Beyond Biomarkers: Blending Copeptin and Clinical Cues to Distinguish Central Diabetes Insipidus from Primary Polydipsia in Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population and Inclusion Criteria
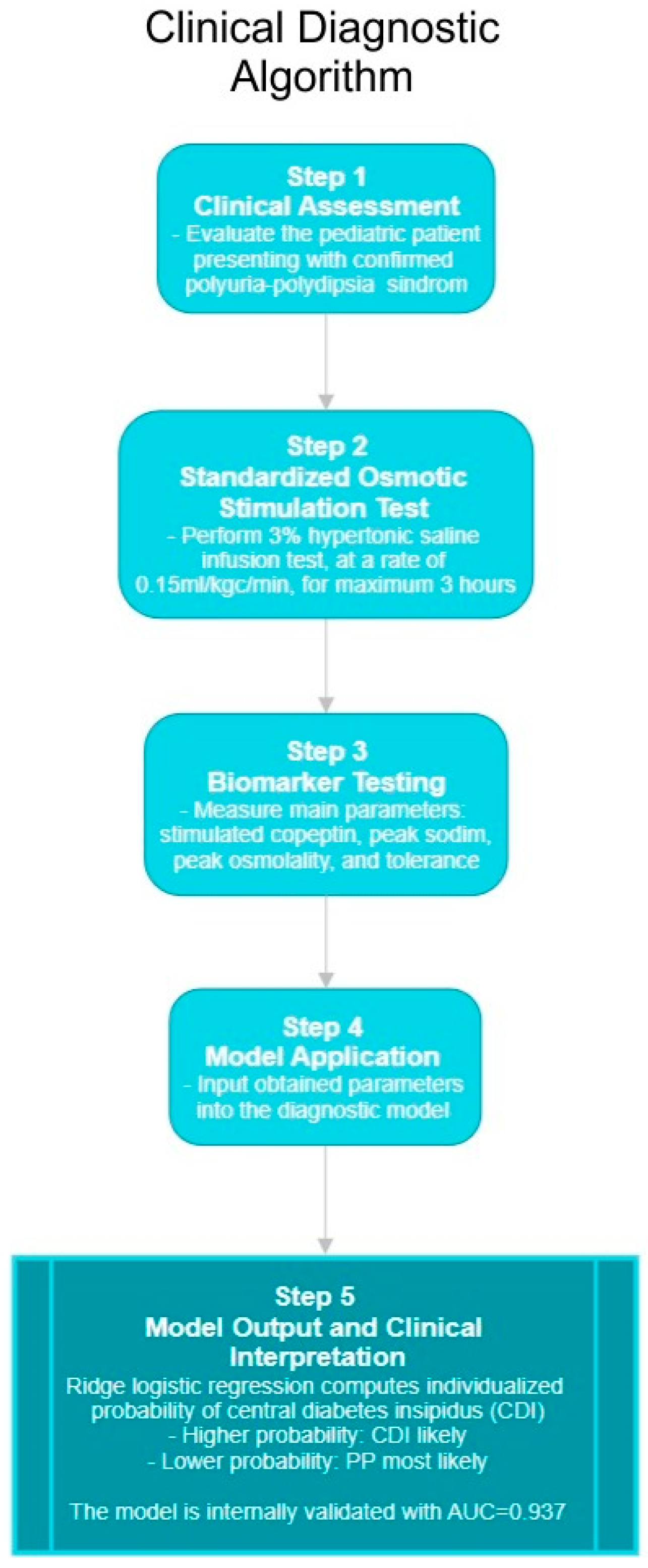
2.3. Diagnostic Protocol and Biomarker Testing
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Comparative Analysis
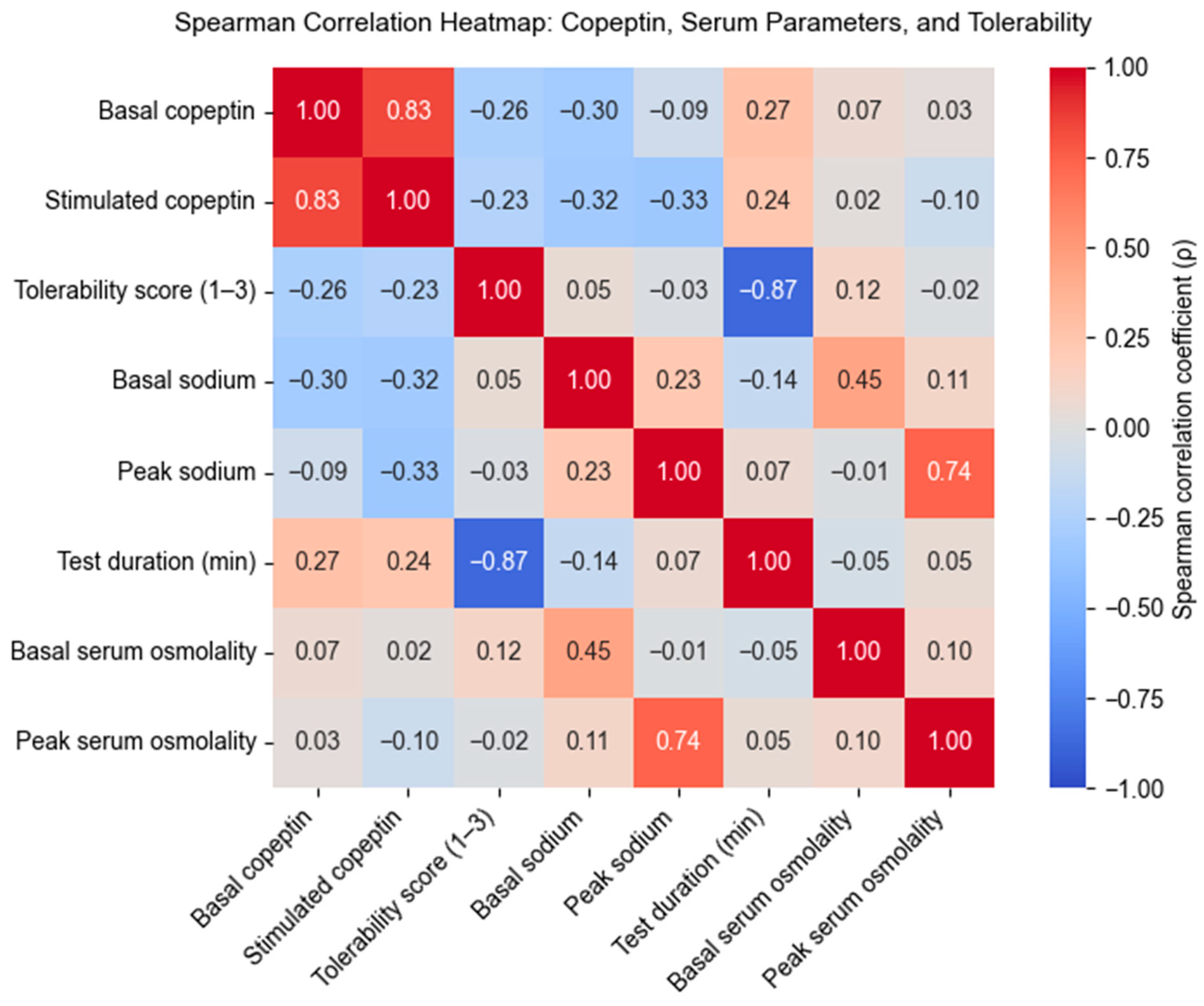
Correlation Analysis Between Copeptin Levels, Serum Parameters, and Test Tolerability
3.2. Multicollinearity Assessment and Predictor Selection
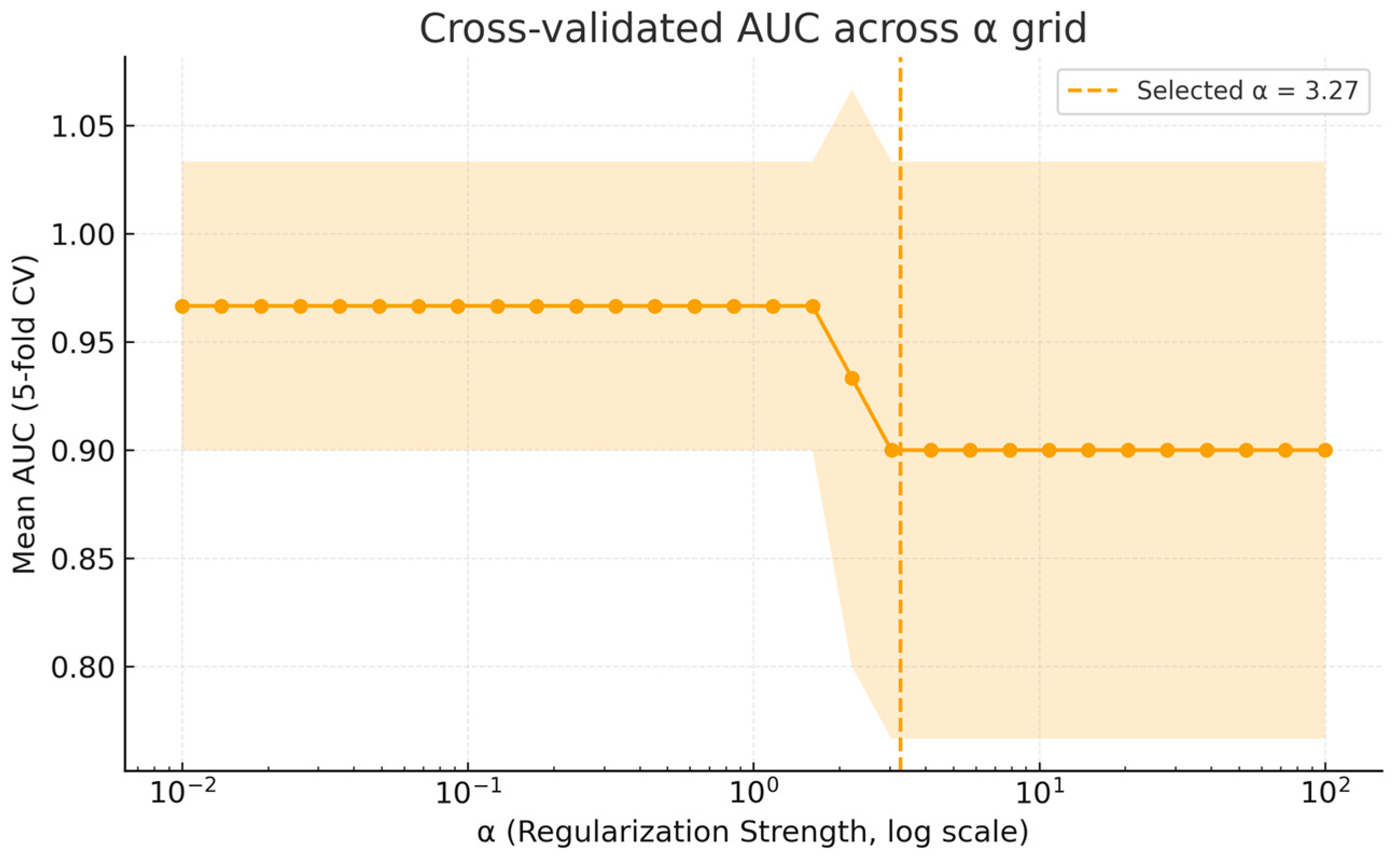
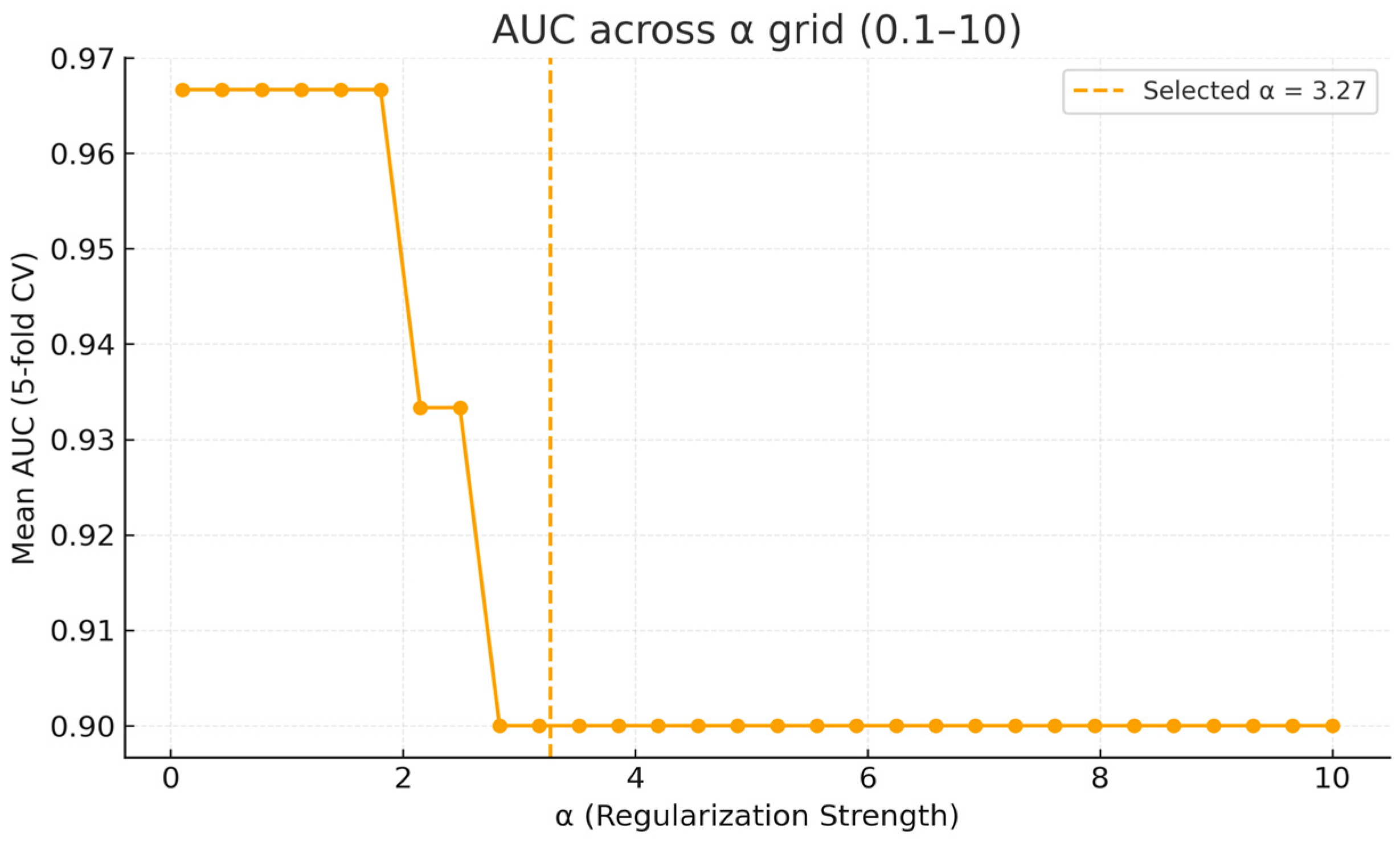
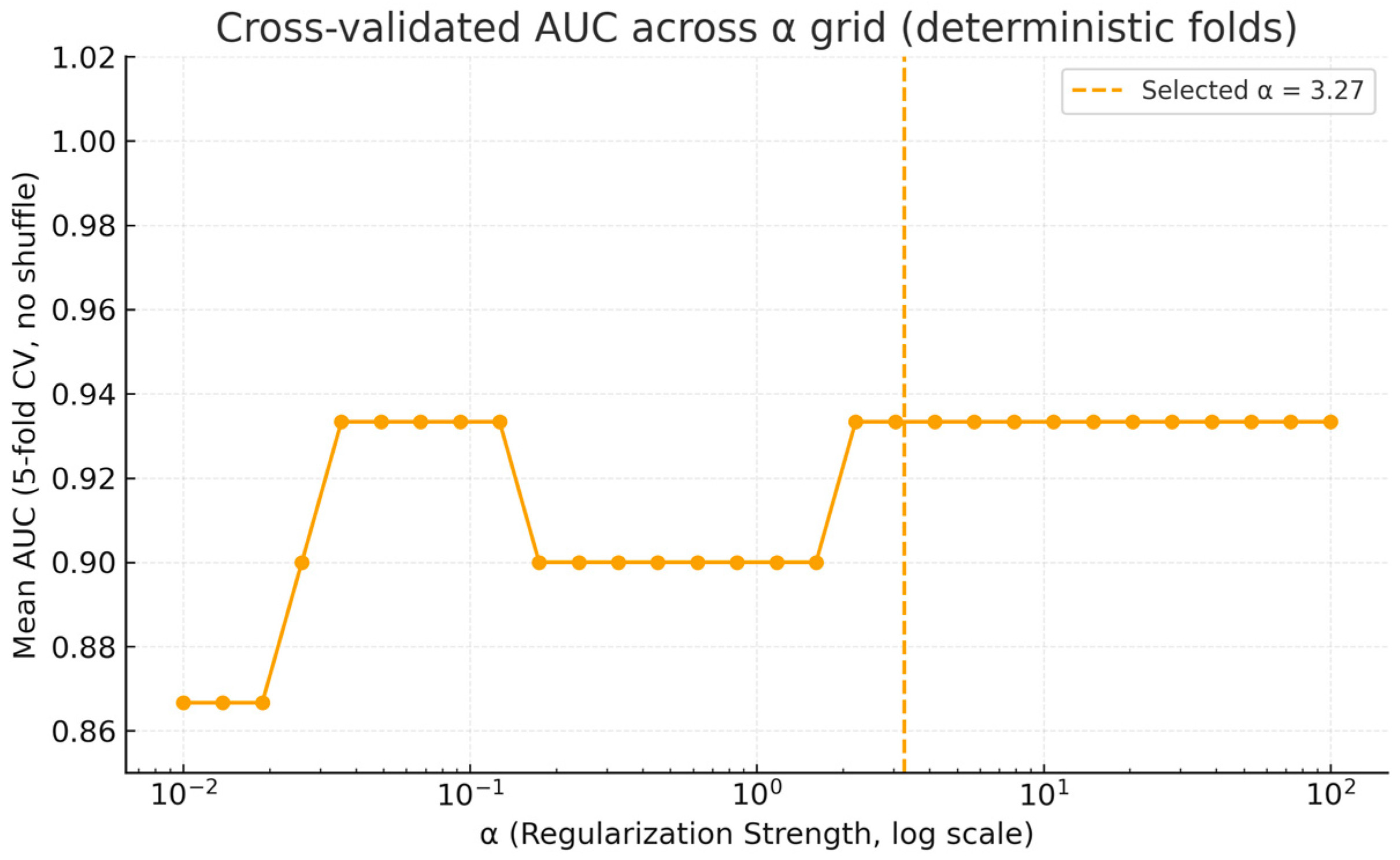
3.3. Ridge Logistic Regression Modeling and Internal Validation
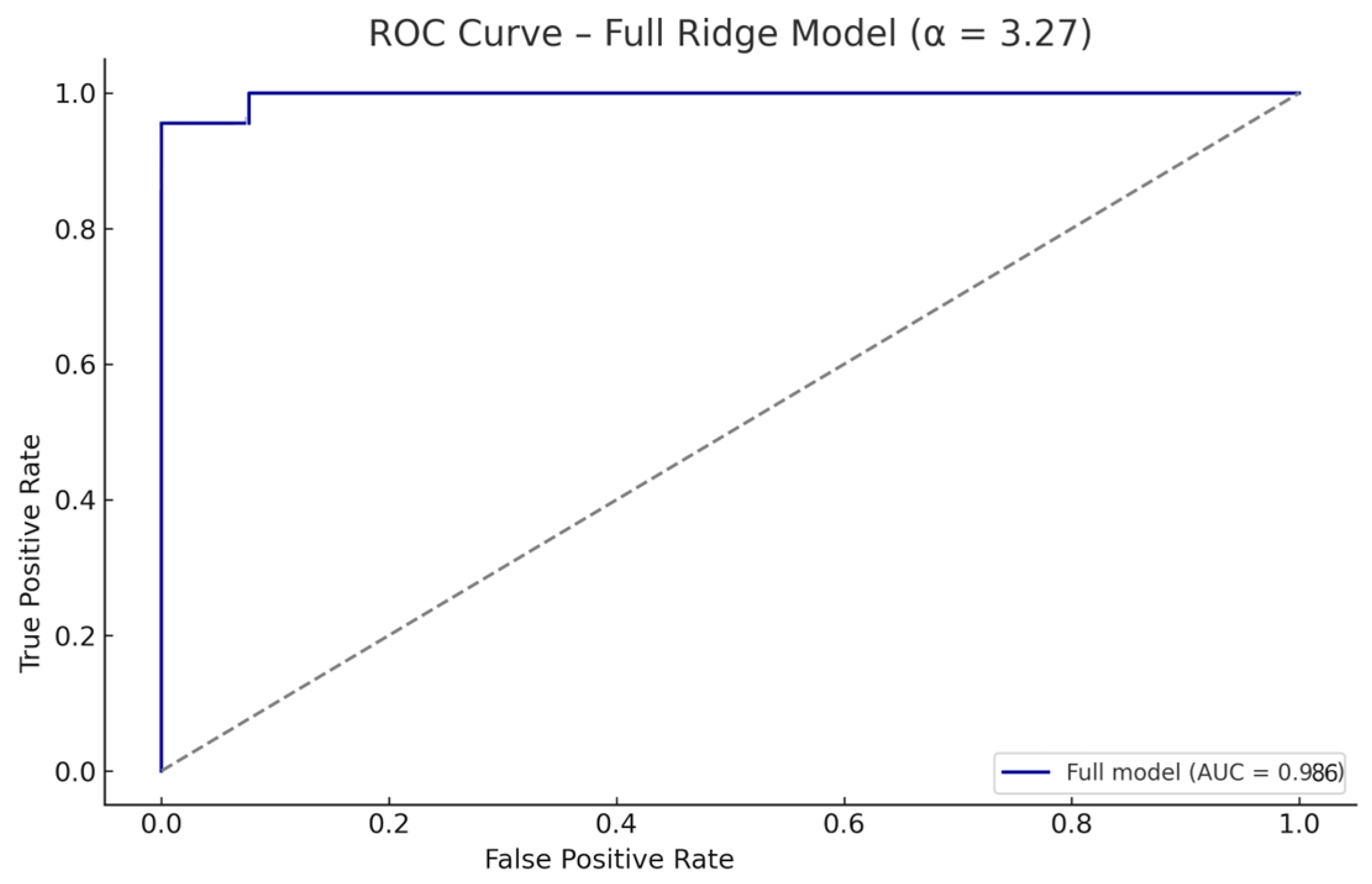
3.3.1. Full-Sample Performance
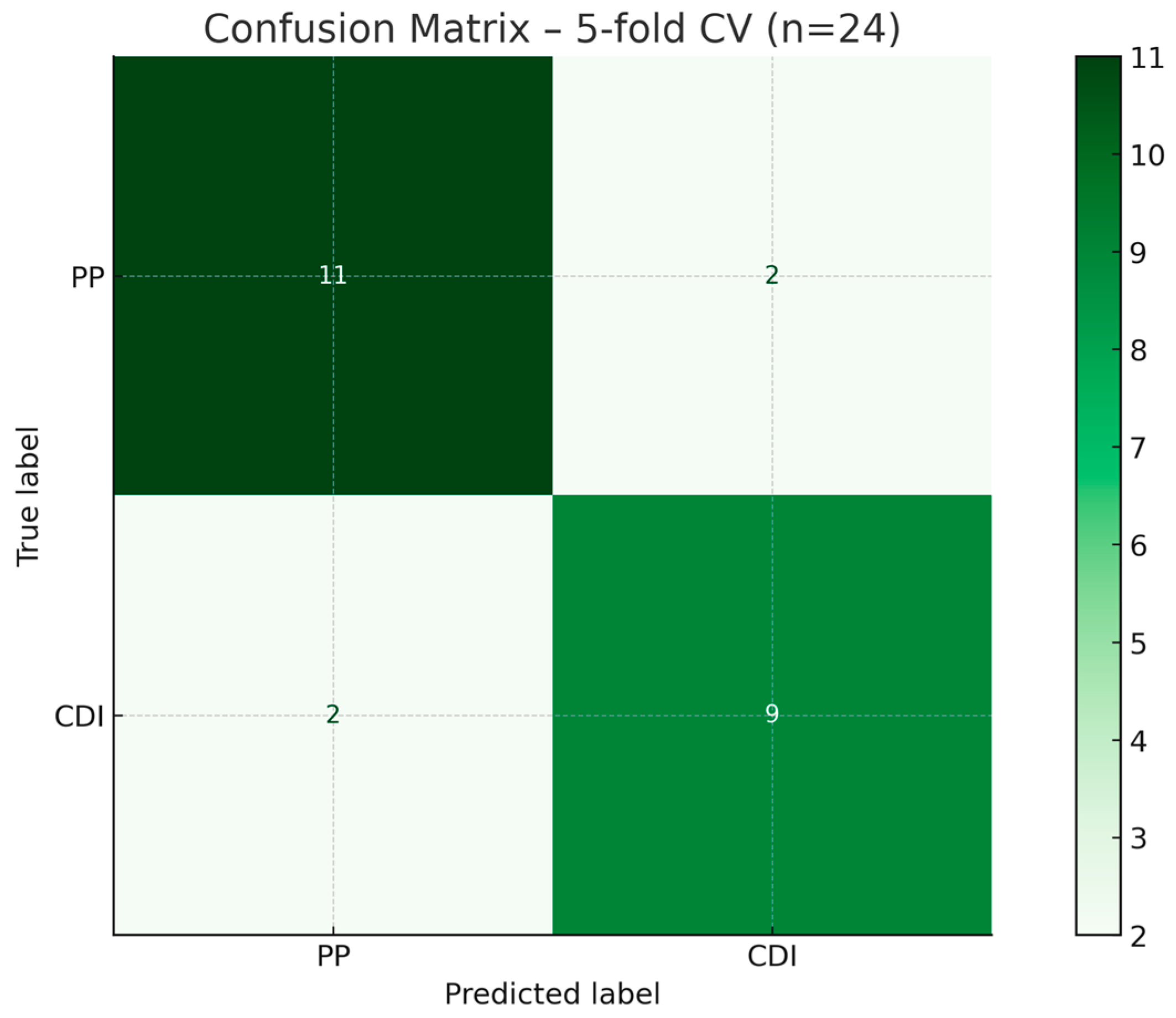
3.3.2. Internal Validation via Stratified Cross-Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AVP | Arginine Vasopressin |
| AUC | Area Under the Curve |
| CDI | Central Diabetes Insipidus |
| EDTA | Ethylenediaminetetraacetic Acid |
| FIA | Fluorescence Immunoassay |
| GDPR | General Data Protection Regulation |
| NDI | Nephrogenic Diabetes Insipidus |
| PP | Primary Polydipsia |
| PPS | Polyuria–Polydipsia Syndrome |
| ROC | Receiver Operating Characteristic |
| STARD | Standards for Reporting Diagnostic Accuracy Studies |
| TRPV1/4 | Transient Receptor Potential Vanilloid 1/4 |
