condition | Molar Proportion Between Vitexin and Reagents | Reaction Time |
|---|---|---|
| 1 | −1 (vit 4 μM) | −1 (12 h) |
| 2 | −1 (vit 4 μM) | +1 (24 h) |
| 3 | +1 (vit 8 μM) | −1 (12 h) |
| 4 | +1 (vit 8 μM) | +1 (24 h) |
| Reaction Condition | Reaction Yield (%) | Loading Capacity (%) |
|---|---|---|
| 1 (−1, −1) | 7.33 | 1.80 ± 0.01 |
| 2 (−1, +1) | 18.73 | 1.91 ± 0.01 |
| 3 (+1, −1) | 26.94 | 4.75 ± 0.015 |
| 4 (+1, +1) | 29.78 | 13.02 ± 0.1 |
| Sample | 1st Event | 2nd Event | 3rd Event | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Onset (°C) | Endset (°C) | Weight loss (%) | Onset (°C) | Endset (°C) | Weight Loss (%) | Onset (°C) | Endset (°C) | Weight Loss (%) | |
| Vitexin | 15.98 | 119.04 | 0.5 | 249.12 | 257.58 | 6.57 | 264.13 | 283.27 | 9.43 |
| ZIF-8 | 33.79 | 72.00 | 1.34 | 181.69 | 320.33 | 8.42 | 446.47 | 554.79 | 16.94 |
| VIT@ZIF-8 | 43.04 | 85.04 | 6.37 | 193.53 | 226.81 | 4.68 | 419.27 | 550.56 | 10.77 |
| Complex | RMSD (Å) | Redocking Energy (Kcal/mol) | BE * with Vitexin (Kcal/mol) |
|---|---|---|---|
| 3R43 (AKR1C3 with mefenamic acid) | 0.348 | −9.1 | −8.9 |
| 8JP1 (AKR1C3 with a flavonoid) | 0.486 | −8.2 | −8.6 |
| 5IKR (COX-2 with mefenamic acid) | 1.848 | −9.1 | −5.9 |
| Energy | AKR1C3/MFA | AKR1C3/VTX | AKR1C3/FLV | AKR1C3/VBE | AKR1C3/VBG | COX-2/ MFA | COX-2/ VTX |
|---|---|---|---|---|---|---|---|
| Binding energy | −131.375 ± 21.313 | −132.220 ± 14.985 | −42.005 ± 18.369 | −111.644 ± 18.416 | −95.586 ± 16.440 | −49.112 ± 68.874 | −18.785 ± 87.254 |
| Van der Waals | −128.159 ± 11.871 | −202.753 ± 12.809 | −89.788 ± 18.554 | −215.007 ± 13.086 | −214.191 ± 10.119 | −45.259 ± 65.358 | −67.208 ± 98.803 |
| Electrostatic | −342.275 ± 29.052 | −28.896 ± 9.564 | −193.187 ± 17.586 | −57.177 ± 11.189 | −63.589 ± 10.840 | −55.516 ± 70.586 | −7.582 ± 12.081 |
| Polar solvation | 354.146 ± 28.074 | 121.333 ± 11.011 | 256.122 ± 17.874 | 182.100 ± 15.582 | 204.050 ± 15.537 | 56.810 ± 93.724 | 63.874 ± 46.987 |
| SASA | −15.087 ± 0.731 | −21.904 ± 1.125 | −15.152 ± 0.737 | −21.560 ± 0.863 | −21.856 ± 1.074 | −5.148 ± 7.636 | −7.870 ± 11.035 |
| Category | Parameter | Obtained Value | Interpretation |
|---|---|---|---|
| Physicochemical | Molecular weight (g/mol) | 432.38 | Moderately high; within acceptable range, but may reduce permeability. |
| TPSA (Å2) | 181.05 | Very high; TPSA > 140 indicates low intestinal and BBB permeability. | |
| H-bond donors | 7 | Exceeds Lipinski’s limit (≤5); may reduce oral absorption. | |
| H-bond acceptors | 10 | At upper limit; contributes to solubility. | |
| Rotatable bonds | 3 | Low flexibility; contributes to conformational stability. | |
| Lipophilicity | Log P (consensus) | −0.02 | Neutral/hydrophilic; high solubility but limited membrane permeability. |
| Solubility (Log S) | ESOL: −2.84; Ali: −3.57; SILICOS-IT: −2.38 | Soluble | Good aqueous solubility. |
