1. Introduction
As populations age globally, the demographic shift brings a heavier burden of infectious disease among older adults. Although many communicable diseases have declined in younger groups, older people remain disproportionately vulnerable or frail. Age-associated immune decline, known as immunosenescence and inflammaging, reduces adaptive immunity and increases baseline systemic inflammation, thereby impairing pathogen response and worsening infection outcomes [
1,
2,
3]. Between 2010 and 2024, hospital admissions of older patients rose nearly 25%, reflecting rising age-related illnesses and infectious complications among older people [
4].
Forecast models estimate that by 2050, the prevalence of chronic diseases and disability among older adults will rise sharply, with related disability nearly doubling [
5]. Thus, the intersection of population ageing and infectious risk is clear: older adults face a higher incidence of infections (e.g., pneumonia, influenza, SARS-CoV-2), a worse mortality rate, and more prolonged or disabling recovery trajectories [
6,
7]. Furthermore, older adults are particularly vulnerable not only to infectious diseases but also to indirect health threats exacerbated by environmental and social stressors, such as climate-related disruptions to food systems and nutrition [
8].
The clinical complexity of older patients presents multiple intertwined challenges. Multimorbidity, often defined as the concomitant presence of 2 or more chronic physical conditions, involving several organ systems rather than isolated conditions, is extremely prevalent: studies report 55% to 98% of older adults presenting with complex multimorbidity, linked to higher mortality and long-term care needs [
9,
10,
11]. Common conditions include hypertension, diabetes, heart disease, chronic lung disease, and sarcopenia, the latter affecting 11–50% of those over 80, and strongly associated with falls, disability, infection vulnerability, and mortality [
12].
Additionally, older patients often present atypically. Instead of textbook symptoms, infections may manifest as confusion, falls, delirium or functional decline, leading to delayed diagnosis and worse outcomes [
13]. Underlying frailty, cognitive impairment, and sensory deficits, using a comprehensive geriatric assessment approach [
14], compound this clinical ambiguity.
Polypharmacy is another major concern: many older patients use multiple medications for comorbid conditions, increasing risks of drug–drug interactions, adverse effects, and reduced physiological reserve. Decisions about stopping potentially inappropriate medications require nuanced, personalized risk-benefit analyses, especially when medications offer marginal benefit or harm in advanced age or multimorbidity [
15].
All these factors combine to make geriatric clinical care highly complex: the same presenting sign may mask multiple underlying conditions; standard protocols may be ill-suited for frail, multimorbid individuals, and routine lab or imaging interpretations may be clouded by age-related changes [
16]. Recent clinical experimental studies increasingly highlight the need for new models of geriatric assessment that account for multimorbidity, frailty, and medication load together.
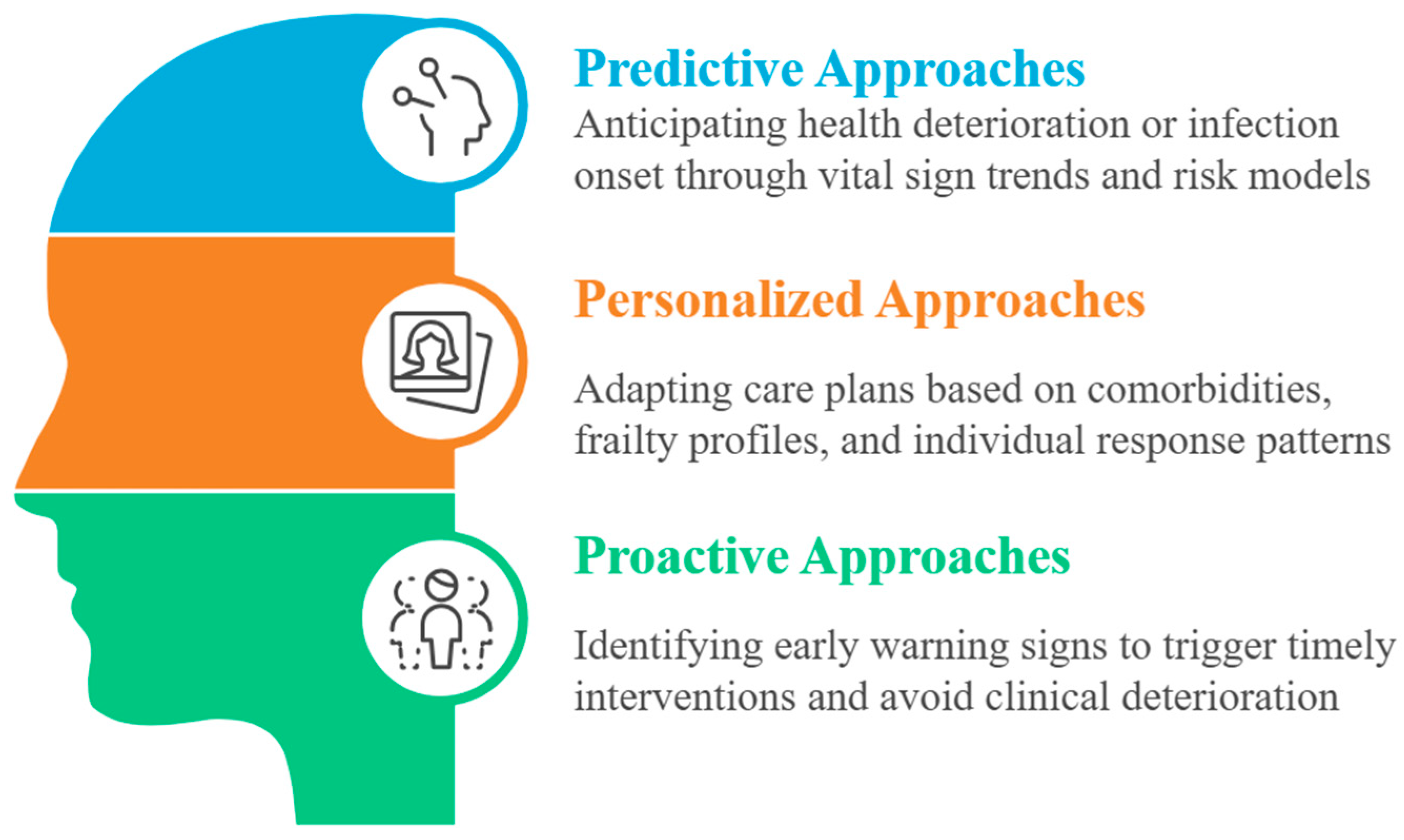
In this regard, Artificial Intelligence (AI) may offer powerful tools to reshape geriatric medicine along three axes: predictive, personalized, and proactive (
Figure 1) [
17].
For example, Machine Learning (ML) models, including deep learning and time-series analysis, can analyse longitudinal datasets and wearable sensor signals to anticipate adverse events. A typical example is the prediction of dementia in people initially free from this condition [
18]. Another broad review of AI in remote patient monitoring (2023) demonstrated the capacity to detect early deterioration in chronic and acute illness via continuous analysis of vital signs and behaviours, using federated learning to preserve privacy and personalize thresholds [
19]. Furthermore, AI methods are already being applied to customize care for older adults. Surveys and clinical trials show older patients respond well to AI-based medication advice systems, though they value transparency and trust in the source [
20]. A recent review highlights applications in fall prediction, cognitive decline tracking, and multi-morbidity management, using integrated patient data, genomics, physiology, and environment to tailor risk assessments and treatment strategies [
21].
Finally, about the topic of proactive care in geriatrics, emerging work on agentic AI, often Large Language Model (LLM)-powered, points toward systems capable of autonomous and pre-emptive decision support. A 2025 theoretical study described AI agents that monitor health, environment, cognition, and daily routines, anticipating declines or hazards and intervening (e.g., suggesting hydration, scheduling check-ins, alerting clinicians), all while preserving autonomy and privacy [
22]. Complementary research in nursing perspectives (2024) emphasized AI’s role in assisting diagnoses, continuous monitoring, and personalized alerts, enabling a proactive rather than reactive model of geriatric care [
23]. Finally, an important 2025 review explicitly addressed the intersection of AI and precision geriatric medicine, citing ongoing trials and pilot studies using predictive analytics to stratify patients, forecast adverse drug effects, tailor therapy plans, and support clinical decision-making in older adult populations [
24]. These systems may integrate genomic, behavioural, physiological, and pharmacological data to optimize interventions and reduce burdens of comorbidity and polypharmacy.
2. Methods
This study followed a narrative review design aimed at synthesizing current evidence on AI applications for the management of infectious diseases in older adults. We searched three major electronic databases, PubMed, Scopus, and Web of Science, for peer-reviewed articles published up to July 2025. The search strategy combined keywords and MeSH terms related to AI, machine learning, deep learning, natural language processing, infectious diseases, older adults, geriatrics, diagnosis, prognosis, antimicrobial stewardship, healthcare-associated infections, and post-discharge care.
We included original studies, systematic reviews, and meta-analyses that reported on the use of AI- or machine learning-based tools in individuals aged ≥65 years, or that provided geriatric-specific analyses. Studies were eligible if they addressed one or more of the following areas: (1) diagnosis of infectious diseases, (2) risk stratification or prognostic modelling, (3) optimization of antimicrobial therapy, (4) prevention and surveillance of healthcare-associated infections (HAIs), or (5) continuity of care after hospital discharge. Articles not available in English, editorials, commentaries, and studies not involving older adult populations were excluded. Titles and abstracts retrieved through the search were screened for relevance, and full texts were reviewed when eligibility was uncertain. Studies meeting the inclusion criteria were synthesized qualitatively and organized by thematic domain (diagnosis, prognosis, antimicrobial stewardship, prevention of HAIs, and continuity of care).
