Clinical and Molecular Insights of Arterial and Venous Thrombosis in Myeloproliferative Diseases—Case-Based Narrative Review
Abstract
1. Introduction
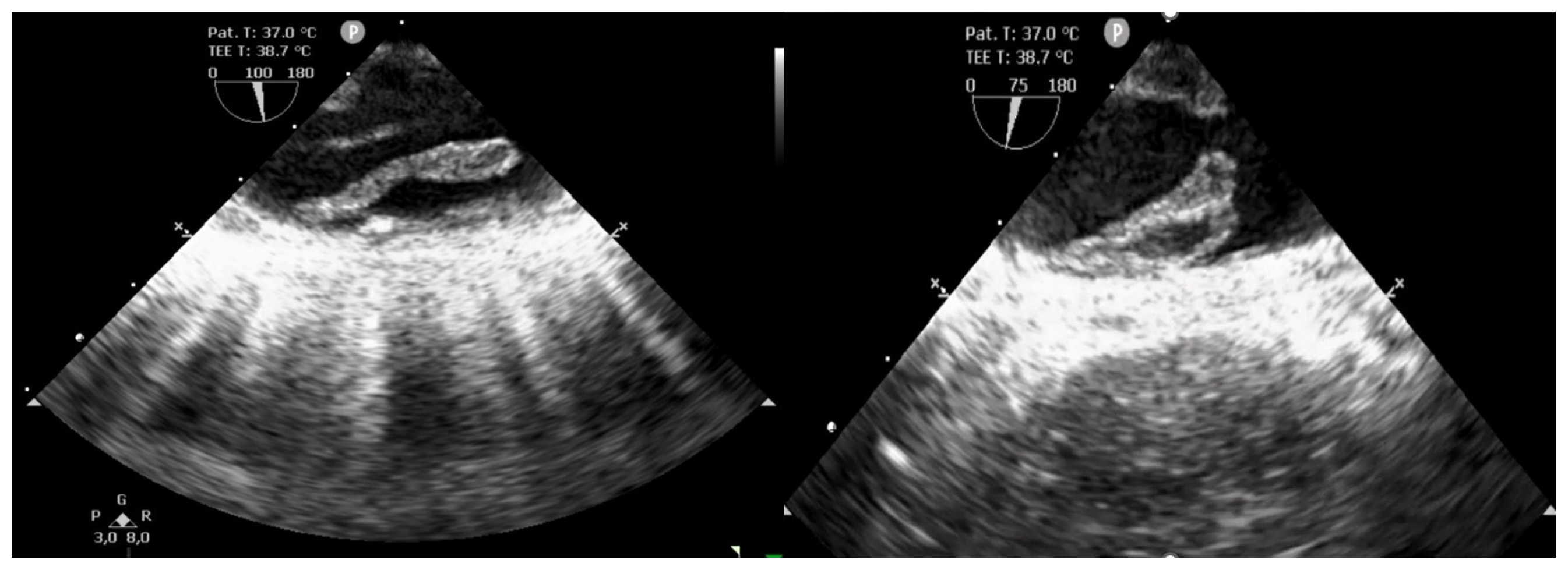
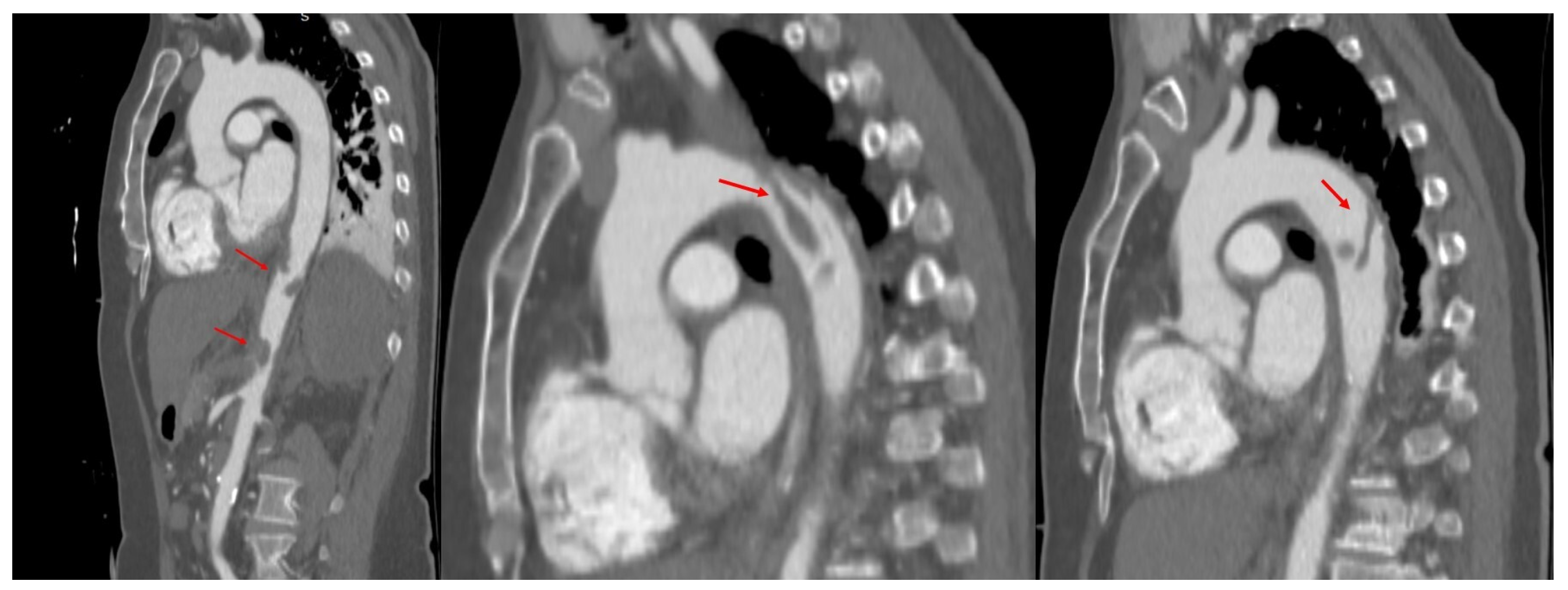
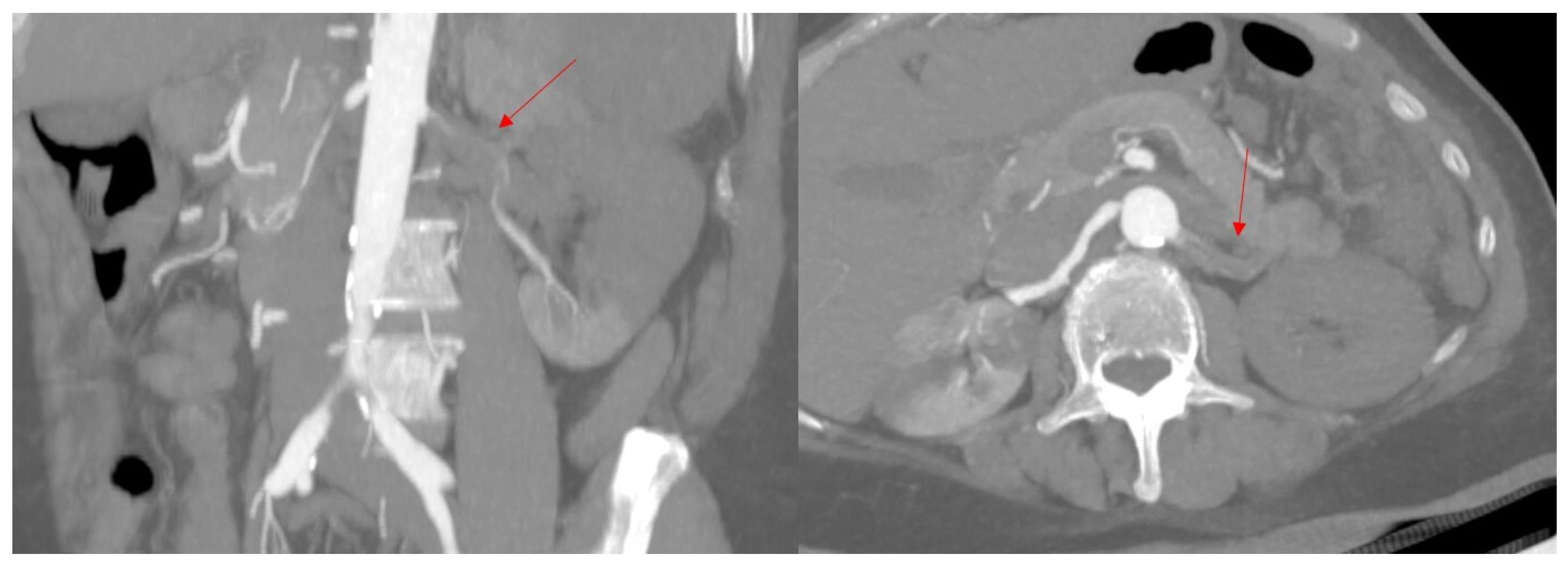
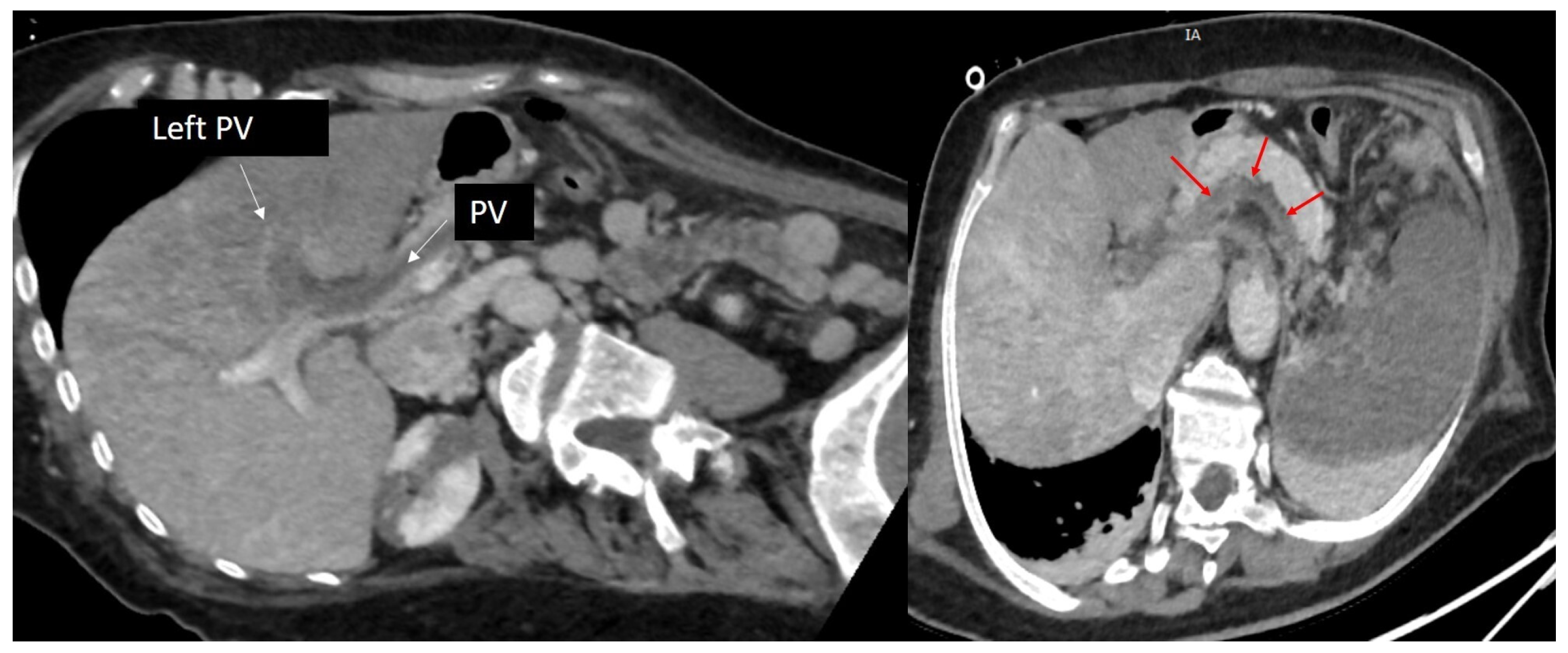
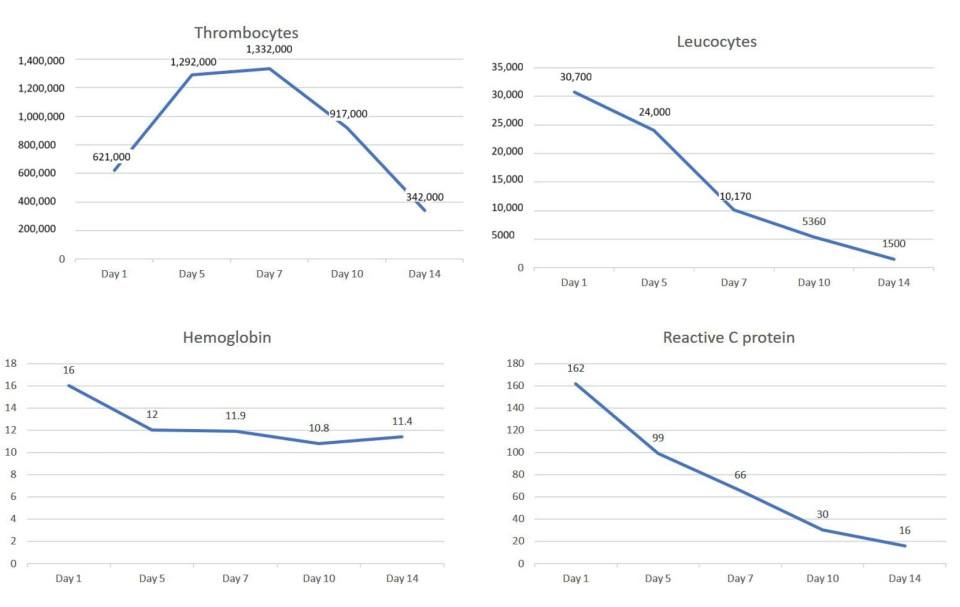
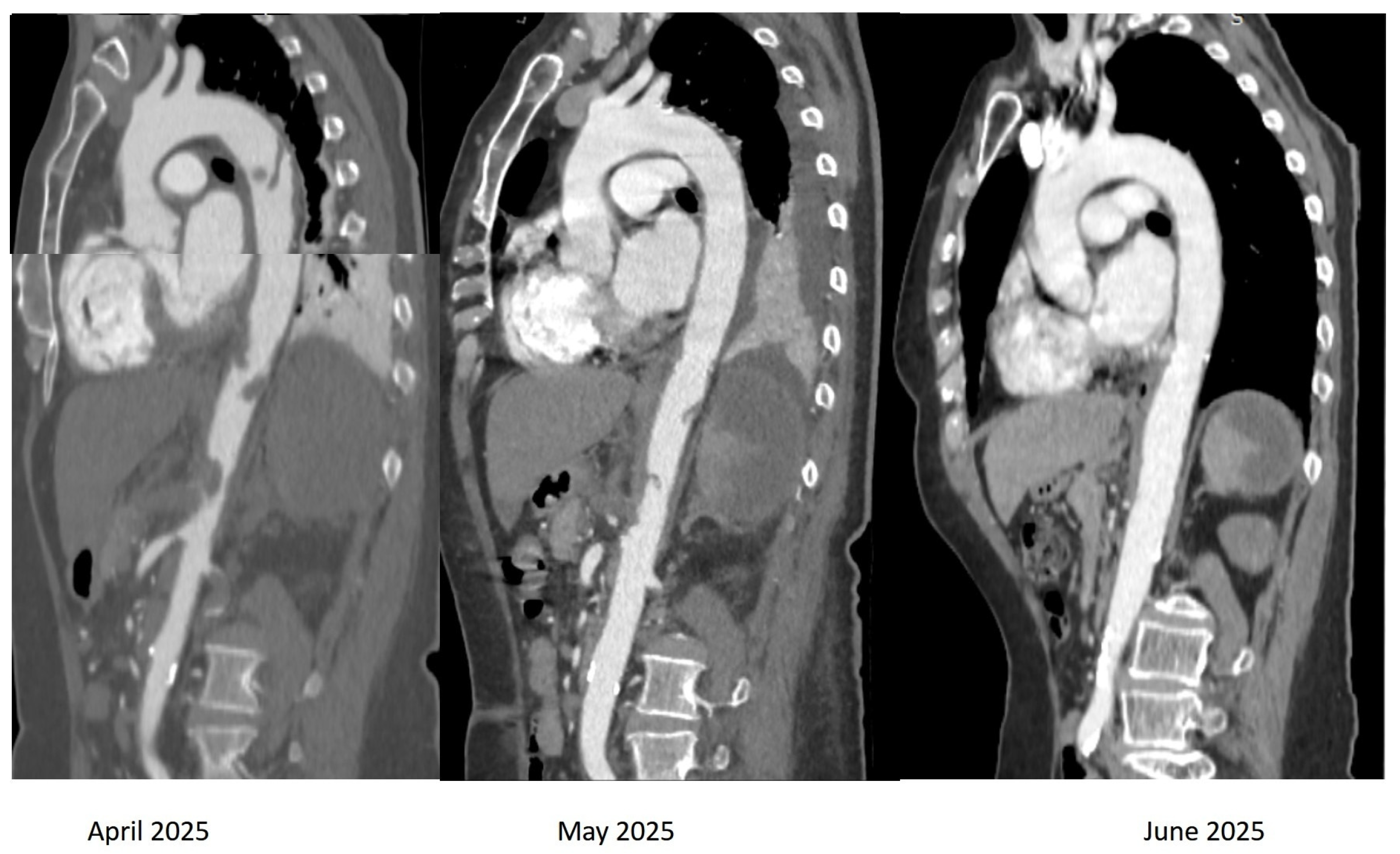
2. Case Presentation
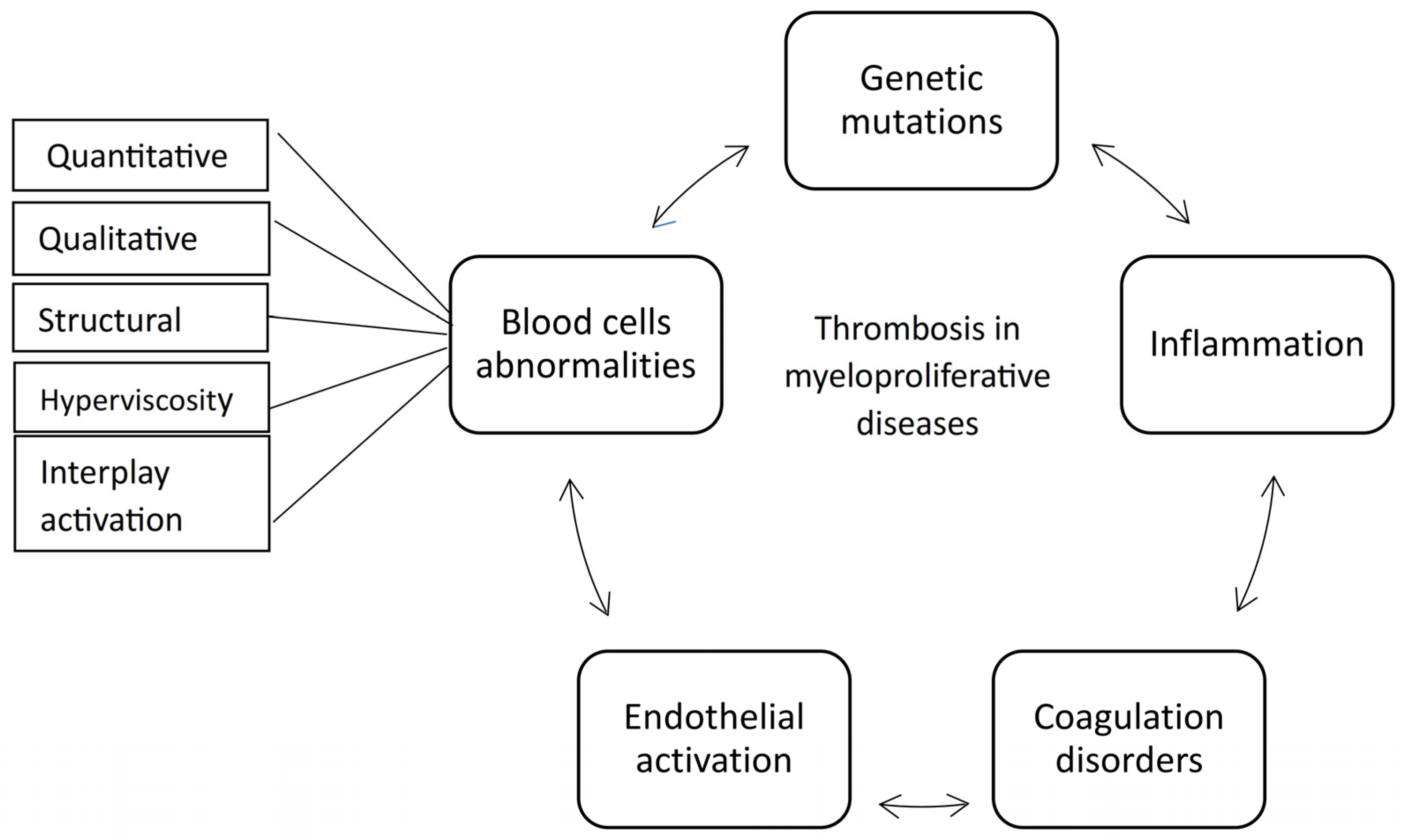
3. Insights into Pathophysiological Mechanisms of Thrombosis in Myeloproliferative Neoplasms
3.1. Genetic Mutations
3.2. The Coagulation Disorders and Platelet Involvement
3.3. The Leukocytes Involvement
3.4. The Thrombo-Inflammatory Process
3.5. The Endothelial Involvement
3.6. Red Blood Cells Involvement
4. Thrombosis Risk Assessment and Stratification
4.1. Clinical Assessment
4.2. Cardiovascular Risk Factors
4.3. Blood Cells Assessment
4.4. Genetic Risk Factors
4.5. Risk Scores
5. Treatment Insights
5.1. Prevention of the Thrombotic Complications in Myeloproliferative Disorders
5.2. Treatment of Acute Thrombotic Events in Myeloproliferative Disorders
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Hasserjian, R.P.; Orazi, A.; Mathews, V.; Roberts, A.W.; Schiffer, C.A.; Roug, A.S.; Cazzola, M.; Döhner, H.; Tefferi, A. Classification of myeloid neoplasms/acute leukemia: Global perspectives and the international consensus classification approach. Am. J. Hematol. 2022, 97, 514–518. [Google Scholar] [CrossRef]
- Moliterno, A.R.; Ratchford, E.V. Shedding New Light on the Magnitude of Thrombosis Risk in Patients with Myeloproliferative Neoplasms. Ann. Intern. Med. 2018, 168, 363–364. [Google Scholar] [CrossRef]
- Zhang, H.; Kafeiti, N.; Masarik, K.; Lee, S.; Yang, X.; Zheng, H.; Zhan, H. Decoding Endothelial MPL and JAK2V617F Mutation: Insight Into Cardiovascular Dysfunction in Myeloproliferative Neoplasms. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1960–1974. [Google Scholar] [CrossRef]
- Barbui, T.; Ghirardi, A.; Carobbio, A.; De Stefano, V.; Rambaldi, A.; Tefferi, A.; Vannucchi, A.M. Thrombosis in myeloproliferative neoplasms: A viewpoint on its impact on myelofibrosis, mortality, and solid tumors. Blood Cancer J. 2024, 14, 188. [Google Scholar] [CrossRef]
- Carobbio, A.; Vannucchi, A.M.; Rumi, E.; De Stefano, V.; Rambaldi, A.; Carli, G.; Randi, M.L.; Gisslinger, H.; Passamonti, F.; Thiele, J.; et al. Survival expectation after thrombosis and overt-myelofibrosis in essential thrombocythemia and prefibrotic myelofibrosis: A multistate model approach. Blood Cancer J. 2023, 13, 115. [Google Scholar] [CrossRef]
- Barbui, T.; Carobbio, A.; Thiele, J.; Gangat, N.; Rumi, E.; Rambaldi, A.; Vannucchi, A.M.; Tefferi, A.; IWG-MRT group; Jeryczynski, G.; et al. The impact of thrombosis on probabilities of death and disease progression in polycythemia vera: A multistate transition analysis of 1545 patients. Blood Cancer J. 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Gerds, A.T.; Yu, J.; Parasuraman, S.; Shah, A.; Xi, A.; Kumar, S.; Scherber, R.M.; Verstovsek, S. Thrombotic events and mortality risk in patients with newly diagnosed polycythemia vera or essential thrombocythemia. Leuk. Res. 2022, 115, e106809. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Carobbio, A. Prediction models for essential thrombocythemia from two longitudinal studies involving 2000 patients. Blood Cancer J. 2024, 14, 17. [Google Scholar] [CrossRef]
- De Stefano, V.; Ghirardi, A.; Masciulli, A.; Carobbio, A.; Palandri, F.; Vianelli, N.; Rossi, E.; Betti, S.; Di Veroli, A.; Iurlo, A.; et al. Arterial thrombosis in philadelphia-negative myeloproliferative neoplasms predicts second cancer: A case-control study. Blood 2020, 135, 381–386. [Google Scholar] [CrossRef]
- Ghirardi, A.; Carobbio, A.; Guglielmelli, P.; Rambaldi, A.; De Stefano, V.; Vannucchi, A.M.; Tetfferi, A.l.; Barbui, T. Age-stratified analysis reveals arterial thrombosis as a predictor for gender-related second cancers in myeloproliferative neoplasms: A case-control study. Blood Cancer J. 2024, 14, 68. [Google Scholar] [CrossRef]
- Hindilerden, F.; Akay, Ö.N.; Aksoy, E.; Dağlar-Aday, A.; Gültürk, E.; Nalçacı, M.; Yönal-Hindilerden, İ. Secondary Solid Cancers in Patients with Philadelphia Chromosome-Negative Myeloproliferative Neoplasms: A Multicenter Study. Turk. J. Haematol. 2024, 41, 246–255. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Björkholm, M.; Dickman, P.W.; Landgren, O.; Derolf, Å.R.; Kristinsson, S.Y.; Andersson, T.M.L. Risk for Arterial and Venous Thrombosis in Patients with Myeloproliferative Neoplasms: A Population-Based Cohort Study. Ann. Intern. Med. 2018, 168, 317–325. [Google Scholar] [CrossRef]