| Pharmacokinetics | GI absorption | Low | Consistent with high polarity and high TPSA. |
| BBB permeation | No | Expected for polar, polyphenolic compounds. | |
| P-gp substrate | No | Low risk of active efflux, but overall absorption still limited. | |
| CYP450 inhibition | No (1A2, 2C19, 2C9, 2D6, 3A4) | Low potential for metabolic drug–drug interactions. | |
| Log Kp (cm/s) | −8.79 | Indicates low skin permeability. | |
| Drug-likeness | Lipinski | 1 violation (H-donors > 5) | Moderate compliance with drug-like profile. |
| Veber | 1 violation (TPSA > 140) | Reduced oral absorption expected. | |
| Bioavailability score | 0.55 | Moderate; suggests limited bioavailability. | |
| Medicinal Chemistry | PAINS / Brenk alerts | 0 | No structural alerts for assay interference. |
| Synthetic accessibility | 5.12 | Moderately complex synthesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Alencar Filho, J.M.T.; França, A.R.d.S.; da Silva, L.B.R.; Sampaio, P.A.; Pereira, E.C.V.; da Silva, A.R.; Alencar, M.V.V.d.O.; Araújo, T.C.d.L.; Menezes, P.M.N.; Costa, S.P.M.; et al. Vitexin as a Potential Antidysmenorrheic Agent: Development of a ZIF-8-Based Immediate-Release System and Evaluation via In Vivo and In Silico Approaches. Biomedicines 2025, 13, 2602. https://doi.org/10.3390/biomedicines13112602
de Alencar Filho JMT, França ARdS, da Silva LBR, Sampaio PA, Pereira ECV, da Silva AR, Alencar MVVdO, Araújo TCdL, Menezes PMN, Costa SPM, et al. Vitexin as a Potential Antidysmenorrheic Agent: Development of a ZIF-8-Based Immediate-Release System and Evaluation via In Vivo and In Silico Approaches. Biomedicines. 2025; 13(11):2602. https://doi.org/10.3390/biomedicines13112602
Chicago/Turabian Stylede Alencar Filho, José Marcos Teixeira, Ana Rita de Sousa França, Luana Beatriz Rocha da Silva, Pedrita Alves Sampaio, Emanuella Chiara Valença Pereira, Ademar Rocha da Silva, Milenna Victória Valentim de Oliveira Alencar, Tarcísio Cícero de Lima Araújo, Pedro Modesto Nascimento Menezes, Salvana Priscylla Manso Costa, and et al. 2025. "Vitexin as a Potential Antidysmenorrheic Agent: Development of a ZIF-8-Based Immediate-Release System and Evaluation via In Vivo and In Silico Approaches" Biomedicines 13, no. 11: 2602. https://doi.org/10.3390/biomedicines13112602
APA Stylede Alencar Filho, J. M. T., França, A. R. d. S., da Silva, L. B. R., Sampaio, P. A., Pereira, E. C. V., da Silva, A. R., Alencar, M. V. V. d. O., Araújo, T. C. d. L., Menezes, P. M. N., Costa, S. P. M., Barreto, I. C., Silva, F. S., Araújo, E. C. d. C., de Alencar Filho, E. B., & Rolim, L. A. (2025). Vitexin as a Potential Antidysmenorrheic Agent: Development of a ZIF-8-Based Immediate-Release System and Evaluation via In Vivo and In Silico Approaches. Biomedicines, 13(11), 2602. https://doi.org/10.3390/biomedicines13112602