Relevant information was categorized according to clinical application areas, AI methodologies used, reported outcomes (e.g., diagnostic accuracy, AUC, sensitivity), and any geriatric-specific adaptations. The results were synthesized qualitatively and presented across thematic domains.
3. Artificial Intelligence Algorithms Applicable to the Diagnosis of Infections in Older People
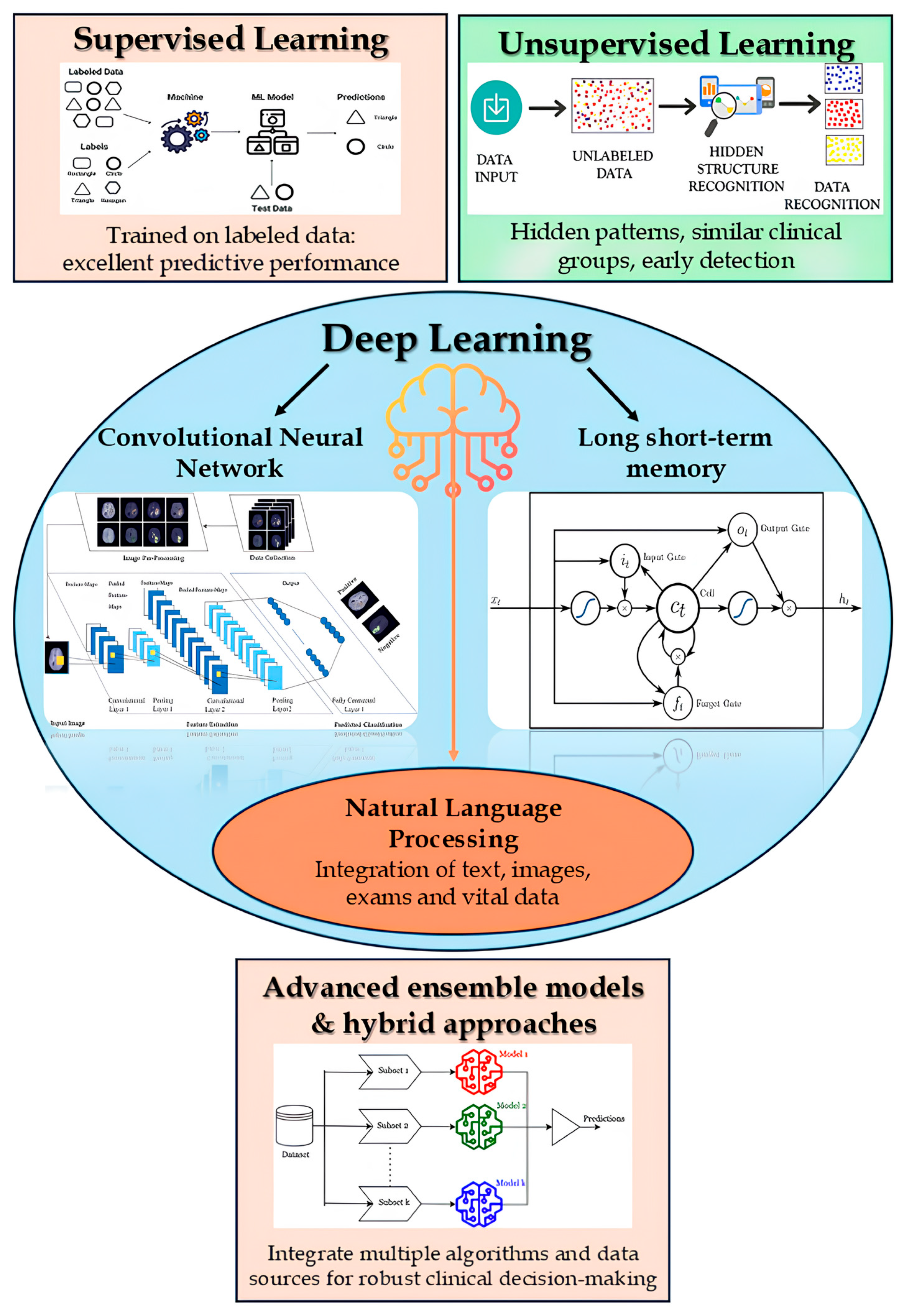
AI methodologies applicable to the diagnosis of infections in older adults encompass a wide spectrum of approaches, ranging from supervised and unsupervised learning to deep learning and natural language processing (NLP) (
Figure 2) [
25].
Within the realm of supervised learning, classical algorithms, such as logistic regression, Support Vector Machines (SVM), and k-nearest neighbors, are trained on labelled clinical datasets to enable early classification of infectious episodes or to estimate individualized risk profiles. In particular, ensemble methods, including Random Forests and boosting-based models such as XGBoost, frequently yield superior performance by integrating the predictive capabilities of multiple decision trees or classifiers [
26]. For instance, Random Forest has achieved an area under the curve (AUC) of approximately predicting nosocomial infections from surveillance data [
27], while an XGBoost-based model reached an AUROC of ~0.97 in the early detection of sepsis among elderly patients [
28].
In contrast, unsupervised learning techniques, such as k-means clustering and anomaly detection models, do not require labeled data and are instrumental in uncovering latent patterns or grouping patients with similar clinical profiles. These methods can aid in the early identification of atypical configurations in vital signs or laboratory results suggestive of infection [
29].
Deep learning further enhances diagnostic capabilities using multilayer neural networks [
30]. Convolutional Neural Networks (CNNs), for example, autonomously extract salient features from complex inputs such as radiological images, enabling the identification of pneumonia on chest X-rays with a diagnostic accuracy comparable to that of experienced radiologists [
31]. Similarly, recurrent neural architectures such as Long Short-Term Memory (LSTM) networks and contemporary Transformer-based models are adept at capturing temporal dependencies within clinical monitoring data. These networks can analyze sequential trends in physiological parameters (e.g., vital signs, laboratory trajectories) to forecast severe infectious events, such as the onset of sepsis, well in advance of clinical manifestation [
32]. Recent studies have demonstrated that Transformer-based models applied to time-series data of vital signs can achieve predictive accuracies of approximately 96% up to 12 h before sepsis onset [
33].
Concurrently, Natural Language Processing (NLP) facilitates the analysis of unstructured clinical texts. Neural models pre-trained on biomedical corpora, such as BERT-based Transformers, can extract infection-related indicators from medical reports, clinical notes, and nursing documentation, often capturing symptomatology and signs of infection overlooked by systems relying solely on structured data [
34]. The integration of NLP techniques with traditional electronic health record (EHR) data has been shown to enhance infection surveillance: for example, the extraction of relevant information from narrative notes via NLP, when combined with structured EHR variables, has improved both specificity and predictive accuracy in the monitoring of surgical site infections [
35].
Finally, advanced ensemble models and hybrid approaches are emerging as effective strategies for multimodal data integration in the clinical domain. Decision-support systems that synthesize diverse data sources, such as real-time vital signs, laboratory results, diagnostic imaging, and free-text documentation, through complementary algorithmic components offer a robust diagnostic framework by leveraging the strengths of multiple methodologies [
36]. These hybrid strategies, such as neural networks capable of fusing heterogeneous data or ensembles that aggregate outputs from diverse models, are explained to enhance the accuracy and reliability of early diagnosis, thereby enabling the timely identification of infections in older adults and guiding proactive clinical interventions [
37].
4. Early Diagnosis and Recognition of Atypical Presentations
AI algorithms represent a strategic asset for the early diagnosis of infections in older adults, owing to their capacity to integrate and simultaneously analyze heterogeneous clinical data from multiple sources. The real-time combination of vital signs, laboratory results, diagnostic imaging, and unstructured text from EHRs enables the overcoming of limitations inherent to traditional clinical interpretation, which is often fragmented and subject to subjective bias [
38,
39]. In particular, infectious risk stratification in clinically complex patients benefits from the multimodal analysis provided by AI systems, which are capable of detecting subtle yet clinically meaningful variations in the patient’s condition. This capability is especially valuable in older adults, in whom infections often present with vague or atypical symptoms (
Figure 3) [
40].
In older adults, infections often manifest through subtle and atypical signs, which may be easily overlooked or misattributed. Algorithms trained on large geriatric datasets can detect these weak signals early and place them within a coherent clinical framework, facilitating the timely initiation of targeted diagnostic pathways.
Numerous studies have highlighted how the implementation of AI-based predictive models can significantly reduce diagnostic delays and contribute to the prevention of inappropriate hospitalizations. For example, an early warning system based on a random forest model, integrated into a university hospital, anticipated the identification of sepsis by over eight hours compared to clinical staff, with a positive impact on the timing of antibiotic therapy and overall clinical outcomes [
41]. In geriatric settings as well, the application of predictive models within internal medicine wards has been associated with a reduction in avoidable emergency department visits and improved appropriateness of hospital admissions.
The applications of such tools extend across various care settings. In hospitals, AI algorithms can support triage, continuous surveillance, and personalized infectious risk management; in long-term care facilities, they enable proactive monitoring of residents and early outbreak detection; in home care and telemedicine, integrated sensors and remote predictive models can detect even minimal clinical changes, allowing for intervention before hospitalization becomes necessary [
42]. These applications underscore the transformative potential of AI in the management of infections among older adults, promoting a predictive, timely, and person-centered model of care.
5. Risk Stratification and Prediction of Clinical Outcomes
The use of AI in risk stratification and prediction of clinical outcomes in older adults with infectious diseases has expanded in recent years, driven by the growing ageing population and the continuous advancements in AI technology [
43]. In this context, AI-based models have proven to be valuable tools for estimating critical outcomes such as sepsis [
44,
45,
46,
47,
48], septic shock [
49], mortality [
50], and the occurrence of complications [
51] in older adults, a population at high risk of adverse events due to comorbidities and increased frailty [
52]. In many cases, current diagnostic methods and predictive algorithms lack specificity for this age group. By leveraging large datasets and advanced algorithms, AI models can instead predict the likelihood of adverse events more accurately, and support clinicians in identifying high-risk patients to enable timely interventions.