| VIF | Variance Inflation Factor |
| WDT | Water Deprivation Test |
Appendix A. Supplementary Analyses of Model Calibration and Regularization Stability
References
- Flynn, K.; Hatfield, J.; Brown, K.; Vietor, N.; Hoang, T. Central and Nephrogenic Diabetes Insipidus: Updates on Diagnosis and Management. Front. Endocrinol. 2025, 15, 1479764. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandri-Silva, C.; Carpenter, M.; Ayoob, R.; Barcia, J.; Chishti, A.; Constantinescu, A.; Dell, K.M.; Goodwin, J.; Hashmat, S.; Iragorri, S.; et al. Diagnosis, Treatment, and Outcomes in Children With Congenital Nephrogenic Diabetes Insipidus: A Pediatric Nephrology Research Consortium Study. Front. Pediatr. 2020, 7, 550. [Google Scholar] [CrossRef]
- Levtchenko, E.; Ariceta, G.; Arguedas Flores, O.; Bichet, D.G.; Bockenhauer, D.; Emma, F.; Hoorn, E.J.; Koster-Kamphuis, L.; Nijenhuis, T.; Trepiccione, F.; et al. International Expert Consensus Statement on the Diagnosis and Management of Congenital Nephrogenic Diabetes Insipidus (Arginine Vasopressin Resistance). Nat. Rev. Nephrol. 2025, 21, 83–96. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Gaisl, O. Diabetes Insipidus. Presse Med. 2021, 50, 104093. [Google Scholar] [CrossRef]
- Kitamura, M.; Nishioka, J.; Matsumoto, T.; Umino, S.; Kawano, A.; Saiki, R.; Tanaka, Y.; Yatsuga, S. Estimate Incidence and Predictive Factors of Pediatric Central Diabetes Insipidus in a Single-Institute Study. Endocr. Metab. Sci. 2022, 7–8, 100119. [Google Scholar] [CrossRef]