- Johnson, M.; Gernsheimer, T.; Johansen, K. Essential thrombocytosis: Underemphasized cause of large-vessel thrombosis. J. Vasc. Surg. 1995, 22, 443–449. [Google Scholar] [CrossRef]
- Koschmieder, S.; Panse, J. Thrombosis at Unusual Sites: Focus on Myeloproliferative Neoplasms and Paroxysmal Nocturnal Hemoglobinuria. Hamostaseologie 2025, 45, 166–174. [Google Scholar] [CrossRef]
- Sohn, V.; Arthurs, Z.; Andersen, C.; Starnes, B. Aortic thrombus due to essential thrombocytosis: Strategies for medical and surgical management. Ann. Vasc. Surg. 2008, 22, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Agha, S.; Lockridge, L.; Lee, R.; Cleary, J.P.; Mazur, E.M. Medical management of a large aortic thrombus in a young woman with essential thrombocythemia. Mayo Clin. Proc. 2001, 76, 427–431. [Google Scholar] [CrossRef]
- Jeon, W.J.; Mehta, A.; Hudson, J.; Castillo, D.R.; Wang, J.; Nguyen, A.; Akhtari, M. Portal vein thrombosis as the presenting manifestation of JAK2 positive myeloproliferative neoplasm. Am. J. Med. Sci. 2023, 365, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Huang, G.; Wu, S.; Liu, X.; Chen, S.; Luo, P.; Liu, C.; Zuo, X. The role of leukocytes in myeloproliferative neoplasm thromboinflammation. J. Leukoc. Biol. 2024, 115, 1020–1028. [Google Scholar] [CrossRef]
- Chia, Y.C.; Siti Asmaa, M.J.; Ramli, M.; Woon, P.Y.; Johan, M.F.; Hassan, R.; Islam, M.A. Molecular Genetics of Thrombotic Myeloproliferative Neoplasms: Implications in Precision Oncology. Diagnostics 2023, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Cordua, S.; Kjaer, L.; Skov, V.; Pallisgaard, N.; Hasselbalch, H.C.; Ellervik, C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood 2019, 134, 469–479, Erratum in Blood 2019, 134, 1195. [Google Scholar] [CrossRef]
- Vignoli, A.; Gambe, S.; van der Meijden, P.E.J.; Marchetti, M.; Russo, L.; Tessarolo, S.; Giaccherini, C.; Swieringa, F.; ten Cate, H.; Finazzi, G.; et al. Increased platelet thrombus formation under flow conditions in whole blood from polycythaemia vera patients. Blood Transfus. 2022, 20, 143–151. [Google Scholar]
- Padda, J.; Khalid, K.; Yadav, J.; Almanie, A.H.; Mehta, K.A.; Al Hennawi, H.; Boddeti, N.L.; Campos, V.Y.M.; Jean-Charles, G. JAK2 and TET2 Mutation in Polycythemia Vera. Cureus 2021, 13, e17854. [Google Scholar] [CrossRef]
- Ortmann, C.A.; Kent, D.G.; Nangalia, J.; Silber, Y.; Wedge, D.C.; Grinfeld, J.; Baxter, E.J.; Massie, C.E.; Papaemmanuil, E.; Menon, S.; et al. Effect of mutation order on myeloproliferative neoplasms. N. Engl. J. Med. 2015, 372, 601–612. [Google Scholar] [CrossRef]
- Morath, O.; Rinke, J.; Walter, A.; Crodel, C.; Meggendorfer, M.; Baer, C.; Hochhaus, A.; Ernst, T. Molecular predictors of venous and arterial thrombotic events in patients with myelofibrosis. Ann. Hematol. 2025, 104, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Parashar, Y.; Kushwaha, R.; Kumar, A.; Agarwal, K.; Singh, U.S.; Jain, M.; Verma, S.P.; Tripathi, A.K. Haemostatic Profile in Patients of Myeloproliferative Neoplasms-A Tertiary Care Centre Experience. J. Clin. Diagn. Res. 2016, 10, EC01–EC04. [Google Scholar] [CrossRef] [PubMed]
- Giaccherini, C.; Verzeroli, C.; Marchetti, M.; Gamba, S.; Piras, F.; Russo, L.; Tessarolo, S.; Vignoli, A.; Finazzi, G.; Rambaldi, A.; et al. PO-26—Whole blood rotational thromboelastometry (ROTEM) to detect hypercoagulability in patients with myeloproliferative neoplasms (MPN). Thromb. Res. 2016, 140, 185–186. [Google Scholar] [CrossRef]
- Şahin, D.G.; Akay, O.M.; Uskudar Teke, H.; Andıc, N.; Gunduz, E. Use of rotational thromboelastometry for a global screening of coagulation profile in patients of myeloproliferative neoplasms. Platelets. 2021, 32, 280–283. [Google Scholar] [CrossRef]
- Lucchesi, A.; Napolitano, R.; Bochicchio, M.T.; Giordano, G.; Napolitano, M. Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The “Circulating Wound” Model. Int. J. Mol. Sci. 2021, 22, 11343. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Liang, H.P.H.; Iqra, Z.; Whittaker, S.; Tan, C.W.; Dale, B.J.; Chen, V.M. Platelets from patients with myeloproliferative neoplasms have increased numbers of mitochondria that are hypersensitive to depolarization by thrombin. Sci. Rep. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Nayak, L.; Sweet, D.R.; Thomas, A.; Lapping, S.D.; Kalikasingh, K.; Madera, A.; Vinayachandran, V.; Padmanabhan, R.; Vasudevan, N.T.; Myers, J.T.; et al. A targetable pathway in neutrophils mitigates both arterial and venous thrombosis. Sci Transl Med. 2022, 14, eabj7465. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Wang, M.; Wang, Z.; Wang, D.; Niu, J.; Yang, E.; Li, Y.; Sun, Y.; Zhao, P.; et al. Activated PRKCD-mediated neutrophil extracellular traps pathway may be the prothrombotic mechanism of neutrophils in polycythemia vera patients based on clinical retrospective analysis and bioinformatics study. Int. Immunopharmacol. 2024, 127, 111366. [Google Scholar] [CrossRef]