A meta-analysis by Zhang et al. (2024) [
44] showed that ML algorithms have excellent diagnostic accuracy in predicting the occurrence of sepsis, suggesting potential for clinical use. Similarly, Lin et al. (2025) [
45] introduced an ML model that utilizes non-invasive routine tests, such as complete blood count data, to accurately predict sepsis in older patients in the intensive care unit (ICU). This model, integrated within an AI-based clinical decision support system (AI-CDSS), enabled early sepsis detection and intervention in critical care settings, improving patient outcomes. Chua et al. (2025) [
49] also emphasized AI’s role in managing sepsis within emergency departments, showing its utility in predicting progression to septic shock and mortality. In terms of mortality prediction, Zhang et al. (2025) [
50] validated an ML model employing sepsis-predictive algorithms capable of estimating 28-day mortality risk in elderly sepsis patients. The model proved to be both rapid and practical, supporting early risk assessment and prompt clinical decision-making. Recent advancements have further refined AI prediction models, extending their utility to conditions such as multi-organ failure (MOF). For example, Rajakaruna et al. (2024) [
51] showed the potential of an AI-driven approach in predicting the onset of MOF in COVID-19 patients.
Another important aspect of AI’s role in infectious disease management is the ability to personalize prognostic assessments based on factors such as frailty, cognitive status, and functional performance, which significantly impact clinical outcomes in older adults but are often challenging if assessed using traditional methods. A review by Velazquez-Diaz et al. (2023) [
53] reported that AI models have been effectively used to identify frailty syndrome using both clinical and non-clinical data, including activity monitoring. Models [
54] incorporating frailty indicators have shown the ability to predict adverse outcomes such as prolonged hospitalization, complications, readmissions, and mortality, underlining the promise of AI in delivering individualized care. However, transitioning these tools from research to clinical practice remains a complex challenge. AI indeed shows promise in predicting cognitive decline [
55], a key concern in geriatric health, opening opportunities for early intervention. In the context of infectious diseases, this application is particularly important: older sepsis survivors are at a significantly higher risk [
56] of long-term cognitive impairment and functional disability. Developing AI models capable of identifying those at risk could enable timely interventions to prevent or mitigate these long-term consequences.
AI-powered clinical decision support systems (CDSS) are increasingly integrated into practice, enhancing decision-making in the management of infectious diseases in older adults. These systems combine patient-specific data to recommend personalized treatment plans. For instance, Düvel et al. (2025) [
57] evaluated the feasibility of an AI-based CDSS prototype for guiding antibiotic therapy in sepsis, while Lin et al. (2025) [
45] found that such systems improved the management of sepsis in elderly patients by aiding clinicians in assessing treatment timing and appropriate care levels. Moreover [
58], AI-based CDSS utilizing EHR data can prioritize treatment alternatives tailored to the complexities of geriatric care. These systems provide clinicians with actionable insights for early, informed decisions about targeted therapies and advanced care planning, helping to align clinical choices with both medical needs and patient preferences.
Beyond individual decision support, AI holds promise for enhancing coordination among multidisciplinary teams, essential in managing complex cases like infectious diseases in older adults. AI models can integrate and synthesize data across various disciplines (geriatrics, infectious diseases, intensive care), offering a comprehensive clinical picture. One study [
59] showcased a digital platform powered by AI that supported care coordination and shared care planning in frailty-focused elder care. Such evidence indicates that integrating AI into multidisciplinary care pathways may lead to improved outcomes and more efficient resource use. By facilitating collaboration and delivering precise, data-driven insights, AI empowers diverse healthcare teams to address the full spectrum of patient needs.
AI-based models for risk stratification and clinical outcome prediction are transforming the management of infectious diseases in older adults. These technologies not only improve predictive accuracy but also support the delivery of personalized, evidence-based care tailored to the complex needs of this vulnerable population. The integration of AI into clinical decision support systems and multidisciplinary care frameworks enhances coordination and efficiency. As AI technologies continue to evolve, they hold the potential to optimize clinical outcomes, streamline resource utilization, and elevate the overall quality of care for older adults facing infectious diseases.
6. Antimicrobial Therapy: Clinical Personalization and AI-Supported Stewardship
AI and ML applications have demonstrated considerable potential in enhancing the clinical personalization of antimicrobial therapy and strengthening antimicrobial stewardship (AMS), particularly relevant in older adult populations. ML models have been effectively employed to guide both empirical and targeted antimicrobial selection by integrating complex clinical and microbiological variables such as comorbidities, prior antibiotic exposure, local resistance patterns, organ function, comorbidities, and real-time Electronic Health Record (HER) data. For instance, a deep learning-based optical system was developed to automate and accelerate antimicrobial susceptibility testing [
60]. The model achieved high discriminative performance (mean AUC: 0.87).
Retrospective case studies reinforce the clinical applicability of these tools. In the United States, the PyTorch EHR deep learning model achieved an AUC of 0.911 for predicting Methicillin-Resistant Staphylococcus aureus (MRSA) positivity using time-series EHR data, outperforming traditional models and enhancing early therapeutic decision-making [
61]. Similarly, in ICU settings, random forest classifiers have been used to predict the risk of carbapenem-resistant Gram-negative infections, demonstrating improved accuracy (84%) and enabling timely interventions [
62].
Moreover, ML algorithms have been used to calculate optimal dosing strategies in the presence of renal or hepatic dysfunctions, especially renal or hepatic impairment, addressing a common clinical need in geriatric care [
63]. In surgical settings, AI models assessing prophylactic adequacy have yielded outstanding predictive performance (AUC > 0.97), supporting context-specific antibiotic management [
64]. At the institutional level, AI has also been applied to detect inappropriate prescribing patterns and to promote AMS. A recent systematic review evaluating AI applications in antimicrobial stewardship programs found that ML models not only outperformed traditional rule-based approaches in sensitivity and negative predictive value but also provided explainable support for early detection of inappropriate prescriptions and multidrug-resistant organisms, particularly in vulnerable populations such as older adults [
65]. For instance, decision-tree-based models have identified misuse of agents such as piperacillin/tazobactam, offering real-time feedback for quality improvement initiatives and educational interventions [
66]. Importantly, AI-driven systems have supported prospective audit-and-feedback mechanisms by offering interpretable predictions to clinicians, thereby facilitating acceptance and appropriate use of AI recommendations. These tools also aid in early identification of candidates for de-escalation, reducing antimicrobial pressure and supporting resistance prevention strategies [
67].
A recent meta-analysis confirms the high diagnostic and predictive performance of AI tools across AMS settings, with pooled AUC, accuracy, and sensitivity estimates ranging from 72% to 77%, and negative predictive values approaching 80% [
68]. Notably, these tools can enhance safety and efficacy in the geriatric population, which is disproportionately affected by adverse drug reactions and multidrug-resistant infections [
69]. In this perspective, AI-enabled antimicrobial therapy represents a major step toward precision medicine, enhances therapeutic safety, reduces drug-related complications, and promotes sustainability in older adults [
70,
71]. By optimizing both patient-level and institution-wide prescriptions, these tools support therapeutic safety, reduce unnecessary antibiotic exposure, and foster sustainable prescribing practices aligned with the complex needs of ageing populations.
7. Prevention, Surveillance and Control of Healthcare Associated Infections (HAIs)
In older adult populations, AI is emerging as a pivotal tool for the prevention and control of HAIs, through early risk stratification, outbreak surveillance, and the implementation of targeted interventions. ML models can analyze EHRs and device-derived data to identify high-risk elderly patients, such as those with indwelling catheters, mechanical ventilation, or prosthetic implants, who are particularly susceptible to HAIs. This enables the timely application of preventive measures, including the reinforcement of hygiene protocols or the early removal of invasive devices.
These predictive algorithms often demonstrate high levels of diagnostic accuracy. A recent meta-analysis reported a pooled sensitivity of approximately 84%, a specificity of 90%, and an AUC of around 0.86 for AI-based models in HAI surveillance, outperforming or matching the accuracy of conventional manual infection tracking [
72]. Specifically, models addressing the most common HAIs, such as urinary tract infections and surgical site infections, frequently exceed an AUC of 0.80, underscoring their robust reliability [
73].
AI-powered surveillance systems, implemented within hospitals and long-term care facilities, can continuously monitor real-time clinical signals to detect outbreaks earlier than traditional methods, triggering timely alerts in geriatric wards or residential care settings. For instance, an AI-driven monitoring platform deployed in nursing homes was able to flag early signs of respiratory or urinary infections 2 to 4 days prior to clinical diagnosis (AUC = 0.86) [
74]. Likewise, an automated syndromic surveillance network across elder care facilities demonstrated earlier outbreak detection and reduced outbreak magnitude compared to conventional reporting systems [
75].
Predictive analytics also inform tailored preventive actions: by identifying which patients or environments carry the highest risk, AI can assist infection control teams in deploying focused interventions, such as targeted decolonization, optimized sanitation schedules, or preemptive isolation, to contain environmental reservoirs of pathogens. In practice, these tools support epidemiological management by synthesizing complex data into actionable insights, enabling infection prevention specialists to allocate resources efficiently and respond swiftly in high-risk geriatric settings [
76].
Initial implementations have shown promising outcomes: one hospital reported an 85% reduction in manual surveillance workload and a halving of HAI incidence following the deployment of an AI-assisted surveillance system [
28]. Although routine clinical adoption remains in its early stages due to challenges related to system integration and workflow alignment, current evidence strongly suggests that AI can significantly enhance the prevention, monitoring, and control of HAIs in geriatric care, offering superior accuracy, timeliness, and adaptability when compared to traditional approaches.