- Mutter, C.M.; Smith, T.; Menze, O.; Zakharia, M.; Nguyen, H. Diabetes Insipidus: Pathogenesis, Diagnosis, and Clinical Management. Cureus 2021, 13, e13523. [Google Scholar] [CrossRef]
- Maghnie, M.; Cosi, G.; Genovese, E.; Manca-Bitti, M.L.; Cohen, A.; Zecca, S.; Tinelli, C.; Gallucci, M.; Bernasconi, S.; Boscherini, B.; et al. Central Diabetes Insipidus in Children and Young Adults. N. Engl. J. Med. 2000, 343, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, J.; Yao, Y.; Zhu, H.; Deng, K.; Lu, L.; Zhang, Y.; Duan, L.; Wang, L.; Yang, H.; et al. Clinical and Pathological Features of 124 Patients with Indistinguishable Sellar Lesions and Central Diabetes Insipidus. J. Clin. Neurosci. 2020, 80, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, L.L.I.; Kaminsky, E.; Kienitz, T.; Quinkler, M. Central Diabetes Insipidus Caused by Arginine Vasopressin Gene Mutation: Report of a Novel Mutation and Review of Literature. Horm. Metab. Res. 2020, 52, 796–802. [Google Scholar] [CrossRef]
- Bockenhauer, D.; Bichet, D.G. Nephrogenic Diabetes Insipidus. Curr. Opin. Pediatr. 2017, 29, 199–205. [Google Scholar] [CrossRef]
- Shah, S.A.; Carter, H.P. New-Onset Nephrotic Syndrome in a Child Associated with COVID-19 Infection. Front. Pediatr. 2020, 8, 471. [Google Scholar] [CrossRef]
- Hureaux, M.; Vargas-Poussou, R. Genetic Basis of Nephrogenic Diabetes Insipidus. Mol. Cell Endocrinol. 2023, 560, 111825. [Google Scholar] [CrossRef]