- Georgatzakou, H.T.; Fortis, S.P.; Papageorgiou, E.G.; Antonelou, M.H.; Kriebardis, A.G. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules 2022, 12, 803. [Google Scholar] [CrossRef]
- Šefer, D.; Miljić, P.; Kraguljac-Kurtović, N.; Bižić-Radulović, S.; Bogdanović, A.; Knežević, V.; Marković, D.; Beleslin-Čokić, B.; Novaković, I.; Marinković, J.; et al. Correlation between leukocyte-platelet aggregates and thrombosis in myeloproliferative neoplasms. Int. J. Lab. Hematol. 2022, 44, 302–312. [Google Scholar] [CrossRef]
- He, F.; Laranjeira, A.B.; Kong, T.; Lin, S.; Ashworth, K.J.; Liu, A.; Lasky, N.M.; Fisher, D.A.; Cox, M.J.; Fulbright, M.C.; et al. Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms. J. Clin. Investig. 2024, 134, e172256. [Google Scholar] [CrossRef]
- Beckman, J.D.; DaSilva, A.; Aronovich, E.; Nguyen, A.; Nguyen, J.; Hargis, G.; Reynolds, D.; Vercellotti, G.M.; Betts, B.; Wood, D.K. JAK-STAT inhibition reduces endothelial prothrombotic activation and leukocyte-endothelial proadhesive interactions. J. Thromb. Haemost. 2023, 21, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.; Gourdou-Latyszenok, V.; Le Lay, N.; Peghaire, C.; Kilani, B.; Dias, J.V.; Duplaa, C.; Renault, M.A.; Denis, C.; Villeval, J.L.; et al. Vascular endothelial cell expression of JAK2V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica 2019, 104, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Diz-Küçükkaya, R.; İyigün, T.; Albayrak, Ö.; Eker, C.; Günel, T. JAK2V617F Mutation in Endothelial Cells of Patients with Atherosclerotic Carotid Disease. Turk. J. Haematol. 2024, 41, 167–174. [Google Scholar] [PubMed]
- Hekimoğlu, H.; Toprak, S.F.; Sözer, S. JAK2V617F-Positive Endothelial Cells Induce Apoptosis and Release JAK2V617F-Positive Microparticles. Turk. J. Haematol. 2022, 39, 13–21. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- Walton, B.L.; Lehmann, M.; Skorczewski, T.; Holle, L.A.; Beckman, J.D.; Cribb, J.A.; Mooberry, M.J.; Wufsus, A.R.; Cooley, B.C.; Homeister, J.W.; et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood 2017, 129, 2537–2546. [Google Scholar] [CrossRef]
- Watson, C.T.; Ward, S.C.; Rizzo, S.A.; Redaelli, A.; Manning, K.B. Influence of Hematocrit Level and Integrin αIIbβIII Function on vWF-Mediated Platelet Adhesion and Shear-Induced Platelet Aggregation in a Sudden Expansion. Cell. Mol. Bioeng. 2024, 17, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.M.P.; El Nemer, W.; De Grandis, M. Red Blood Cell Contribution to Thrombosis in Polycythemia Vera and Essential Thrombocythemia. Int. J. Mol. Sci. 2024, 25, 1417. [Google Scholar] [CrossRef]
- Wautier, M.P.; El Nemer, W.; Gane, P.; Rain, J.D.; Cartron, J.P.; Colin, Y.; Le Van Kim, C.; Wautier, J.L. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood 2007, 110, 894–901. [Google Scholar] [CrossRef]
- Novitzky-Basso, I.; Spring, F.; Anstee, D.; Tripathi, D.; Chen, F. Erythrocytes from patients with myeloproliferative neoplasms and splanchnic venous thrombosis show greater expression of Lu/BCAM. Int. J. Lab. Hematol. 2018, 40, 473–477. [Google Scholar] [CrossRef]
- Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; Vannucchi, A.M.; Antonioli, E.; et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: An international study of 891 patients. Blood 2011, 117, 5857–5859. [Google Scholar] [CrossRef]
- Beleva, E.A. Splanchnic Vein Thrombosis in Myelofibrosis—An Underappreciated Hallmark of Disease Phenotype. Int. J. Mol. Sci. 2023, 24, 15717. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, M.; Andreescu, B. A Review About the Assessment of the Bleeding and Thrombosis Risk for Patients with Myeloproliferative Neoplasms Scheduled for Surgery. Cureus 2024, 16, e56008. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Barosi, G.; Birgegard, G.; Cervantes, F.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Hehlmann, R.; Hoffman, R.; et al. Philadelphianegative classical myeloproliferative neoplasms: Critical concepts and management recommendations from European LeukemiaNet. J. Clin. Oncol. 2011, 29, 761–770. [Google Scholar] [CrossRef]
- Marchioli, R.; Finazzi, G.; Specchia, G.; Cacciola, R.; Cavazzina, R.; Cilloni, D.; De Stefano, V.; Elli, E.; Iurlo, A.; Latagliata, R.; et al. Cardiovascular events and intensity of treatment in polycythemia Vera. N. Engl. J. Med. 2013, 368, 22–33. [Google Scholar] [CrossRef]
- Marchioli, R.; Finazzi, G.; Landolfi, R.; Kutti, J.; Gisslinger, H.; Patrono, C.; Marilus, R.; Villegas, A.; Tognoni, G.; Barbui, T. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 2005, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Enblom Larsson, A.; Renlund, H.; Andréasson, B.; Holmberg, H.; Liljeholm, M.; Själander, A. Thrombosis, major bleeding, and mortality in 1079 patients with myelofibrosis: A matched population-based study. Blood Adv. 2025, 9, 2783–2793. [Google Scholar] [CrossRef]