8. Continuity of Care and Reduction in Readmissions
Older adults recovering from infection are vulnerable to early deterioration and unplanned rehospitalization because of frailty, multimorbidity, polypharmacy, cognitive impairment, and atypical recovery patterns. Aligning continuity-of-care tools with these geriatric constructs is essential for safe discharge and sustained recovery (
Figure 4).
8.1. AI Algorithms to Predict Risk of Post-Infection Readmission
Post-sepsis and post-pneumonia readmissions remain frequent and costly; ML models using EHR and claims data can identify patients at high risk within 30 days of discharge [
77]. In multicenter evaluations of AI-enabled sepsis prediction embedded in workflow, implementation was associated with reductions in in-hospital mortality, length of stay, and 22.7% lower 30-day readmission, suggesting a downstream impact on continuity outcomes [
78]. ML models tailored to older adults and multimorbidity are emerging: recent geriatric readmission models and ML frameworks have shown improved discrimination over traditional scores, focusing on social and functional determinants. For pneumonia, national-database analyses demonstrate that gradient-boosting and rule-based models identify comorbidity, illness severity, discharge disposition, and payer type as key drivers [
79]. High-performing models combine structured data with unstructured notes via natural language processing to evaluate geriatric complexity, functional status and social risk. This, coupled with age- or frailty-calibrated thresholds, enables earlier identification of frail patients needing intensified follow-up and risk-tiered actions, such as pharmacist review for polypharmacy, early home visits, telemonitoring enrolment, etc. [
77]. Implemented in this way, AI-guided, risk-stratified transitional care is associated with measurable reductions in 30-day post-infection readmissions in older adults.
8.2. Planning Assisted Discharge and Home Patient Monitoring
Assisted discharge bundles (discharge reconciliation, patient/caregiver teaching, and scheduled follow-up) reduce readmissions in older adults; augmenting transitional-care programs with AI-derived risk and needs assessments further decreases rehospitalization [
80]. Systematic evidence indicates that EHR-based interventions (alerts, care-plan automation, post-discharge tasking) are associated with 17–28% reductions in 30-/90-day readmissions across RCTs [
81]. Discharge planning tools should: (i) embed AI risk at the point of discharge, (ii) auto-generate individualized follow-up (infectious-disease reviews, renal labs for nephrotoxic agents, vaccine appointments) [
82], (iii) flag red-flag symptoms tailored to atypical presentations (e.g., delirium, falls), and (iv) route tasks to community nurses and caregivers.
Home patient monitoring after infectious discharge relies on simple vital-sign kits and symptom diaries. Often those records are not integrated with the EHR, and they are corrected and analyzed in a comprehensive way only during hospital readmission [
83]. Implementing AI-driven trend detection and more frequent home visits allow the interpretation of early relapses (tachycardia, hypoxia, fever) and medication-related toxicity.
8.3. Integration with Telemedicine, Wearable Devices, and Digital Platforms
Remote patient monitoring (RPM) and telehealth can detect deterioration early, but effects on readmissions vary by condition and program design. Systematic reviews in hospital-at-home and post-acute populations highlight feasibility, early detection, and heterogeneity of impact [
84]. Telemonitoring reduces readmissions in chronic obstructive pulmonary disease (COPD) and pneumonia, with less consistent benefit in heart failure [
85,
86]. Telemedicine can extend post-infection follow-up for older adults by combining scheduled tele-visits with real-time teleconsultation between home-visiting nurses and supervising physicians. In Hospital-at-Home programs, remote visits were non-inferior to in-home visits for safety and experience [
87].
Remote patient monitoring ranges from non-wearable home kits (pulse oximetry, Blood Pressure, temperature) to wearables that stream physiology (heart rate, respiratory rate, nocturnal SpO
2, activity) [
88]. During and beyond COVID-19, home oximetry programs proved feasible and generally safe [
89]. Wearables for older adults are increasingly studied: reviews document usability considerations and sensor choices relevant to detecting infectious relapse [
90].
Digital care platforms can close the hospital-to-community gap by unifying tele-visits, RPM feeds, medication surveillance, and shared care plans across hospital, primary care, and community nursing. World Health Organization (WHO) guidance emphasizes that digital interventions should strengthen, not replace, care systems, with attention to feasibility and equity [
91].
8.4. Support for Integrated Territorial Care Models and Safe Hospital-to-Community Transition
Integrated territorial care aligns with WHO’s Integrated Care for Older People (ICOPE) approach, emphasizing person-centered assessment, shared care plans, and community follow-up, now supported by digital tools and decision support [
92]. AI and IoT-enabled transitional pathways can coordinate primary care, home nursing, and social services, with early pilot evidence of feasibility and proposed outcome frameworks [
88,
93].
9. Current Limitations and Prospects
The application of AI in the geriatric infectious disease domain offers both substantial promise and notable limitations. On the one hand, ML and deep learning algorithms have demonstrated encouraging capabilities in enhancing diagnostic accuracy, prognostic stratification, and the personalization of therapeutic strategies for older adults, for instance, by optimizing antimicrobial stewardship and enabling the early identification of patients at risk for infectious complications. On the other hand, the current literature underscores that evidence specifically tailored to the geriatric population remains limited and fragmented.
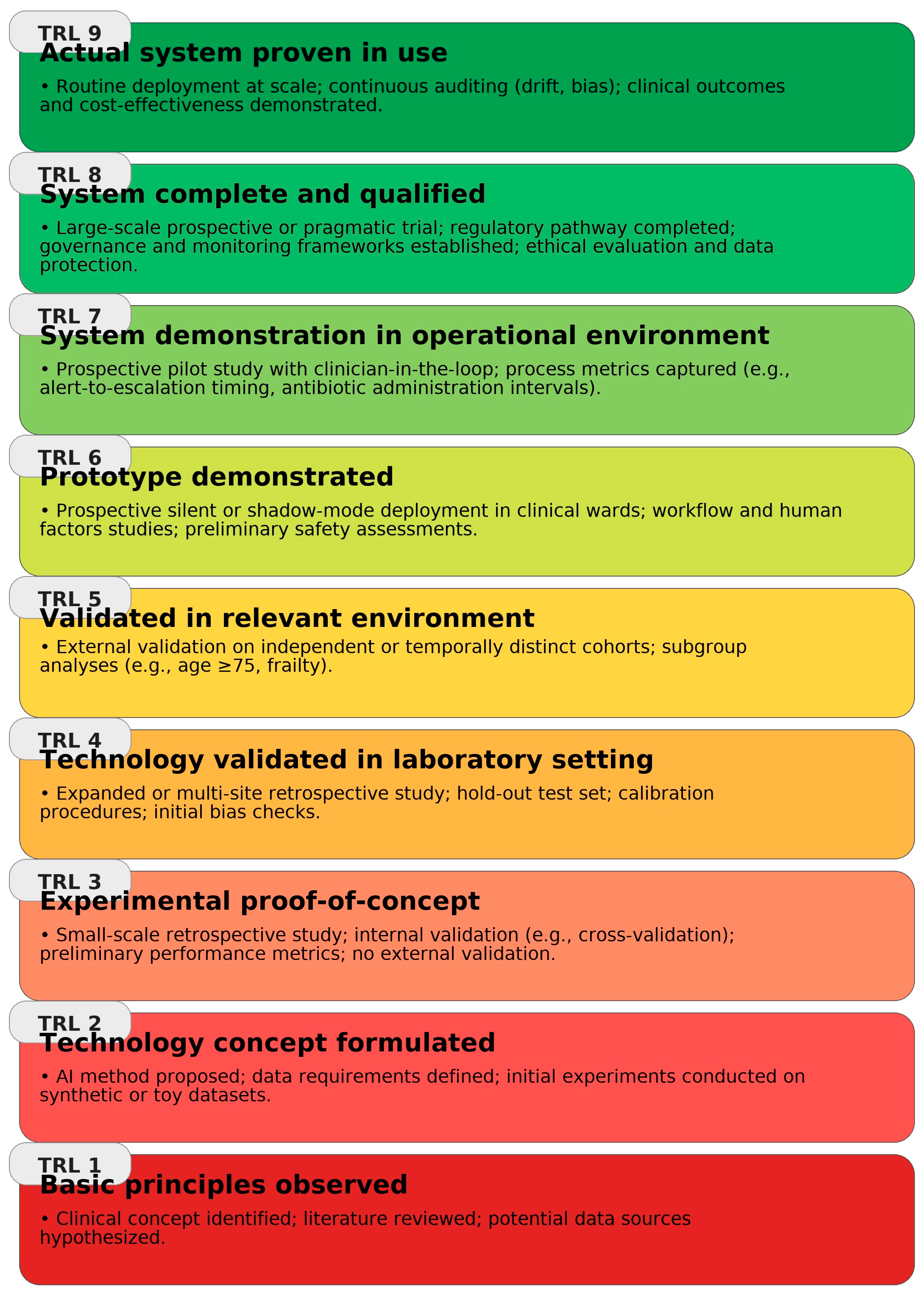
Building on NASA’s Technology Readiness Level (TRL) framework [
94],
Figure 5 synthesizes the implementation maturity of clinical AI use-cases across the geriatric infection pathway, highlighting domains with stronger external/prospective evidence versus those where deployment remains preliminary.
To better delineate the scope and maturity of the current body of evidence,
Table 1 systematically synthesizes the principal study designs, target populations, evaluated endpoints, and reported methodological limitations. This tabular overview complements the qualitative synthesis by providing a concise framework for assessing both the strengths and the translational gaps of AI applications in geriatric infectious disease care.