- Bockenhauer, D.; van’t Hoff, W.; Dattani, M.; Lehnhardt, A.; Subtirelu, M.; Hildebrandt, F.; Bichet, D.G. Secondary Nephrogenic Diabetes Insipidus as a Complication of Inherited Renal Diseases. Nephron Physiol. 2010, 116, p23–p29. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, R.; Kutti Sridharan, G. Primary Polydipsia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sailer, C.O.; Winzeler, B.; Nigro, N.; Suter-Widmer, I.; Arici, B.; Bally, M.; Schuetz, P.; Mueller, B.; Christ-Crain, M. Characteristics and Outcomes of Patients with Profound Hyponatraemia Due to Primary Polydipsia. Clin. Endocrinol. 2017, 87, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Petrea (Cliveți), C.L.; Ciortea, D.-A.; Candussi, I.-L.; Gurău, G.; Matei, N.M.; Bergheș, S.-E.; Chirila, S.I.; Berbece, S.I. A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion. Curr. Issues Mol. Biol. 2024, 46, 11438–11459. [Google Scholar] [CrossRef]
- Gubbi, S.; Hannah-Shmouni, F.; Koch, C.A.; Verbalis, J.G. Diagnostic Testing for Diabetes Insipidus. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Stockand, J.D. Vasopressin Regulation of Renal Sodium Excretion. Kidney Int. 2010, 78, 849–856. [Google Scholar] [CrossRef]