- Gerds, A.T.; Mesa, R.; Burke, J.M.; Grunwald, M.R.; Stein, B.L.; Squier, P.; Yu, J.; Hamer-Maansson, J.E.; Oh, S.T. Association between elevated white blood cell counts and thrombotic events in polycythemia vera: Analysis from REVEAL. Blood 2024, 143, 1646–1655. [Google Scholar] [CrossRef]
- Ripamonti, A.; Cavalca, F.; Montelisciani, L.; Antolini, L.; Gambacorti-Passerini, C.; Elli, E.M. Neutrophil-to-lymphocyte ratio (NLR) at diagnosis in essential thrombocythemia: A new promising predictor of thrombotic events. Cancer 2025, 131, e35638. [Google Scholar] [CrossRef]
- Shimano, K.A.; Vanderpoel, V.; Stone, H.; Resar, L.; Kucine, N. Clinical features associated with thrombotic events in children with myeloproliferative neoplasms. Am. J. Hematol. 2022, 97, E353–E355. [Google Scholar] [CrossRef]
- Wille, K.; Deventer, E.; Sadjadian, P.; Becker, T.; Kolatzki, V.; Hünerbein, K.; Meixner, R.; Jiménez-Muñoz, M.; Fuchs, C.; Griesshammer, M. Arterial and Venous Thromboembolic Complications in 832 Patients with BCR-ABL-Negative Myeloproliferative Neoplasms. Hamostaseologie 2024, 44, 386–392. [Google Scholar] [CrossRef]
- Chiasakul, T.; Baker, R.I. Management of Bleeding, Thrombotic and Pregnancy-Related Complications in Women with Myeloproliferative Neoplasms: A Case-Based Review Focusing on Sex-Specific Challenges. J. Clin. Med. 2025, 14, 1537. [Google Scholar] [CrossRef]
- Szuber, N.; Guglielmelli, P.; Gangat, N. Topics of Interest in Women with Myeloproliferative Neoplasms. Am. J. Hematol. 2025, 100, 74–87. [Google Scholar] [CrossRef]
- Karantanos, T.; Chaturvedi, S.; Braunstein, E.M.; Spivak, J.; Resar, L.; Karanika, S.; Williams, D.M.; Rogers, O.; Gocke, C.D.; Moliterno, A.R. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020, 4, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Cerquozzi, S.; Barraco, D.; Lasho, T.; Finke, C.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Risk factors for arterial vs venous thrombosis in polycythemia vera: A single center experience in 587 patients. Blood Cancer J. 2017, 7, 662. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhang, Y.; Sun, T.; Ju, M.; Liu, X.; Xue, F.; Chen, Y.; Liu, W.; Li, H.; Wang, W.; et al. Prediction of thrombosis in polycythemia vera: Development and validation of a multiple factor-based prognostic score system. Res. Pract. Thromb. Haemost. 2023, 7, 100132. [Google Scholar] [CrossRef]
- Aswad, M.H.; Kissova, J.; Ovesna, P.; Penka, M. Risk factors of thrombosis in a cohort of 206 patients with BCR-ABL1 negative myeloproliferative neoplasms. Neoplasma 2021, 68, 1341–1350. [Google Scholar] [CrossRef]
- Gecht, J.; Tsoukakis, I.; Kricheldorf, K.; Stegelmann, F.; Klausmann, M.; Griesshammer, M.; Schulz, H.; Hollburg, W.; Göthert, J.R.; Sockel, K.; et al. Kidney Dysfunction Is Associated with Thrombosis and Disease Severity in Myeloproliferative Neoplasms: Implications from the German Study Group for MPN Bioregistry. Cancers 2021, 13, 4086. [Google Scholar] [CrossRef] [PubMed]
- How, J.; Leiva, O.; Redd, R.; Marneth, A.E.; DeAngelo, D.J.; Dieli-Conwright, C.M.; El-Jawahri, A.; Kamaz, B.; Kim, C.; Lindsley, R.C.; et al. Cardiovascular risk factors in myeloproliferative neoplasms: Associations with survival and thrombotic outcomes. Blood Vessel. Thromb. Hemost. 2025, 2, 100051. [Google Scholar] [CrossRef] [PubMed]
- Krecak, I.; Lekovic, D.; Grohovac, D.; Sabljic, A.; Holik, H.; Zekanovic, I.; Moric Peric, M.; Galusic, D.; Perisa, V.; Krecak, F.; et al. Glycated hemoglobin (HbA1c) and thrombotic risk in polycythemia vera and essential thrombocythemia. Leuk. Lymphoma 2024, 65, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Furuya, C.; Hashimoto, Y.; Morishita, S.; Inano, T.; Ochiai, T.; Shirane, S.; Edahiro, Y.; Araki, M.; Ando, M.; Komatsu, N. Reevaluation of cardiovascular risk factors for thrombotic events in 580 Japanese patients with essential thrombocythemia. J. Thromb. Thrombolysis 2023, 55, 263–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Wang, Y.; Teng, G.; Li, D.; Du, C.; Chen, Y.; Zhang, H.; Li, Y.; Fu, L.; et al. Thrombosis among 1537 patients with JAK2(V617F)-mutated myeloproliferative neoplasms: Risk factors and development of a predictive model. Cancer Med. 2020, 9, 2096–2105. [Google Scholar] [CrossRef]
- Lai, H.; Tu, Y.; Zhang, S.; Liao, C.; Tu, H.; Li, J. Association of inflammation and abnormal lipid metabolism with risk of thrombosis and thrombosis progression in patients with polycythemia vera: A retrospective study. Ann. Hematol. 2023, 102, 3413–3426. [Google Scholar] [CrossRef]