9.1. Limits of Current Evidence and Applications
Despite the growing body of research on AI in medicine, there remains a striking paucity of studies specifically targeting older adults, leading to the underrepresentation of geriatric patients in the training datasets of AI models. This demographic gap can introduce systematic biases and compromise the generalizability of algorithms to frail populations, thereby undermining both the equity and reliability of AI-driven clinical decisions [
96]. In particular, the underrepresentation of older and frail adults in AI training datasets represents a critical source of bias, as models trained primarily on younger or healthier populations may fail to capture the complexity and heterogeneity of geriatric patients. Indeed, geriatricians and data scientists have warned that AI may inadvertently exacerbate health disparities in geriatric care due to biased model development and the lack of external validation on appropriately aged populations [
97]. This limitation can reduce model accuracy, compromise external validity, and risk exacerbating health disparities. To date, only a handful of prospective multicenter studies have evaluated the impact of AI systems on clinically meaningful outcomes in older adults, and the absence of such rigorous trials continues to cast uncertainty on the true clinical benefit and cost-effectiveness of these tools in geriatric practice. Future research should therefore prioritize the development of dedicated, representative datasets and multicenter, externally validated studies that include sufficient numbers of frail and very old individuals to ensure equitable and generalizable AI applications in geriatric infectious disease care.
Another limitation concerns the methodological rigor underlying the reported high performance of many AI models. AUC values approaching 0.98, while impressive, may conceal overfitting when derived from retrospective analyses on restricted or homogeneous datasets. Few studies explicitly report how missing data, variable selection, or hyperparameter tuning were handled, limiting the assessment of model robustness and reproducibility. Furthermore, calibration analyses, essential to determine whether predicted risks align with observed outcomes, are rarely presented, making it difficult to evaluate clinical reliability beyond discrimination metrics. The lack of standardized benchmarking against optimally specified conventional statistical models also risks inflating the apparent superiority of AI approaches. Importantly, the gap between algorithmic performance in controlled research settings and effectiveness in routine geriatric care remains wide: most systems have not been tested prospectively within clinical workflows, where multimorbidity, frailty, and heterogeneous data quality may markedly degrade performance. Bridging this translational gap will require prospective studies embedded in real-world care pathways, systematic calibration and reproducibility assessments, and the inclusion of clinically meaningful endpoints such as treatment adequacy, timeliness of intervention, and patient-centered outcomes.
Beyond high-level considerations, three practical domains shape adoption in geriatric settings: (i) data quality and interoperability: heterogeneous EHR schemas, non-random missingness, and site-to-site microbiology variability limit generalizability; multi-institutional or federated data and standardized pipelines can mitigate this [
98]; (ii) prospective, externally validated evidence: most studies remain retrospective/single-center; few pilots or pragmatic trials report calibration and fairness auditing, constraining real-world reliability [
99]; (iii) organizational readiness and workflow fit: limited leadership support, and insufficient clinician training hinder EHR integration and sustained use; explainability is essential to clinician trust and to avoid alert fatigue. Targeted enablers include governance for privacy/ethics, interpretable models with routine calibration (e.g., SHapley Additive exPlanations SHAP/Local Interpretable Model-agnostic Explanations LIME, calibration plots), EHR-native deployment, and structured education [
100].
An additional limitation concerns the practical implementation of AI across various geriatric care settings. Long-term care facilities and community-based home care services frequently suffer from low levels of digitalization and poorly integrated health information infrastructures, posing significant barriers to the deployment of advanced data-driven tools. Even in hospital environments, integrating AI algorithms into existing clinical workflows can encounter organizational and resource-related obstacles, particularly in the absence of dedicated managerial support [
101]. Furthermore, current AI models are often opaque and difficult to interpret, so-called “black boxes”, which hinders their usability in everyday geriatric clinical practice [
102]. Clinicians often express understandable reluctance to rely on recommendations generated by non-explainable algorithms, fearing unforeseen errors and challenges in reconciling such outputs with their clinical judgment. The lack of interpretable and transparent models thus hampers trust among healthcare professionals and constitutes a major barrier to the widespread adoption of AI in the care of frail older adults [
103]. Furthermore, many of the available studies are limited by small sample sizes, single-center settings, and restricted inclusion of very old or frail patients, which further constrains the generalizability of their findings. Lastly, the included studies are highly heterogeneous in terms of objectives, design, populations, and analytical approaches, which limits direct comparability and makes it difficult to draw definitive conclusions. Future research should therefore prioritize well-designed, multicenter, and externally validated studies that adhere to rigorous scientific methodology to ensure reproducible and generalizable results in geriatric infectious disease care.
9.2. Development Perspectives
Considering these limitations, several avenues for development have emerged to ensure that AI becomes a truly effective and safe tool for the management of infections in geriatric settings. Foremost among these is the urgent need to build dedicated datasets for the frail elderly population, enriching AI models with specific data (clinical, laboratory, and functional) pertinent to individuals aged 75 and older. Targeted data collection initiatives, ideally supported by multicenter consortia and geriatric biobanks, may enhance representativeness and mitigate age-related biases in algorithmic performance.
Concurrently, the co-design of AI tools in close collaboration with multidisciplinary teams, including geriatricians, infectious disease specialists, pharmacologists, nurses, and data scientists, is essential to ensure that the solutions developed address real-world clinical needs. Such participatory approaches foster the creation of models attuned to the geriatric context, accounting for factors such as multimorbidity, polypharmacy, and frailty, while also facilitating user acceptance from the early stages of development [
104]. In the broader context of combating antimicrobial resistance, it has likewise been emphasized that research must be steered toward more interpretable AI models and that interdisciplinary collaboration is key to integrating these technologies equitably and sustainably into care pathways [
95].
Another critical perspective involves the integration of AI into regional and national health information systems. This requires the development of interoperable infrastructures that allow algorithms to access electronic health records, public health registries, and other relevant data sources securely, while fully complying with privacy regulations. Investing in advanced digital platforms within long-term care facilities and community healthcare settings will be instrumental in bridging the digital divide, thereby ensuring that AI-based surveillance and decision support tools can also be deployed in resource-limited or peripheral contexts [
105].
Simultaneously, the development of “explainable” AI models, endowed with transparent and interpretable decision-making logic, will be imperative. The adoption of principles from Explainable AI (XAI) and ethics-by-design can help overcome the “black box” problem, enhancing the perceived trustworthiness of these technologies. The ability to trace and communicate the rationale behind a prediction (e.g., using techniques such as LIME or SHAP) is, in fact, crucial for fostering clinician and patient acceptance of algorithmic support.
Finally, greater emphasis must be placed on the training of healthcare professionals in the critical use of AI. Physicians, nurses, and other practitioners will need to acquire AI literacy and develop the competencies necessary to interpret algorithmic recommendations appropriately and integrate them into clinical decision-making. A recent systematic review on the subject underscored that clinician education, coupled with the implementation of XAI techniques, constitutes a foundational step toward improving both understanding and acceptance of artificial intelligence in routine practice [
106].
From an implementation standpoint, workflow integration requires EHR-native deployment with outputs available early in the admission episode; Ali et al. [
107] illustrate ML models trained on admission clinical data with SHAP explanation, an approach conducive to timely decision-making. Training should build AI literacy around feature-attribution to support clinician acceptance and safe escalation pathways. Cost-effectiveness ultimately requires prospective/pragmatic evaluations linking model-driven actions to resource use and outcomes. Ethical/legal safeguards entail Institutional Review Board (IRB) oversight and robust data-protection/governance across sites, acknowledging missingness and documentation variability that affect reliability and accountability in AI-supported decisions [
107].
Taken together, these measures, when guided by clear ethical and governance frameworks, will lay the groundwork for the effective, safe, and equitable implementation of AI in infectious disease management for older adults, ultimately advancing a model of care that is both data-driven and profoundly person-centered.
10. Conclusions
AI is increasingly emerging as a high-potential resource to enhance diagnostic accuracy, refine prognostic stratification, and personalize the therapeutic management of infections in older adults, thus addressing the growing clinical complexity of the geriatric population. However, for these tools to yield tangible and measurable benefits, rigorous validation, seamless integration into care pathways, and careful adaptation to the infectious and pathophysiological specificities of advanced age are indispensable.
This trajectory demands a structured alliance of expertise: only through the synergistic collaboration of clinicians, data scientists, epidemiologists, public health professionals, and institutional decision-makers can implementation be rendered effective, safe, and equitable. The future of geriatric infectious disease medicine will thus be increasingly data-driven and AI-supported, yet also deeply centered on the individual, on their clinical history, personal values, and inherent vulnerabilities.
Technological progress must be guided by an ethics of care: the algorithm must remain in service of the person, and not the other way around. Ultimately, the future of geriatric infectiology will be shaped by powerful algorithms—but it must always speak the language of human frailty.