- Atila, C.; Loughrey, P.B.; Garrahy, A.; Winzeler, B.; Refardt, J.; Gildroy, P.; Hamza, M.; Pal, A.; Verbalis, J.G.; Thompson, C.J.; et al. Central Diabetes Insipidus from a Patient’s Perspective: Management, Psychological Co-Morbidities, and Renaming of the Condition: Results from an International Web-Based Survey. Lancet Diabetes Endocrinol. 2022, 10, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Quinkler, M.; Lorenz, D.; Zopf, K.; Haagen, U.; Papassotiriou, J.; Pfeiffer, A.F.H.; Fassnacht, M.; Störk, S.; Allolio, B. Copeptin in the Differential Diagnosis of the Polydipsia-Polyuria Syndrome—Revisiting the Direct and Indirect Water Deprivation Tests. J. Clin. Endocrinol. Metab. 2011, 96, 1506–1515. [Google Scholar] [CrossRef]
- Pedrosa, W.; Drummond, J.B.; Soares, B.S.; Ribeiro-Oliveira, A. A Combined Outpatient and Inpatient Overnight Water Deprivation Test Is Effective and Safe in Diagnosing Patients with Polyuria-Polydipsia Syndrome. Endocr. Pract. 2018, 24, 963–972. [Google Scholar] [CrossRef]
- Morelle, J.; Goffin, E.; Devuyst, O. Molecular Physiology of Water Balance. N. Engl. J. Med. 2015, 373, 196. [Google Scholar] [CrossRef]
- Verbalis, J.G. Disorders of Water Balance. In Harrison’s Principles of Internal Medicine; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Bichet, D.G. Regulation of Thirst and Vasopressin Release. Annu. Rev. Physiol. 2019, 81, 359–373. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Ball, S. The Neurohypophysis: Endocrinology of Vasopressin and Oxytocin. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279157/ (accessed on 31 August 2025).