- Găman, M.A.; Srichawla, B.S.; Chen, Y.F.; Roy, P.; Dhali, A.; Nahian, A.; Manan, M.R.; Kipkorir, V.; Suteja, R.C.; Simhachalam Kutikuppala, L.V.; et al. Overview of dyslipidemia and metabolic syndrome in myeloproliferative neoplasms. World J. Clin. Oncol. 2024, 15, 717–729. [Google Scholar] [CrossRef]
- Leiva, O.; Campia, U.; Snyder, J.; Barns, B.M.; Rizzo, S.; Khairani, C.D.; Brunner, A.; Al-Samkari, H.; Leaf, R.K.; Rosovsky, R.; et al. Patients with myeloproliferative neoplasms and COVID-19 have increased rates of arterial thrombosis. Res. Pract. Thromb. Haemost. 2022, 6, e12752. [Google Scholar] [CrossRef]
- Harrison, C.N.; Nangalia, J.; Boucher, R.; Jackson, A.; Yap, C.; O’Sullivan, J.; Fox, S.; Ailts, I.; Dueck, A.C.; Geyer, H.L.; et al. Ruxolitinib Versus Best Available Therapy for Polycythemia Vera Intolerant or Resistant to Hydroxycarbamide in a Randomized Trial. J. Clin. Oncol. 2023, 41, 3534–3544. [Google Scholar] [CrossRef]
- De Stefano, V.; Passamonti, F.; Palandri, F.; Ramundo, F.; Rossi, E.; Betti, S.; Fagiolo, L.; Guglielmelli, P.; Abagnale, D.P.; Pugliese, N.; et al. Exploring thromboembolic risk factors in polycythemia vera: From current evidence to PROSPERO study design. Ann. Hematol. 2025, 104, 3553–3566. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Barbui, T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2024, 98, 1465–1487. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, R.; Finazzi, G.; Specchia, G.; Masciulli, A.; Mennitto, M.R.; Barbui, T. The CYTO-PV: A Large-Scale Trial Testing the Intensity of CYTOreductive Therapy to Prevent Cardiovascular Events in Patients with Polycythemia Vera. Thrombosis 2011, 2011, 794240. [Google Scholar] [CrossRef] [PubMed]
- Chojecki, A.; Boselli, D.; Dortilus, A.; Hamadeh, I.; Begley, S.; Chen, T.; Bose, R.; Podoltsev, N.; Zeidan, A.M.; Balmaceda, N.B.; et al. Hematocrit control and thrombotic risk in patients with polycythemia vera treated with ruxolitinib in clinical practice. Ann. Hematol. 2024, 103, 2837–2843. [Google Scholar] [CrossRef]
- Shimoda, K.; Yasunaga, H.; Sugimoto, Y.; Uenaka, H.; Dochi, T.; Jun, G. The association between blood cell counts and thrombotic events in Japanese patients with polycythemia vera: A retrospective database study. Blood 2023, 142, 3191. [Google Scholar] [CrossRef]
- Carobbio, A.; Ferrari, A.; Masciulli, A.; Ghirardi, A.; Barosi, G.; Barbui, T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: A systematic review and meta-analysis. Blood Adv. 2019, 3, 1729–1737. [Google Scholar] [CrossRef]
- Galvez, C.; Stein, B.L. Thrombocytosis and Thrombosis: Is There Really a Correlation? Curr. Hematol. Malig. Rep. 2020, 15, 261–267. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ito, T.; Gotoh, A.; Nakamae, M.; Kimura, F.; Koike, M.; Kirito, K.; Wada, H.; Usuki, K.; Tanaka, T.; et al. Clinical characteristics, prognostic factors, and outcomes of patients with essential thrombocythemia in Japan: The JSH-MPN-R18 study. Int. J. Hematol. 2022, 115, 208–221. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sabljic, A.; Galusic, D.; Holik, H.; Perisa, V.; Moric Peric, M.; Zekanovic, I.; Budimir, J.; et al. Evaluation of Absolute Neutrophil, Lymphocyte and Platelet Count and Their Ratios as Predictors of Thrombotic Risk in Patients with Prefibrotic and Overt Myelofibrosis. Life 2024, 14, 523. [Google Scholar] [CrossRef]
- Manz, K.; Bahr, J.; Ittermann, T.; Döhner, K.; Koschmieder, S.; Brümmendorf, T.H.; Griesshammer, M.; Nauck, M.; Völzke, H.; Heidel, F.H. Validation of myeloproliferative neoplasms associated risk factor RDW as predictor of thromboembolic complications in healthy individuals: Analysis on 6849 participants of the SHIP-study. Leukemia 2023, 37, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Febra, C.; Spinu, V.; Ferreira, F.; Gil, V.; Maio, R.; Penque, D.; Macedo, A. Predictive Value for Increased Red Blood Cell Distribution Width in Unprovoked Acute Venous Thromboembolism at the Emergency Department. Clin. Appl. Thromb./Hemost. 2023, 29. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Xu, Z.; Zhang, P.; Qin, T.; Qu, S.; Pan, L.; Sun, X.; Shi, Z.; Huang, H.; et al. RBC distribution width predicts thrombosis risk in polycythemia vera. Leukemia 2022, 36, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, A.; Vannucchi, A.M.; De Stefano, V.; Masciulli, A.; Guglielmelli, P.; Loscocco, G.G.; Ramundo, F.; Rossi, E.; Kanthi, Y.; Tefferi, A.; et al. Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J. 2022, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.S.; Kim, Y.K.; Jung, S.I.; Jung, H.R.; Chung, I.S. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann. Lab. Med. 2012, 32, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Chatambudza, M.; Skhosana, L.; Ketseoglou, I.; Wiggill, T. High Risk Janus Kinase 2 V617F Allele Burden in a Seven-Year Cohort of Patients with Myeloproliferative Neoplasms. Clin. Lab. 2021, 67, 11. [Google Scholar] [CrossRef]