Author Contributions
Conceptualization, A.P., F.P., and V.G.; methodology, A.P., and F.P.; software, A.P., and S.O.; validation, F.P., and V.G.; formal analysis, C.S., and V.G.; investigation, A.P., F.P., S.O., E.D.P., N.V., and V.G.; resources, A.P., F.P., S.O., E.D.P., and N.V.; data curation, A.P., F.P., S.O., E.D.P., N.V., and V.G.; writing—original draft preparation, A.P., F.P., S.O., E.D.P., N.V., and V.G.; writing—review and editing, F.P., A.P., C.S., V.B. and V.G.; visualization, A.P., and S.O.; supervision, C.S., V.B. and V.G.; project administration, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Pennisi, F.; Maggi, S.; Veronese, N.; Soysal, P. Aging, longevity, and healthy aging: The Public health approach. Aging Clin. Exp. Res. 2025, 37, 125. [Google Scholar] [CrossRef]
- Shreya, D.; Fish, P.N.; Du, D. Navigating the Future of Elderly Healthcare: A Comprehensive Analysis of Aging Populations and Mortality Trends Using National Inpatient Sample (NIS) Data (2010–2024). Cureus 2025, 17, e80442. [Google Scholar] [CrossRef]
- Brown, C.; Losina, E. Epidemiology of Aging (Socioeconomic Impact). In Medical Radiology; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–22. [Google Scholar] [CrossRef]
- Veronese, N.; Polidori, M.C.; Maggi, S.; Zamora, J.; Ruiz-Calvo, G.; Bangert, M.; Bourron, P.; Bausch, A.; Avilés-Hernández, J.D.; López-Soto, A.; et al. Measuring the impact of hospitalization for infectious diseases on the quality of life of older patients in four european countries: The AEQUI longitudinal matched cohort study (2020–2023). Clin. Microbiol. Infect. 2025, 31, 847–854. [Google Scholar] [CrossRef]
- Abbadi, A.; Gentili, S.; Tsoumani, E.; Brandtmüller, A.; Hendel, M.K.; Salomonsson, S.; Calderón-Larrañaga, A.; Vetrano, D.L. Impact of lower-respiratory tract infections on healthcare utilization and mortality in older adults: A Swedish population-based cohort study. Aging Clin. Exp. Res. 2024, 36, 146. [Google Scholar] [CrossRef]
- Nucci, D.; Pennisi, F.; Pinto, A.; De Ponti, E.; Ricciardi, G.E.; Signorelli, C.; Veronese, N.; Castagna, A.; Maggi, S.; Cadeddu, C.; et al. Impact of extreme weather events on food security among older people: A systematic review. Aging Clin. Exp. Res. 2025, 37, 137. [Google Scholar] [CrossRef]
- Onder, G.; Vetrano, D.L.; Palmer, K.; Trevisan, C.; Amato, L.; Berti, F.; Campomori, A.; Catalano, L.; Corsonello, A.; Kruger, P.; et al. Italian guidelines on management of persons with multimorbidity and polypharmacy. Aging Clin. Exp. Res. 2022, 34, 989–996. [Google Scholar] [CrossRef]
- Pennisi, F.; Buzzoni, C.; Russo, A.G.; Gervasi, F.; Braga, M.; Renzi, C. Comorbidities, Socioeconomic Status, and Colorectal Cancer Diagnostic Route. JAMA Netw. Open 2025, 8, e258867. [Google Scholar] [CrossRef]
- Pennisi, F.; Ricciardi, G.E.; Von Wagner, C.; Smith, L.; Kaushal, A.; Lyratzopoulos, G.; Merriel, S.W.D.; Hamilton, W.; Abel, G.; Valderas, J.M.; et al. Impact of Self-Reported Long-Term Mental Health Morbidity on Help-Seeking and Diagnostic Testing for Bowel-Related Cancer Symptoms: A Vignette Study. Cancer Med. 2024, 13, e70426. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Cawthon, P.M.; Arai, H.; Ávila-Funes, J.A.; Barazzoni, R.; Bhasin, S.; Binder, E.F.; Bruyère, O.; Cederholm, T.; Chen, L.-K.; et al. An executive summary on the Global conceptual definition of Sarcopenia. Aging Clin. Exp. Res. 2024, 36, 153. [Google Scholar] [CrossRef]
- Schoevaerdts, D.; Sibille, F.-X.; Gavazzi, G. Infections in the older population: What do we know? Aging Clin. Exp. Res. 2021, 33, 689–701. [Google Scholar] [CrossRef]
- Pilotto, A.; Aprile, P.L.; Veronese, N.; Lacorte, E.; Morganti, W.; Custodero, C.; Piscopo, P.; Fabrizi, E.; Gatta, F.D.; Merlo, A.; et al. The Italian guideline on comprehensive geriatric assessment (CGA) for the older persons: A collaborative work of 25 Italian Scientific Societies and the National Institute of Health. Aging Clin. Exp. Res. 2024, 36, 121. [Google Scholar] [CrossRef]
- Veronese, N.; Gallo, U.; Boccardi, V.; Demurtas, J.; Michielon, A.; Taci, X.; Zanchetta, G.; Campbell Davis, S.E.; Chiumente, M.; Venturini, F.; et al. Efficacy of deprescribing on health outcomes: An umbrella review of systematic reviews with meta-analysis of randomized controlled trials. Ageing Res. Rev. 2024, 95, 102237. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, B.; Hu, Q.; Chen, X.; Du, J.; Shao, S. The health care needs of multidimensional frail elderly patients with multimorbidity in primary health-care settings: A qualitative study. BMC Prim. Care 2025, 26, 128. [Google Scholar] [CrossRef]
- Abadir, P.; Chellappa, R. Artificial Intelligence in Geriatrics: Riding the Inevitable Tide of Promise, Challenges, and Considerations. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2024, 79, glad279. [Google Scholar] [CrossRef] [PubMed]
- Mohanannair Geethadevi, G.; Quinn, T.J.; George, J.; Anstey, K.J.; Bell, J.S.; Sarwar, M.R.; Cross, A.J. Multi-domain prognostic models used in middle-aged adults without known cognitive impairment for predicting subsequent dementia. Cochrane Database Syst. Rev. 2023, 6, CD014885. [Google Scholar] [CrossRef] [PubMed]
- Shaik, T.; Tao, X.; Higgins, N.; Li, L.; Gururajan, R.; Zhou, X.; Acharya, U.R. Remote patient monitoring using artificial intelligence: Current state, applications, and challenges. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2023, 13, e1485. [Google Scholar] [CrossRef]
- Vordenberg, S.E.; Nichols, J.; Marshall, V.D.; Weir, K.R.; Dorsch, M.P. Investigating Older Adults’ Perceptions of AI Tools for Medication Decisions: Vignette-Based Experimental Survey. J. Med. Internet Res. 2024, 26, e60794. [Google Scholar] [CrossRef]
- Peddi, S.; Narla, S.; Valivarthi, D.T. Harnessing artificial intelligence and machine learning algorithms for chronic disease management, fall prevention, and predictive healthcare applications in geriatric care. Int. J. Eng. Res. Sci. Technol. 2019, 15, 1–15. [Google Scholar]
- Khalil, R.A.; Ahmad, K.; Ali, H. Redefining Elderly Care with Agentic AI: Challenges and Opportunities. arXiv 2025, arXiv:2507.14912. [Google Scholar] [CrossRef]
- Badawy, W.; Shaban, M. Exploring Geriatric nurses’ perspectives on the adoption of AI in elderly care a qualitative study. Geriatr. Nurs. 2025, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Garg, R. Smart Aging: Harnessing Artificial Intelligence to Enhance Elderly Health Care and Independence. J. Indian Acad. Geriatr. 2025, 21, 143–146. [Google Scholar] [CrossRef]
- Graham, S.A.; Lee, E.E.; Jeste, D.V.; Van Patten, R.; Twamley, E.W.; Nebeker, C.; Yamada, Y.; Kim, H.-C.; Depp, C.A. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef]
- Ma, X.; Mai, Y.; Ma, Y.; Ma, X. Constructing an early warning model for elderly sepsis patients based on machine learning. Sci. Rep. 2025, 15, 10580. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Nie, S.; Ning, J.; Wang, Q.; Yuan, H.; Wu, H.; Li, B.; Hu, W.; Wu, C. Risk assessment and prediction of nosocomial infections based on surveillance data using machine learning methods. BMC Public Health 2024, 24, 1780. [Google Scholar] [CrossRef]
- Radaelli, D.; Di Maria, S.; Jakovski, Z.; Alempijevic, D.; Al-Habash, I.; Concato, M.; Bolcato, M.; D’Errico, S. Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review. Healthcare 2024, 12, 1996. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Ghassemi, M.M.; Nemati, S.; Niehaus, K.E.; Clifton, D.; Clifford, G.D. Machine Learning and Decision Support in Critical Care. Proc. IEEE 2016, 104, 444–466. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Irvin, J.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.; Shpanskaya, K. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv 2017, arXiv:1711.05225. [Google Scholar] [CrossRef]
- Li, Y.; Rao, S.; Solares, J.R.A.; Hassaine, A.; Ramakrishnan, R.; Canoy, D.; Zhu, Y.; Rahimi, K.; Salimi-Khorshidi, G. BEHRT: Transformer for Electronic Health Records. Sci. Rep. 2020, 10, 7155. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, J. A time series driven model for early sepsis prediction based on transformer module. BMC Med. Res. Methodol. 2024, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, W.; Kim, S.; Kim, D.; Kim, S.; So, C.H.; Kang, J. BioBERT: A pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 2020, 36, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- El Arab, R.A.; Almoosa, Z.; Alkhunaizi, M.; Abuadas, F.H.; Somerville, J. Artificial intelligence in hospital infection prevention: An integrative review. Front. Public Health 2025, 13, 1547450. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.T.; Chen, Z.; Li, P. A survey on deep learning for big data. Inf. Fusion 2018, 42, 146–157. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Pennisi, F.; Buzzoni, C.; Gervasi, F.; Russo, A.G.; Renzi, C. Emergency colon cancer diagnosis in people with mental health conditions: A population-based cohort study in northern Italy. BMJ Ment. Health 2025, 28, e301733. [Google Scholar] [CrossRef]