- D’Acierno, M.; Fenton, R.A.; Hoorn, E.J. The Biology of Water Homeostasis. Nephrol. Dial. Transplant. 2025, 40, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Fenske, W.; Refardt, J.; Chifu, I.; Schnyder, I.; Winzeler, B.; Drummond, J.; Ribeiro-Oliveira, A.; Drescher, T.; Bilz, S.; Vogt, D.R.; et al. A Copeptin-Based Approach in the Diagnosis of Diabetes Insipidus. N. Engl. J. Med. 2018, 379, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Gippert, S.; Brune, M.; Dirksen, R.L.; Choukair, D.; Bettendorf, M. Arginine-Stimulated Copeptin-Based Diagnosis of Central Diabetes Insipidus in Children and Adolescents. Horm. Res. Paediatr. 2023, 97, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sarkarinejad, A.; Paydar, S.; Khosrojerdi, A.; Hosseini, M. Copeptin: A Novel Prognostic Biomarker in Trauma: A Review Article. J. Health Popul. Nutr. 2023, 42, 128. [Google Scholar] [CrossRef]
- Refardt, J.; Atila, C.; Chifu, I.; Ferrante, E.; Erlic, Z.; Drummond, J.B.; Indirli, R.; Drexhage, R.C.; Sailer, C.O.; Widmer, A.; et al. Arginine or Hypertonic Saline–Stimulated Copeptin to Diagnose AVP Deficiency. N. Engl. J. Med. 2023, 389, 1877–1887. [Google Scholar] [CrossRef]
- Ciortea, D.-A.; Petrea (Cliveți), C.L.; Bujoreanu Bezman, L.; Vivisenco, I.C.; Berbece, S.I.; Gurău, G.; Matei, M.N.; Nechita, A. Diagnostic Utility of Copeptin in Pediatric Patients with Polyuria-Polydipsia Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 10743. [Google Scholar] [CrossRef]
- Giannakopoulos, A.; Kritikou, D.; Chrysis, D. Evaluation of copeptin in children after stimulation with clonidine or L-Dopa. J. Pediatr. Endocrinol. Metab. 2024, 37, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, L.; Ferrulli, A.; García-Alonso, M. Copeptin in the diagnosis and management of renal tubular disorders. Pediatr. Nephrol. 2025. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Refardt, J.; Winzeler, B. Approach to the Patient: "Utility of the Copeptin Assay". J. Clin. Endocrinol. Metab. 2022, 107, 1727–1738. [Google Scholar] [CrossRef]
- Lustenberger, S.; Atila, C.; Baumgartner, J.; Monnerat, S.; Beck, J.; Santos de Jesus, J.; Christ-Crain, M. Urea-Stimulated Copeptin: A Novel Diagnostic Approach in Polyuria Polydipsia Syndrome. Eur. J. Endocrinol. 2025, 192, 437–444. [Google Scholar] [CrossRef]
- Ciortea, D.-A.; Petrea, C.L.; Vivisenco, I.C.; Berbece, S.I.; Gurău, G.; Matei, M.N.; Nechita, A. Comparative Diagnostic Performance of Copeptin After Hypertonic Saline Infusion Versus Water Deprivation Test in Pediatric Patients with Polyuria–Polydipsia Syndrome. Int. J. Mol. Sci. 2025, 26, 5449. [Google Scholar] [CrossRef]
- Teare, H.; Argente, J.; Dattani, M.; Leger, J.; Maghnie, M.; Sherlock, M.; Ali, G.-C.; Francombe, J.; Marjanovic, S. Challenges and Improvement Needs in the Care of Patients with Central Diabetes Insipidus. Orphanet J. Rare Dis. 2022, 17, 58. [Google Scholar] [CrossRef]
- Lyu, P.; Xie, N.; Shao, X.; Xing, S.; Wang, X.; Duan, L.; Zhao, X.; Lu, J.; Liu, R.; Zhang, D.; et al. Integrating Bioinformatics and Machine Learning for Comprehensive Analysis and Validation of Diagnostic Biomarkers and Immune Cell Infiltration Characteristics in Pediatric Septic Shock. Sci. Rep. 2025, 15, 10456. [Google Scholar] [CrossRef]
- Krones, F.; Marikkar, U.; Parsons, G.; Szmul, A.; Mahdi, A. Review of Multimodal Machine Learning Approaches in Healthcare. Inf. Fusion. 2025, 114, 102690. [Google Scholar] [CrossRef]
- Jandoubi, B.; Akhloufi, M.A. Multimodal Artificial Intelligence in Medical Diagnostics. Information 2025, 16, 591. [Google Scholar] [CrossRef]
- Friedrich, S.; Groll, A.; Ickstadt, K.; Kneib, T.; Pauly, M.; Rahnenführer, J.; Friede, T. Regularization Approaches in Clinical Biostatistics: A Review of Methods and Their Applications. Stat. Methods Med. Res. 2023, 32, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Wilimitis, D.; Walsh, C.G. Practical Considerations and Applied Examples of Cross-Validation for Model Development and Evaluation in Health Care: Tutorial. JMIR AI 2023, 2, e49023. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Corben, L.A.; Selvadurai, L.P.; Harding, I.H.; Georgiou-Karistianis, N. Predictive Machine Learning and Multimodal Data to Develop Highly Sensitive, Composite Biomarkers of Disease Progression in Friedreich Ataxia. Sci. Rep. 2025, 15, 17629. [Google Scholar] [CrossRef]
- Ciortea, D.-A.; Petrea (Cliveți), C.L.; Berbece, S.I.; Fotea, S.; Vivisenco, I.C.; Gurău, G.; Matei, M.N.; Nechita, A. Impact of Hyponatremia and ADH Secretion in MIS-C and COVID-19: An Integrative Approach of Prognostic and Diagnostic Markers. Curr. Issues Mol. Biol. 2024, 46, 11749–11771. [Google Scholar] [CrossRef]
- Christ-Crain, M. EJE AWARD 2019: New Diagnostic Approaches for Patients with Polyuria Polydipsia Syndrome. Eur. J. Endocrinol. 2019, 181, R11–R21. [Google Scholar] [CrossRef] [PubMed]