- Moliterno, A.R.; Kaizer, H.; Reeves, B.N. JAK2 V617F allele burden in polycythemia vera: Burden of proof. Blood 2023, 141, 1934–1942. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Loscocco, G.G.; Mannarelli, C.; Rossi, E.; Mannelli, F.; Ramundo, F.; Coltro, G.; Betti, S.; Maccari, C.; Ceglie, S.; et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021, 11, 199. [Google Scholar] [CrossRef]
- Soudet, S.; Le Roy, G.; Cadet, E.; Michaud, A.; Morel, P.; Marolleau, J.P.; Sevestre, M.A. JAK2 allele burden is correlated with a risk of venous but not arterial thrombosis. Thromb. Res. 2022, 211, 1–5. [Google Scholar] [CrossRef]
- Nikolova, D.; Radinov, A. Thrombotic incidents in patients with myelofibrosis suggest to be independent of JAK2 V617f mutational status. Folia Med. 2022, 64, 655–660. [Google Scholar] [CrossRef]
- Pasquer, H.; Daltro de Oliveira, R.; Vasseur, L.; Soret-Dulphy, J.; Maslah, N.; Zhao, L.P.; Marcault, C.; Cazaux, M.; Gauthier, N.; Verger, E.; et al. Distinct clinico-molecular arterial and venous thrombosis scores for myeloproliferative neoplasms risk stratification. Leukemia 2024, 38, 326–339. [Google Scholar] [CrossRef]
- Narlı Özdemir, Z.; İpek, Y.; Patır, P.; Ermiş, G.; Çiftçiler, R.; Özmen, D.; Baysal, M.; Gürsoy, V.; Yıldızhan, E.; Güven, S.; et al. Impact of CALR and JAK2V617F Mutations on Clinical Course and Disease Outcomes in Essential Thrombocythemia: A Multicenter Retrospective Study in Turkish Patients. Turk. J. Haematol. 2024, 41, 26–36. [Google Scholar] [CrossRef]
- Pérez Encinas, M.M.; Sobas, M.; Gómez-Casares, M.T.; Abuin Blanco, A.; Noya Pereira, M.S.; Raya, J.M.; Andrade-Campos, M.M.; Álvarez Larrán, A.; Lewandowski, K.; Łukasz, S.; et al. The risk of thrombosis in essential thrombocythemia is associated with the type of CALR mutation: A multicentre collaborative study. Eur. J. Haematol. 2021, 106, 371–379. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Wang, D.; Yang, E.; Li, Y.; Li, Y.; Sun, Y.; Wang, M.; Lv, Y.; Hu, X. TET2 Mutation May Be More Valuable in Predicting Thrombosis in ET Patients Compared to PV Patients: A Preliminary Report. J. Clin. Med. 2022, 11, 6615. [Google Scholar] [CrossRef]
- Mroczkowska-Bękarciak, A.; Szeremet, A.; Chyrko, O.; Wróbel, T. CALR-mutant myeloproliferative neoplasms: Insights from next-generation sequencing. J. Appl. Genet. 2025. [Google Scholar] [CrossRef]
- Passamonti, F.; Thiele, J.; Girodon, F.; Rumi, E.; Carobbio, A.; Gisslinger, H.; Kvasnicka, H.M.; Ruggeri, M.; Randi, M.L.; Gangat, N.; et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: A study by the International Working Group on Myelofibrosis Research and Treatment. Blood 2012, 120, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; Bertozzi, I.; et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012, 120, 5128–5133. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Carobbio, A.; Rumi, E.; De Stefano, V.; Mannelli, L.; Mannelli, F.; Rotunno, G.; Coltro, G.; Betti, S.; Cavalloni, C.; et al. Validation of the IPSET score for thrombosis in patients with prefibrotic myelofibrosis. Blood Cancer J. 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Vannucchi, A.M.; Buxhofer-Ausch, V.; De Stefano, V.; Betti, S.; Rambaldi, A.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015, 5, e369. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zeinah, G.; Krichevsky, S.; Silver, R.T.; Taylor, E.; Tremblay, D.; Srisuwananukorn, A.; Mascarenhas, J.; Scandura, J. A Novel Machine Learning-Derived Dynamic Scoring System Predicts Risk of Thrombosis in Polycythemia Vera (PV) Patients. Blood 2021, 138, 3619. [Google Scholar] [CrossRef]
- Khosla, A.A.; Batra, N.; Singh, R.; Jatwani, K.; Batra, A.; Zhang, Y.; Zhang, Z.; Ahmed, A.; Roy, M.; Ramamoorthy, V.; et al. Machine Learning to Predict Risk of Venous Thromboembolism Among Patients with Myeloproliferative Neoplasms in the Intensive Care Unit. Blood 2023, 142, 1840. [Google Scholar] [CrossRef]
- Verstovsek, S.; Krečak, I.; Heidel, F.H.; De Stefano, V.; Bryan, K.; Zuurman, M.W.; Zaiac, M.; Morelli, M.; Smyth, A.; Redondo, S.; et al. Identifying Patients with Polycythemia Vera at Risk of Thrombosis after Hydroxyurea Initiation: The Polycythemia Vera—Advanced Integrated Models (PV-AIM) Project. Biomedicines 2023, 11, 1925. [Google Scholar] [CrossRef]
- Haider, M.; Gangat, N.; Lasho, T.; Abou Hussein, A.K.; Elala, Y.C.; Hanson, C.; Tefferi, A. Validation of the revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic patients. Am. J. Hematol. 2016, 91, 390–394. [Google Scholar] [CrossRef]
