BMP3 Deficiency Accelerates Cartilage-to-Bone Transition in Ectopic Bone
Abstract
1. Introduction
2. Methods
2.1. Animals
2.2. Flow Cytometry
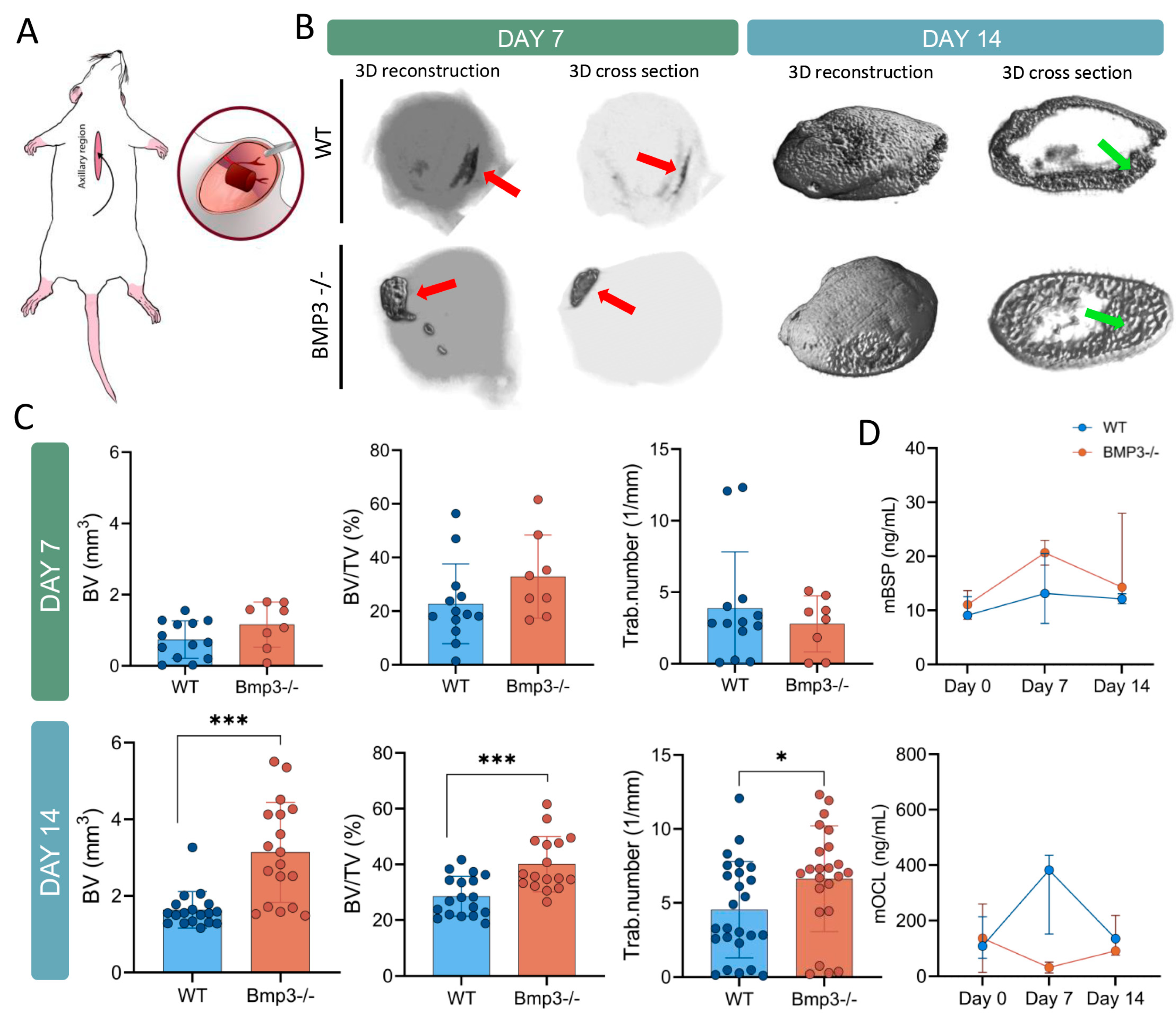
2.3. Subcutaneous Ectopic Bone Formation Assay
2.4. Micro-CT Analysis
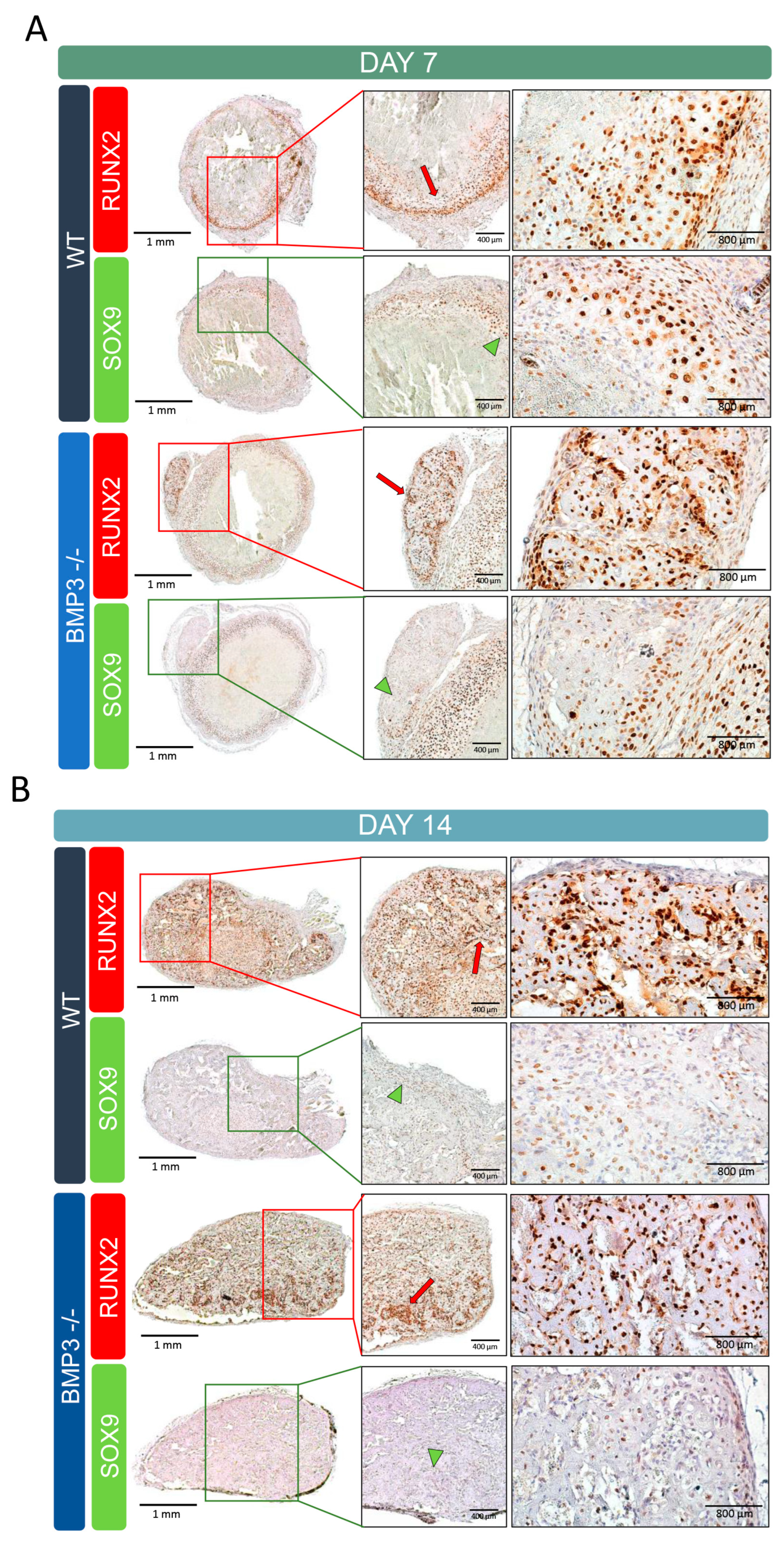
2.5. Histology and Immunohistochemistry
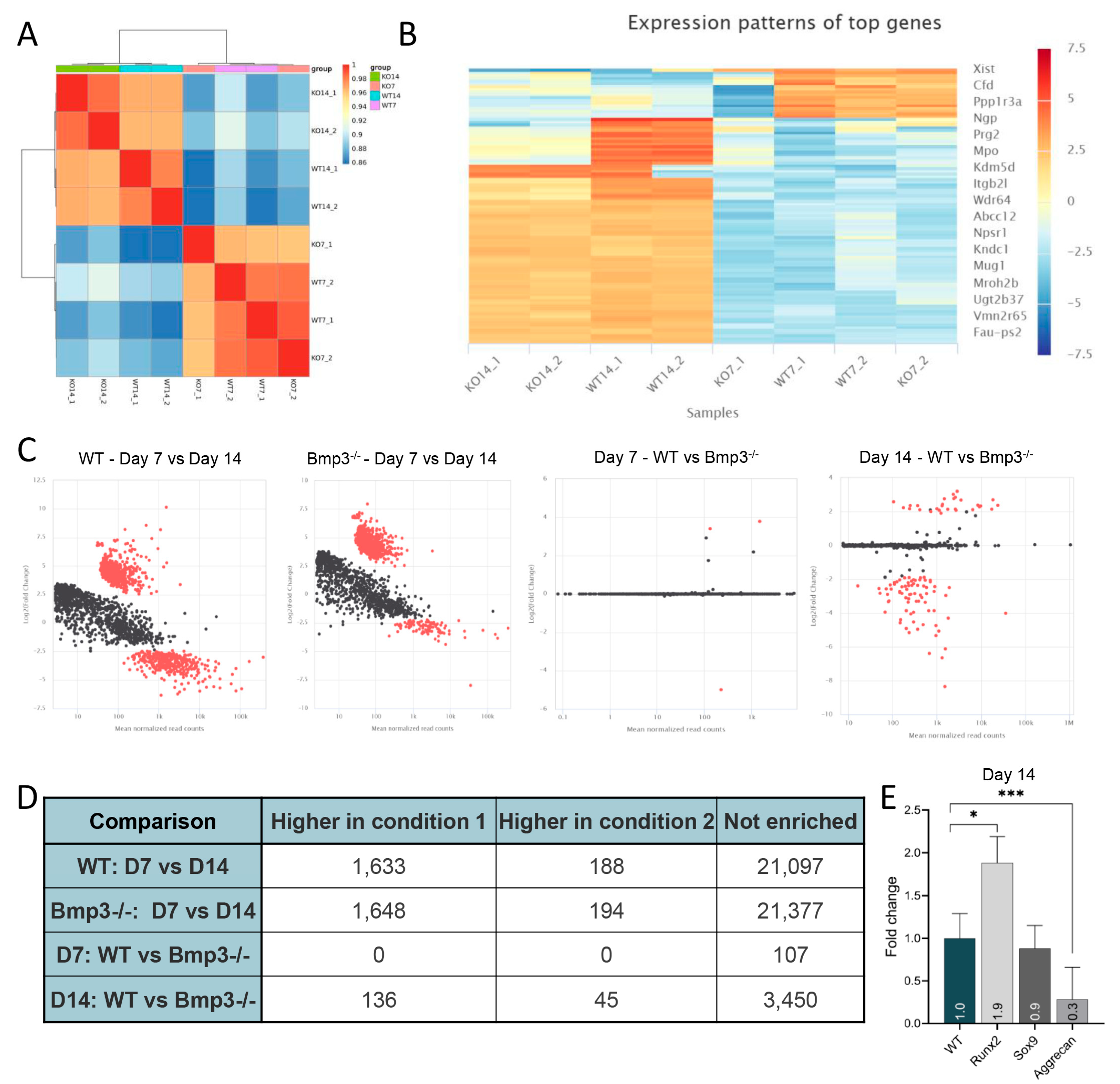
2.6. RNA Sequencing
2.7. RT-qPCR
2.8. ELISA
2.9. Statistical Analysis
3. Results
3.1. Flow Cytometry
3.2. Ectopic Bone Micro-CT Analysis
3.3. Bone Turnover Markers
3.4. Ectopic Bone Immunohistochemistry Analysis
3.5. Gene Expression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddi, A.H. Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef]