- Winzeler, B.; Cesana-Nigro, N.; Refardt, J.; Vogt, D.R.; Imber, C.; Morin, B.; Popovic, M.; Steinmetz, M.; Sailer, C.O.; Szinnai, G.; et al. Arginine-Stimulated Copeptin Measurements in the Differential Diagnosis of Diabetes Insipidus: A Prospective Diagnostic Study. Lancet 2019, 394, 587–595. [Google Scholar] [CrossRef]
- Timper, K.; Fenske, W.; Kühn, F.; Frech, N.; Arici, B.; Rutishauser, J.; Kopp, P.; Allolio, B.; Stettler, C.; Müller, B.; et al. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of the Polyuria-Polydipsia Syndrome: A Prospective Multicenter Study. J. Clin. Endocrinol. Metab. 2015, 100, 2268–2274. [Google Scholar] [CrossRef]
- Ugoji, C.; Heidt, J.; Largent, J.; Bratton, E.; Hester, L.; Keshavarzi, S.; Turner, S.; Mack, C. Important Tool in Our Rare Disease Toolbox: Hybrid Retrospective-Prospective Natural History Studies Serve Well as External Comparators for Rare Disease Studies. Front. Drug Saf. Regul. 2024, 4, 1418050. [Google Scholar] [CrossRef]
- Shu, X.; Cai, F.; Li, W.; Shen, H. Copeptin as a Diagnostic and Prognostic Biomarker in Pediatric Diseases. Clin. Chem. Lab. Med. 2024, 63, 483–498. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the Measurement of Copeptin, a Stable Peptide Derived from the Precursor of Vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Physiology, Vasopressin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Driano, J.E.; Lteif, A.N.; Creo, A.L. Vasopressin-Dependent Disorders: What Is New in Children? Pediatrics 2021, 147, e2020022848. [Google Scholar] [CrossRef]
- Miller, A.; Panneerselvam, J.; Liu, L. A Review of Regression and Classification Techniques for Analysis of Common and Rare Variants and Gene-Environmental Factors. Neurocomputing 2022, 489, 466–485. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Boal, R.L.; Hughes, J.; Matthews, D.; Johnstone, H.; Boot, C.; Cheetham, T.D. Copeptin: Utility in Paediatric Patients with Hyponatraemia. Horm. Res. Paediatr. 2022, 95, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Binder, G.; Weber, K.; Peter, A.; Schweizer, R. Arginine-stimulated Copeptin in Children and Adolescents. Clin. Endocrinol. 2023, 98, 548–553. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Fenske, W. Copeptin in the Diagnosis of Vasopressin-Dependent Disorders of Fluid Homeostasis. Nat. Rev. Endocrinol. 2016, 12, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Tuli, G.; Tessaris, D.; Einaudi, S.; Matarazzo, P.; De Sanctis, L. Copeptin Role in Polyuria-polydipsia Syndrome Differential Diagnosis and Reference Range in Paediatric Age. Clin. Endocrinol. 2018, 88, 873–879. [Google Scholar] [CrossRef]
- Sailer, C.O.; Refardt, J.; Blum, C.A.; Schnyder, I.; Molina-Tijeras, J.A.; Fenske, W.; Christ-Crain, M. Validity of Different Copeptin Assays in the Differential Diagnosis of the Polyuria-Polydipsia Syndrome. Sci. Rep. 2021, 11, 10104. [Google Scholar] [CrossRef]
- Bonnet, L.; Marquant, E.; Fromonot, J.; Hamouda, I.; Berbis, J.; Godefroy, A.; Vierge, M.; Tsimaratos, M.; Reynaud, R. Copeptin Assays in Children for the Differential Diagnosis of Polyuria-polydipsia Syndrome and Reference Levels in Hospitalized Children. Clin. Endocrinol. 2022, 96, 47–53. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Y.; Cheng, J.; Qiu, L.; Chen, S.; Cheng, X. Diagnostic Accuracy of Copeptin in the Differential Diagnosis of Patients With Diabetes Insipidus: A Systematic Review and Meta-Analysis. Endocr. Pract. 2023, 29, 644–652. [Google Scholar] [CrossRef]
- Lam, J.Y.; Shimizu, C.; Tremoulet, A.H.; Bainto, E.; Roberts, S.C.; Sivilay, N.; Gardiner, M.A.; Kanegaye, J.T.; Hogan, A.H.; Salazar, J.C.; et al. A Machine-Learning Algorithm for Diagnosis of Multisystem Inflammatory Syndrome in Children and Kawasaki Disease in the USA: A Retrospective Model Development and Validation Study. Lancet Digit. Health 2022, 4, e717–e726. [Google Scholar] [CrossRef]
- Kigo, J.; Kamau, S.; Mawji, A.; Mwaniki, P.; Dunsmuir, D.; Pillay, Y.; Zhang, C.; Pallot, K.; Ogero, M.; Kimutai, D.; et al. External Validation of a Paediatric Smart Triage Model for Use in Resource Limited Facilities. PLoS Digit. Health 2024, 3, e0000293. [Google Scholar] [CrossRef]
- Steyaert, S.; Qiu, Y.L.; Zheng, Y.; Mukherjee, P.; Vogel, H.; Gevaert, O. Multimodal Deep Learning to Predict Prognosis in Adult and Pediatric Brain Tumors. Commun. Med. 2023, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhou, Z.; Li, S.; Huang, T.; Yin, H.; Lyu, J. Developing an Ensemble Machine Learning Model for Early Prediction of Sepsis-Associated Acute Kidney Injury. iScience 2022, 25, 104932. [Google Scholar] [CrossRef]
- Riley, R.D.; Snell, K.I.E.; Martin, G.P.; Whittle, R.; Archer, L.; Sperrin, M.; Collins, G.S. Penalization and Shrinkage Methods Produced Unreliable Clinical Prediction Models Especially When Sample Size Was Small. J. Clin. Epidemiol. 2021, 132, 88–96. [Google Scholar] [CrossRef]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A Systematic Review Shows No Performance Benefit of Machine Learning over Logistic Regression for Clinical Prediction Models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, Z.; Ruan, T.; Zhou, R.; Liu, C.; Li, Q.; Cheng, W.; Wang, J.; Wang, F.; Xie, H.; et al. Development and External Validation of a Machine Learning Model for Brain Injury in Pediatric Patients on Extracorporeal Membrane Oxygenation. Crit. Care 2025, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Turriziani, L.; Currò, A.; Gagliano, A.; Di Rosa, G.; Caccamo, D.; Tonacci, A.; Gangemi, S. A Machine Learning Approach to the Diagnosis of Autism Spectrum Disorder and Multi-Systemic Developmental Disorder Based on Retrospective Data and ADOS-2 Score. Brain Sci. 2023, 13, 883. [Google Scholar] [CrossRef]