- Mancuso, S.; Accurso, V.; Santoro, M.; Raso, S.; Contrino, A.D.; Perez, A.; Di Piazza, F.; Florena, A.M.; Russo, A.; Siragusa, S. The Essential Thrombocythemia, Thrombotic Risk Stratification, and Cardiovascular Risk Factors. Adv. Hematol. 2020, 2020, 9124821. [Google Scholar] [CrossRef] [PubMed]
- Saelue, P.; Thongsukhsai, P. Risk of thrombosis in essential thrombocythemia according to three prediction models: An external validation study. J. Thromb. Thrombolysis 2023, 55, 527–535. [Google Scholar] [CrossRef]
- Alvarez-Larrán, A.; Cuevas, B.; Velez, P.; Noya, S.; Caballero-Navarro, G.; Ferrer-Marín, F.; Carbonell, S.; Pérez-Encinas, M.; Gómez-Casares, M.T.; Pérez-López, R.; et al. Application of IPSET-thrombosis in 1366 Patients Prospectively Followed From the Spanish Registry of Essential Thrombocythemia. Hemasphere 2023, 7, e936. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, B.; Elshoeibi, A.M.; Elhadary, M.; Ferih, K.; Elsabagh, A.A.; Rahhal, A.; Abu-Tineh, M.; Afana, M.S.; Abdulgayoom, M.; Yassin, M. Applications of Artificial Intelligence in Philadelphia-Negative Myeloproliferative Neoplasms. Diagnostics 2023, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Barbui, T. Polycythemia Vera and Essential Thrombocythemia: 2021 Update on Diagnosis, Risk-Stratification and Management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Dillinger, J.G.; Sollier, C.B.D.; Sideris, G.; Ronez, E.; Henry, P.; Drouet, L. Twice Daily Aspirin to Improve Biological Aspirin Efficacy in Patients with Essential Thrombocytemia. Thromb. Res. 2012, 129, 91–94. [Google Scholar] [CrossRef]
- Landolfi, R.; Marchioli, R.; Kutti, J.; Gisslinger, H.; Tognoni, G.; Patrono, C.; Barbui, T. Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. N. Engl. J. Med. 2004, 350, 114–124. [Google Scholar] [CrossRef]
- Pascale, S.; Petrucci, G.; Dragani, A.; Habib, A.; Zaccardi, F.; Pagliaccia, F.; Pocaterra, D.; Ragazzoni, E.; Rolandi, G.; Rocca, B.; et al. Aspirin-Insensitive Thromboxane Biosynthesis in Essential Thrombocythemia Is Explained by Accelerated Renewal of the Drug Target. Blood 2012, 119, 3595–3603. [Google Scholar] [CrossRef]
- Tremblay, D.; Kosiorek, H.E.; Dueck, A.C.; Hoffman, R. Evaluation of Therapeutic Strategies to Reduce the Number of Thrombotic Events in Patients with Polycythemia Vera and Essential Thrombocythemia. Front. Oncol. 2021, 10, 636675. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus Standard Therapy for the Treatment of Polycythemia Vera. N. Engl. J. Med. 2015, 372, 426–435. [Google Scholar] [CrossRef]
- Passamonti, F.; Griesshammer, M.; Palandri, F.; Egyed, M.; Benevolo, G.; Devos, T.; Callum, J.; Vannucchi, A.M.; Sivgin, S.; Bensasson, C.; et al. Ruxolitinib for the Treatment of Inadequately Controlled Polycythaemia Vera without Splenomegaly (RESPONSE-2): A Randomised, Open-Label, Phase 3b Study. Lancet Oncol. 2017, 18, 88–99. [Google Scholar] [CrossRef]
- Masciulli, A.; Ferrari, A.; Carobbio, A.; Ghirardi, A.; Barbui, T. Ruxolitinib for the Prevention of Thrombosis in Polycythemia Vera: ASystematic Review and Meta-Analysis. Blood Adv. 2020, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Zeng, Q.C.; Zhao, W.H.; Li, B.S.; Chen, R.-L. Interferon-2b Gains High Sustained Response Therapy for Advanced Essential Thrombocythemia and Polycythemia Vera with JAK2V617F Positive Mutation. Leuk. Res. 2014, 38, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Patel, K.P.; Newberry, K.J.; Cortes, J.; Borthakur, G.; Konopleva, M.; Estrov, Z.; Kantarjian, H.; Verstovsek, S. Pegylated Interferon Alfa-2a in Patients with Essential Thrombocythaemia or Polycythaemia Vera: A Post-Hoc, Median 83 Month Follow-up of an Open-Label, Phase 2 Trial. Lancet Haematol. 2017, 4, e165–e175, Erratum in Lancet Haematol. 2017, 4, e257. [Google Scholar] [CrossRef] [PubMed]
- Kiladjian, J.J.; Mesa, R.A.; Hoffman, R. The Renaissance of Interferon Therapy for the Treatment of Myeloid Malignancies. Blood 2011, 117, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Bhuria, V.; Baldauf, C.K.; Schraven, B.; Fischer, T. Thromboinflammation in Myeloproliferative Neoplasms (MPN)—A Puzzle Still to Be Solved. Int. J. Mol. Sci. 2022, 23, 3206. [Google Scholar] [CrossRef]
- Baysal, M.; Aksoy, E.; Bedir, K.H.; Özmen, D.; Patır, P.; Demirci, U.; Yaman, S.; Özdemir, Z.N.; Gürsoy, V.; Yıldızhan, E.; et al. Real-world data on direct oral anticoagulants in BCR::ABL1-negative myeloproliferative neoplasms (MPNs): A multicenter retrospective study on behalf of scientific subcommittee on MPNs for Turkish society of hematology. J. Thromb. Thrombolysis 2025, 58, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.H.; Park, W.; Seo, J.; Kang, M.; Jung, E.H.; Kim, S.A.; Suh, K.J.; Kim, J.W.; Kim, S.H.; et al. The Role of Direct Oral Anticoagulants in Managing Myeloproliferative Neoplasms Patients. Cancer Res. Treat. 2025, 57, 612–620. [Google Scholar] [CrossRef]