- High, K.P.; Bradley, S.F.; Gravenstein, S.; Mehr, D.R.; Quagliarello, V.J.; Richards, C.; Yoshikawa, T.T. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 149–171. [Google Scholar] [CrossRef]
- Henry, K.E.; Hager, D.N.; Pronovost, P.J.; Saria, S. A targeted real-time early warning score (TREWScore) for septic shock. Sci. Transl. Med. 2015, 7, 299ra122. [Google Scholar] [CrossRef]
- Kooli, C.; Al Muftah, H. Artificial intelligence in healthcare: A comprehensive review of its ethical concerns. TECHS 2022, 1, 121–131. [Google Scholar] [CrossRef]
- Woodman, R.J.; Mangoni, A.A. A comprehensive review of machine learning algorithms and their application in geriatric medicine: Present and future. Aging Clin. Exp. Res. 2023, 35, 2363–2397. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Yang, N. Diagnostic performance of machine-learning algorithms for sepsis prediction: An updated meta-analysis. Technol. Health Care 2024, 32, 4291–4307. [Google Scholar] [CrossRef]
- Lin, T.-H.; Chung, H.-Y.; Jian, M.-J.; Chang, C.-K.; Lin, H.-H.; Yen, C.-T.; Tang, S.-H.; Pan, P.-C.; Perng, C.-L.; Chang, F.-Y.; et al. AI-Driven Innovations for Early Sepsis Detection by Combining Predictive Accuracy With Blood Count Analysis in an Emergency Setting: Retrospective Study. J. Med. Internet Res. 2025, 27, e56155. [Google Scholar] [CrossRef]
- Fleuren, L.M.; Klausch, T.L.T.; Zwager, C.L.; Schoonmade, L.J.; Guo, T.; Roggeveen, L.F.; Swart, E.L.; Girbes, A.R.J.; Thoral, P.; Ercole, A.; et al. Machine learning for the prediction of sepsis: A Systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020, 46, 383–400. [Google Scholar] [CrossRef]
- Moor, M.; Bennett, N.; Plečko, D.; Horn, M.; Rieck, B.; Meinshausen, N.; Bühlmann, P.; Borgwardt, K. Predicting sepsis using deep learning across international sites: A retrospective development and validation study. eClinicalMedicine 2023, 62, 102124. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Shanmugam, H.; Airen, L. Machine Learning and Deep Learning Models for Early Sepsis Prediction: A Scoping Review. Indian J. Crit. Care Med. 2025, 29, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.T.; Boon, Y.; Lee, Z.Y.; Kok, J.H.J.; Lim, C.K.W.; Cheung, N.M.T.; Yong, L.P.X.; Kuan, W.S. The role of artificial intelligence in sepsis in the Emergency Department: A narrative review. Ann. Transl. Med. 2025, 13, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Ji, Y.; Wei, B.; Guo, S.; Mei, X.; Wang, J. Machine Learning-Based Mortality Risk Prediction Model in Patients with Sepsis. J. Inflamm. Res. 2025, 18, 6427–6437. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, I.; Amirhosseini, M.H.; Li, Y.; Arachchillage, D.J. AI-Enhanced Prediction of Multi Organ Failure in COVID-19 Patients. In Proceedings of the 12th International IEEE Conference on Intelligent Systems (IS), Varna, Bulgaria, 29–31 August 2024; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar]
- Ecarnot, F.; Boccardi, V.; Calcagno, A.; Franceschi, C.; Fülop, T.; Itzhaki, R.F.; Michel, J.-P.; Panza, F.; Rainero, I.; Solfrizzi, V.; et al. Dementia, infections and vaccines: 30 years of controversy. Aging Clin. Exp. Res. 2023, 35, 1145–1160. [Google Scholar] [CrossRef]
- Velazquez-Diaz, D.; Arco, J.E.; Ortiz, A.; Pérez-Cabezas, V.; Lucena-Anton, D.; Moral-Munoz, J.A.; Galán-Mercant, A. Use of Artificial Intelligence in the Identification and Diagnosis of Frailty Syndrome in Older Adults: Scoping Review. J. Med. Internet Res. 2023, 25, e47346. [Google Scholar] [CrossRef]
- Bai, C.; Mardini, M.T. Advances of artificial intelligence in predicting frailty using real-world data: A scoping review. Ageing Res. Rev. 2024, 101, 102529. [Google Scholar] [CrossRef]
- Ren, H.; Zheng, Y.; Li, C.; Jing, F.; Wang, Q.; Luo, Z.; Li, D.; Liang, D.; Tang, W.; Liu, L.; et al. Using Machine Learning to Predict Cognitive Decline in Older Adults from the Chinese Longitudinal Healthy Longevity Survey: Model Development and Validation Study. JMIR Aging 2025, 8, e67437. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010, 304, 1787. [Google Scholar] [CrossRef] [PubMed]
- Düvel, J.A.; Lampe, D.; Kirchner, M.; Elkenkamp, S.; Cimiano, P.; Düsing, C.; Marchi, H.; Schmiegel, S.; Fuchs, C.; Claßen, S.; et al. An AI-Based Clinical Decision Support System for Antibiotic Therapy in Sepsis (KINBIOTICS): Use Case Analysis. JMIR Hum. Factors 2025, 12, e66699. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, A. AI-driven decision-making for personalized elderly care: A fuzzy MCDM-based framework for enhancing treatment recommendations. BMC Med. Inform. Decis. Mak. 2025, 25, 119. [Google Scholar] [CrossRef]
- Kouroubali, A.; Kondylakis, H.; Logothetidis, F.; Katehakis, D.G. Developing an AI-Enabled Integrated Care Platform for Frailty. Healthcare 2022, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Tseng, D.; Larkin, P.M.K.; Realegeno, S.; Mortimer, L.; Subramonian, A.; Di Carlo, D.; Garner, O.B.; Ozcan, A. Automated, Cost-Effective Optical System for Accelerated Antimicrobial Susceptibility Testing (AST) Using Deep Learning. ACS Photonics 2020, 7, 2527–2538. [Google Scholar] [CrossRef]
- Nigo, M.; Rasmy, L.; Mao, B.; Kannadath, B.S.; Xie, Z.; Zhi, D. Deep learning model for personalized prediction of positive MRSA culture using time-series electronic health records. Nat. Commun. 2024, 15, 2036. [Google Scholar] [CrossRef]
- Liang, Q.; Zhao, Q.; Xu, X.; Zhou, Y.; Huang, M. Early prediction of carbapenem-resistant Gram-negative bacterial carriage in intensive care units using machine learning. J. Glob. Antimicrob. Resist. 2022, 29, 225–231. [Google Scholar] [CrossRef]
- Arjmandmazidi, S.; Heidari, H.R.; Ghasemnejad, T.; Mori, Z.; Molavi, L.; Meraji, A.; Kaghazchi, S.; Mehdizadeh Aghdam, E.; Montazersaheb, S. An In-depth overview of artificial intelligence (AI) tool utilization across diverse phases of organ transplantation. J. Transl. Med. 2025, 23, 678. [Google Scholar] [CrossRef]
- Tacconelli, E.; Górska, A.; De Angelis, G.; Lammens, C.; Restuccia, G.; Schrenzel, J.; Huson, D.H.; Carević, B.; Preoţescu, L.; Carmeli, Y.; et al. Estimating the association between antibiotic exposure and colonization with extended-spectrum β-lactamase-producing Gram-negative bacteria using machine learning methods: A multicentre, prospective cohort study. Clin. Microbiol. Infect. 2020, 26, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Pennisi, F.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. Evaluating the impact of artificial intelligence in antimicrobial stewardship: A comparative meta-analysis with traditional risk scoring systems. Infect. Dis. Now 2025, 55, 105090. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, M.; Kabanza, F.; Nault, V.; Valiquette, L. Evaluation of a machine learning capability for a clinical decision support system to enhance antimicrobial stewardship programs. Artif. Intell. Med. 2016, 68, 29–36. [Google Scholar] [CrossRef]
- Elalouf, A.; Elalouf, H.; Rosenfeld, A.; Maoz, H. Artificial intelligence in drug resistance management. 3 Biotech. 2025, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; Pinto, A.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. Artificial intelligence in antimicrobial stewardship: A systematic review and meta-analysis of predictive performance and diagnostic accuracy. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 463–513. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Boufeas, I.Z.; Sakagianni, A.; Paxinou, E.; Verykios, V.S.; et al. Antibiotic Resistance in the Elderly: Mechanisms, Risk Factors, and Solutions. Microorganisms 2024, 12, 1978. [Google Scholar] [CrossRef]
- Bilal, H.; Khan, M.N.; Khan, S.; Shafiq, M.; Fang, W.; Khan, R.U.; Rahman, M.U.; Li, X.; Lv, Q.-L.; Xu, B. The role of artificial intelligence and machine learning in predicting and combating antimicrobial resistance. Comput. Struct. Biotechnol. J. 2025, 27, 423–439. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohammed, M.; Oleiwi, J.K.; Osman, A.F.; Adam, T.; Betar, B.O.; Gopinath, S.C.B.; Ihmedee, F.H. Enhancing antimicrobial resistance strategies: Leveraging artificial intelligence for improved outcomes. South Afr. J. Chem. Eng. 2025, 51, 272–286. [Google Scholar] [CrossRef]
- Cozzolino, C.; Mao, S.; Bassan, F.; Bilato, L.; Compagno, L.; Salvò, V.; Chiusaroli, L.; Cocchio, S.; Baldo, V. Are AI-based surveillance systems for healthcare-associated infections ready for clinical practice? A systematic review and meta-analysis. Artif. Intell. Med. 2025, 165, 103137. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Riela, P.M.; Gallo, G.; Mura, I.; Agodi, A. A machine learning approach to predict healthcare-associated infections at intensive care unit admission: Findings from the SPIN-UTI project. J. Hosp. Infect. 2021, 112, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Jiménez, A.; Polo-Luque, M.-L.; Gómez-Pulido, J.A.; Rodríguez-Puyol, D.; Gómez-Pulido, J.M. Predictive health monitoring: Leveraging artificial intelligence for early detection of infectious diseases in nursing home residents through discontinuous vital signs analysis. Comput. Biol. Med. 2024, 174, 108469. [Google Scholar] [CrossRef] [PubMed]