- Trimpou, P.; Bounias, I.; Ehn, O.; Hammarsten, O.; Ragnarsson, O. The Influence of Insulin-Induced Hypoglycemia on Copeptin Concentrations. Peptides 2024, 176, 171185. [Google Scholar] [CrossRef]
- Malicka, K.; Horzelski, W.; Lewiński, A.; Lewandowski, K.C. Limited Role of Endogenous Vasopressin/Copeptin in Stimulation of ACTH–Cortisol Secretion during Glucagon Stimulation Test in Humans. Biomedicines 2022, 10, 2857. [Google Scholar] [CrossRef]
- Yousaf, Z.; Al-Shokri, S.D.; Al-Soub, H.; Mohamed, M.F.H. COVID-19-Associated SIADH: A Clue in the Times of Pandemic! Am. J. Physiol. Endocrinol. Metab. 2020, 318, E882–E885. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Snell, K.I.; Ensor, J.; Burke, D.L.; Harrell, F.E.; Moons, K.G.; Collins, G.S. Minimum Sample Size for Developing a Multivariable Prediction Model: PART II—Binary and Time-to-Event Outcomes. Stat. Med. 2019, 38, 1276–1296. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Li, J. A Time Series Driven Model for Early Sepsis Prediction Based on Transformer Module. BMC Med. Res. Methodol. 2024, 24, 23. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Liu, H.; Jin, B.; Cui, X.; Wang, D. Predicting Pediatric Age from Chest X-Rays Using Deep Learning: A Novel Approach. Insights Imaging 2025, 16, 184. [Google Scholar] [CrossRef]
- Benevenuta, S.; Capriotti, E.; Fariselli, P. Calibrating Variant-Scoring Methods for Clinical Decision Making. Bioinformatics 2021, 36, 5709–5711. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Sokou, R.; Tsantes, A.G.; Vitello, A.S.; Bonovas, S. Optimizing Clinical Decision Making with Decision Curve Analysis: Insights for Clinical Investigators. Healthcare 2023, 11, 2244. [Google Scholar] [CrossRef] [PubMed]



| Variable | PP (n = 13) | CDI (n = 11) | p-Value (Mann–Whitney U) |
|---|---|---|---|
| Age (years) | 14.58 (9.67–16.58) | 7.92 (4.83–10.42) | - |
| Weight (kg) | 41.0 (35.0–55.0) | 26.0 (20.0–30.0) | - |
| Stimulated copeptin (pmol/L) | 19.0 (14.0–23.4) | 4.92 (3.1–6.8) | <0.001 |
| Basal copeptin (pmol/L) | 4.05 (3.23–5.20) | 1.86 (1.23–2.65) | <0.001 |
| Baseline sodium (mmol/L) | 141.0 (138.0–142.0) | 144.0(142.0–146.0) | 0.0807 |
| Maximum sodium (mmol/L) | 150.0 (149.1–150.0) | 150.0 (150.0–151.0) | 0.3294 |
| Baseline osmolality (mOsm/kg) | 283.0 (276.6–286.0) | 283.0 (280.0–285.0) | 0.5615 |
| Maximum osmolality (mOsm/kg) | 301.0 (300.0–303.0) | 302.0 (301.0–304.0) | 0.5998 |
| Test duration (min) | 180.0 (180.0–180.0) | 120.0 (120.0–150.0) | 0.1074 |
| Tolerance (1–3) 1 | 1.0 (1.0–1.0) | 2.0 (1.0–2.0) | 0.0329 |
| Variable | VIF 1 |
|---|---|
| Basal copeptin | 3.44 |
| Stimulated copeptin | 3.53 |
| Baseline sodium | 1.62 |
| Peak sodium | 1.89 |
| Baseline osmolality | 2.42 |
| Peak osmolality | 2.86 |
| Test duration | 1.71 |
| Tolerability score | 1.75 |
| Predictor | Coefficient (β) |
|---|---|
| Intercept | –0.083 |
| Stimulated copeptin (standardized) | –0.660 |
| Peak serum sodium (standardized) | –0.154 |
| Peak serum osmolality (standardized) | +0.158 |
| Test duration (standardized) | –0.152 |
| Tolerability score (standardized) | +0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciortea, D.-A.; Petrea, C.L.; Verga, G.I.; Berbece, S.I.; Gurău, G.; Fotea, S.; Matei, M.N. Beyond Biomarkers: Blending Copeptin and Clinical Cues to Distinguish Central Diabetes Insipidus from Primary Polydipsia in Children. Biomedicines 2025, 13, 2573. https://doi.org/10.3390/biomedicines13102573
Ciortea D-A, Petrea CL, Verga GI, Berbece SI, Gurău G, Fotea S, Matei MN. Beyond Biomarkers: Blending Copeptin and Clinical Cues to Distinguish Central Diabetes Insipidus from Primary Polydipsia in Children. Biomedicines. 2025; 13(10):2573. https://doi.org/10.3390/biomedicines13102573
Chicago/Turabian StyleCiortea, Diana-Andreea, Carmen Loredana Petrea (Cliveți), Gabriela Isabela Verga (Răuță), Sorin Ion Berbece, Gabriela Gurău, Silvia Fotea, and Mădălina Nicoleta Matei. 2025. "Beyond Biomarkers: Blending Copeptin and Clinical Cues to Distinguish Central Diabetes Insipidus from Primary Polydipsia in Children" Biomedicines 13, no. 10: 2573. https://doi.org/10.3390/biomedicines13102573
APA StyleCiortea, D.-A., Petrea, C. L., Verga, G. I., Berbece, S. I., Gurău, G., Fotea, S., & Matei, M. N. (2025). Beyond Biomarkers: Blending Copeptin and Clinical Cues to Distinguish Central Diabetes Insipidus from Primary Polydipsia in Children. Biomedicines, 13(10), 2573. https://doi.org/10.3390/biomedicines13102573