- Barbui, T.; De Stefano, V.; Carobbio, A.; Iurlo, A.; Alvarez-Larran, A.; Cuevas, B.; Ferrer Marín, F.; Vannucchi, A.M.; Palandri, F.; Harrison, C.; et al. Direct oral anticoagulants for myeloproliferative neoplasms: Results from an international study on 442 patients. Leukemia 2021, 35, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.A.; Green, A.R. Pathogenesis and management of essential thrombocythemia. Hematology Am. Soc. Hematol. Educ. Program. 2009, 1, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Penchas, S.; Eliakim, M. Thrombocytosis in rheumatoid arthritis—Recurrent arterial thromboembolism and death. Ann. Rheum. Dis. 1977, 36, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, S.M.; Isiklar, H.; Sreeram, S.; Weiss, V.J.; Lumsden, A.B. Acute occlusion of the abdominal aorta. Am. J. Surg. 1998, 176, 193–197. [Google Scholar] [CrossRef]
| Score | MPN | Variables | Categories | Reference |
|---|---|---|---|---|
| Conventional approach | ET | age > 60 years history of thrombosis | Low risk (no risk factors) High risk | Barbui et al. (2011) [49] |
| IPSET | ET | age ≥ 60 years (2 points) history of thrombosis (1 point) leukocyte count ≥ 11 × 109/L. (1 point) | Low (points 0) Intermediate (points 1–2) High (points 3–4) | Passamonti et al. (2012) [96] |
| IPSET-thrombosis | ET | age > 60 years (1 point) thrombosis history (2 points) cardiovascular risk factors (1 point) JAK2V617F (2 points) - | Low-risk < 2 points; Intermediate risk 2 points High-risk > 2 points | Barbui et al. (2012) [97] |
| pre-PMF | Guglielmelli et al. (2020) [98] | |||
| Revised IPSET-thrombosis | ET | age > 60 years history of thrombosis JAK2V617F | Very low (age ≤ 60 years, JAK2 wild type, no prior thrombosis) Low (age ≤ 60 years, JAK2 V617F+, no prior thrombosis) Intermediate (age > 60 years, JAK2 wild type, no prior thrombosis) High (age > 60 years and JAK2 V617F+, or prior thrombosis history regardless of other factors) | Barbui et al. (2015) [99] |
| Machine learning | PV | age ≥ 60 years—1 point; prior thrombosis—1 point; leukocyte count ≥ 12 × 10 9/L—1 point peri-diagnosis thrombosis (<2 years from diagnosis)—1 point peri-thrombosis (<2 years from last thrombosis)—1 point | High-risk (score ≥ 2) Intermediate-risk (score = 1) Low-risk (score = 0) | Abu-Zeinah et al. (2021) [100] |
| Machine learning | PV | intrinsic factors (age, blood type), disease events (time since diagnosis/thrombosis), and short-term changes (body mass index) | - | Krichevsky et al. (2023) [101] |
| Machine learning (PV-AIM) | ET | included demographic, clinical, and laboratory data | Verstovsek et al. (2023) [102] | |
| MFPS-PV | PV | age ≥ 60 years cardiovascular risk factors mutation for thrombosis (DNMT3A, ASXL1, or BCOR/BCORL1) previous thrombosis | - | Gu et al. (2023) [61] |
| Arterial Risk Score (ARTS) | ET PV MF MDS unclassified | cardiovascular risk factors (male sex, tobacco use, hypertension, diabetes, or hypercholesterolemia), 1 point; TET2 or DNMT3A mutation, 1 point; age at diagnosis > 60 years, 2 points; arterial thrombosis prior to or at diagnosis, 2 points. | Low risk (score 0–1) High risk (score 2–6). | Pasquer et. al. (2024) [91] |
| Venous Risk Score | ET PV MF MDS unclassified | venous thrombosis prior to or at diagnosis, 1 point; JAK2 V617F mutation, 1 point. | Low risk (score 0) High risk (score 1–2). | Pasquer et al. (2024) [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drăgan, A.; Găvănescu, M.; Drăgan, A.Ş.; Bardaş, A.; Dobrovie, M.; Mateescu, A.D. Clinical and Molecular Insights of Arterial and Venous Thrombosis in Myeloproliferative Diseases—Case-Based Narrative Review. Biomedicines 2025, 13, 2543. https://doi.org/10.3390/biomedicines13102543
Drăgan A, Găvănescu M, Drăgan AŞ, Bardaş A, Dobrovie M, Mateescu AD. Clinical and Molecular Insights of Arterial and Venous Thrombosis in Myeloproliferative Diseases—Case-Based Narrative Review. Biomedicines. 2025; 13(10):2543. https://doi.org/10.3390/biomedicines13102543
Chicago/Turabian StyleDrăgan, Anca, Mădălina Găvănescu, Adrian Ştefan Drăgan, Alexandru Bardaş, Monica Dobrovie, and Anca Doina Mateescu. 2025. "Clinical and Molecular Insights of Arterial and Venous Thrombosis in Myeloproliferative Diseases—Case-Based Narrative Review" Biomedicines 13, no. 10: 2543. https://doi.org/10.3390/biomedicines13102543
APA StyleDrăgan, A., Găvănescu, M., Drăgan, A. Ş., Bardaş, A., Dobrovie, M., & Mateescu, A. D. (2025). Clinical and Molecular Insights of Arterial and Venous Thrombosis in Myeloproliferative Diseases—Case-Based Narrative Review. Biomedicines, 13(10), 2543. https://doi.org/10.3390/biomedicines13102543