- Kurita, J.; Hori, M.; Yamaguchi, S.; Ogiwara, A.; Saito, Y.; Sugiyama, M.; Sunadori, A.; Hayashi, T.; Hara, A.; Kawana, Y.; et al. Effectiveness of the Facility for Elderly Surveillance System (FESSy) in Two Public Health Center Jurisdictions in Japan: Prospective Observational Study. JMIR Med. Inform. 2025, 13, e58509. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, S.; Zhou, L. The Implications of Artificial Intelligence on Infection Prevention and Control: Current Progress and Future Perspectives. China CDC Wkly. 2024, 5, 901–904. [Google Scholar] [CrossRef]
- Baig, M.M.; Hua, N.; Zhang, E.; Robinson, R.; Spyker, A.; Armstrong, D.; Whittaker, R.; Robinson, T.; Ullah, E. A machine learning model for predicting risk of hospital readmission within 30 days of discharge: Validated with LACE index and patient at risk of hospital readmission (PARR) model. Med. Biol. Eng. Comput. 2020, 58, 1459–1466. [Google Scholar] [CrossRef]
- Shimabukuro, D.W.; Barton, C.W.; Feldman, M.D.; Mataraso, S.J.; Das, R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: A randomised clinical trial. BMJ Open Resp. Res. 2017, 4, e000234. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Wu, F.-H.; Lin, H.-H.; Lee, D.-J.; Chen, Y.-F.; Lin, C.-S. AI Models for Predicting Readmission of Pneumonia Patients within 30 Days after Discharge. Electronics 2022, 11, 673. [Google Scholar] [CrossRef]
- Brown, Z.; Bergman, D.; Holt, L.; Miller, K.; Frownfelter, J.; Bleau, H.; Flynn, A.; Ball, T. Augmenting a Transitional Care Model With Artificial Intelligence Decreased Readmissions. J. Am. Med. Dir. Assoc. 2023, 24, 958–963. [Google Scholar] [CrossRef]
- Pattar, B.S.B.; Ackroyd, A.; Sevinc, E.; Hecker, T.; Turino Miranda, K.; McClurg, C.; Weekes, K.; James, M.T.; Pannu, N.; Ravani, P.; et al. Electronic Health Record Interventions to Reduce Risk of Hospital Readmissions: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2521785. [Google Scholar] [CrossRef]
- Signorelli, C.; Pennisi, F.; D’Amelio, A.C.; Conversano, M.; Cinquetti, S.; Blandi, L.; Rezza, G. Vaccinating in Different Settings: Best Practices from Italian Regions. Vaccines 2024, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Jacobsohn, G.C.; Jones, C.M.C.; Green, R.K.; Cochran, A.L.; Caprio, T.V.; Cushman, J.T.; Kind, A.J.H.; Lohmeier, M.; Mi, R.; Shah, M.N. Effectiveness of a care transitions intervention for older adults discharged home from the emergency department: A randomized controlled trial. Acad. Emerg. Med. 2022, 29, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Thornton-Swan, T.D.; Armitage, L.C.; Vollam, S.; Tarassenko, L.; Lasserson, D.S.; Farmer, A.J. Remote Vital Sign Monitoring in Admission Avoidance Hospital at Home: A Systematic Review. J. Am. Med. Dir. Assoc. 2024, 25, 105080. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, G.M.; Elayadi, A.N.; Chen, E.S.; Galiatsatos, P. The effect of telemedicine employing telemonitoring instruments on readmissions of patients with heart failure and/or COPD: A systematic review. Front. Digit. Health 2024, 6, 1441334. [Google Scholar] [CrossRef]
- Stevens, S. Preventing 30-day readmissions. Nurs. Clin. N. Am. 2015, 50, 123–137. [Google Scholar] [CrossRef]
- Levine, D.M.; Paz, M.; Burke, K.; Beaumont, R.; Boxer, R.B.; Morris, C.A.; Britton, K.A.; Orav, E.J.; Schnipper, J.L. Remote vs In-Home Physician Visits for Hospital-Level Care at Home: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2229067. [Google Scholar] [CrossRef]
- Ali, A.; Montanaro, T.; Sergi, I.; Carrisi, S.; Galli, D.; Distante, C.; Patrono, L. An Innovative IoT and Edge Intelligence Framework for Monitoring Elderly People Using Anomaly Detection on Data from Non-Wearable Sensors. Sensors 2025, 25, 1735. [Google Scholar] [CrossRef]
- Alboksmaty, A.; Beaney, T.; Elkin, S.; Clarke, J.M.; Darzi, A.; Aylin, P.; Neves, A.-L. Effectiveness and safety of pulse oximetry in remote patient monitoring of patients with COVID-19: A systematic review. Lancet Digit. Health 2022, 4, e279–e289. [Google Scholar] [CrossRef]
- Olmedo-Aguirre, J.O.; Reyes-Campos, J.; Alor-Hernández, G.; Machorro-Cano, I.; Rodríguez-Mazahua, L.; Sánchez-Cervantes, J.L. Remote Healthcare for Elderly People Using Wearables: A Review. Biosensors 2022, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Recommendations on Digital Interventions for Health System Strengthening; World Health Organization: Albany, NY, USA, 2019. [Google Scholar]
- World Health Organization. Integrated Care for Older People (ICOPE): Guidance for Person-Centred Assessment and Pathways in Primary Care, 2nd ed.; World Health Organization: Albany, NY, USA, 2024. [Google Scholar]
- Anghel, I.; Cioara, T.; Bevilacqua, R.; Barbarossa, F.; Grimstad, T.; Hellman, R.; Solberg, A.; Boye, L.T.; Anchidin, O.; Nemes, A.; et al. New care pathways for supporting transitional care from hospitals to home using AI and personalized digital assistance. Sci. Rep. 2025, 15, 18247. [Google Scholar] [CrossRef]
- Mankins, J.C. Technology Readiness Levels—A White Paper; Office of Space Access and Technology, NASA: Washington, DC, USA, 1995.
- Pennisi, F.; Pinto, A.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. The Role of Artificial Intelligence and Machine Learning Models in Antimicrobial Stewardship in Public Health: A Narrative Review. Antibiotics 2025, 14, 134. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Shiwani, T.; Relton, S.; Evans, R.; Kale, A.; Heaven, A.; Clegg, A.; Ageing Data Research Collaborative (Geridata) AI group; Abuzour, A.; Alderman, J.; Anand, A.; et al. New Horizons in artificial intelligence in the healthcare of older people. Age Ageing 2023, 52, afad219. [Google Scholar] [CrossRef]
- Albers, C.A.W.; Wieland-Jorna, Y.; De Bruijne, M.C.; Smalbrugge, M.; Joling, K.J.; De Boer, M.E. Enhancing Standardized and Structured Recording by Elderly Care Physicians for Reusing Electronic Health Record Data: Interview Study. JMIR Med. Inform. 2024, 12, e63710. [Google Scholar] [CrossRef]
- Li, E.; Clarke, J.; Ashrafian, H.; Darzi, A.; Neves, A.L. The Impact of Electronic Health Record Interoperability on Safety and Quality of Care in High-Income Countries: Systematic Review. J. Med. Internet Res. 2022, 24, e38144. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Lounsbury, O.; Hasnain, M.; Ashrafian, H.; Darzi, A.; Neves, A.L.; Clarke, J. Physician experiences of electronic health record interoperability and its practical impact on care delivery in the English NHS: A cross-sectional survey study. BMJ Open 2025, 15, e096669. [Google Scholar] [CrossRef]
- Signorelli, C.; Pennisi, F.; Lunetti, C.; Blandi, L.; Pellissero, G.; Working Group Fondazione Sanità Futura. Quality of hospital care and clinical outcomes: A comparison between the Lombardy Region and the Italian National data. Ann. Ig. Med. Prev. Comunità 2024, 36, 234–249. [Google Scholar] [CrossRef]
- Susnjak, T.; Griffin, E. Towards clinical prediction with transparency: An explainable AI Approach to survival modelling in residential aged care. Comput. Methods Programs Biomed. 2025, 263, 108653. [Google Scholar] [CrossRef]
- Wiens, J.; Saria, S.; Sendak, M.; Ghassemi, M.; Liu, V.X.; Doshi-Velez, F.; Jung, K.; Heller, K.; Kale, D.; Saeed, M.; et al. Author Correction: Do no harm: A roadmap for responsible machine learning for health care. Nat. Med. 2019, 25, 1627. [Google Scholar] [CrossRef]
- Denecke, K.; Gabarron, E.; Grainger, R.; Konstantinidis, S.T.; Lau, A.; Rivera-Romero, O.; Miron-Shatz, T.; Merolli, M. Artificial Intelligence for Participatory Health: Applications, Impact, and Future Implications: Contribution of the IMIA Participatory Health and Social Media Working Group. Yearb Med. Inf. 2019, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Panch, T.; Pearson-Stuttard, J.; Greaves, F.; Atun, R. Artificial intelligence: Opportunities and risks for public health. Lancet Digit. Health 2019, 1, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Weng, F.; Lu, W.; Xu, L.; Zhu, W.; Tan, M.; Weng, P. Artificial Intelligence in Chronic Disease Management for Aging Populations: A Systematic Review of Machine Learning and NLP Applications. Int. J. Gen. Med. 2025, 18, 3105–3115. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Alsayed, A.R.; Seder, N.; Jarrar, Y.; Altabanjeh, R.H.; Zihlif, M.; Abu Ata, O.; Samara, A.; Zihlif, M. Unveiling etiology and mortality risks in community-acquired pneumonia: A machine learning approach. Biomol. Biomed. 2025, 26, 333–353. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).