- Hu, W.; Guo, Z.; Tang, W.; Long, J. Mechanoresponsive regulation of tissue regeneration during distraction osteogenesis. FASEB J. 2024, 38, e70056. [Google Scholar] [CrossRef]
- Lv, N.; Zhou, Z.; Hou, M.; Hong, L.; Li, H.; Qian, Z.; Gao, X.; Liu, M. Research progress of vascularization strategies of tissue-engineered bone. Front. Bioeng. Biotechnol. 2023, 11, 1291969. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Levi, B.; Askarinam, A.; Nguyen, A.; Rackohn, T.; Ting, K.; Soo, C.; James, A.W. Brief review of models of ectopic bone formation. Stem Cells Dev. 2012, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Epari, D.R.; Duda, G.N.; Thompson, M.S. Mechanobiology of bone healing and regeneration: In vivo models. Proc. Inst. Mech. Eng. H 2010, 224, 1543–1553. [Google Scholar] [CrossRef]
- Cullinane, D.M.; Einhorn, T.A. Biomechanics of Bone. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. Bone morphogenetic protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Vukičević, S.; Sampath, T.K. Bone Morphogenetic Proteins: Regeneration of Bone and Beyond; Birkhäuser Verlag: Basel, Switzerland, 2004. [Google Scholar]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Akiyama, T.; Raftery, L.A.; Wharton, K.A. Bone morphogenetic protein signaling: The pathway and its regulation. Genetics 2024, 226, iyad200. [Google Scholar] [CrossRef]
- Sampath, T.K.; Vukicevic, S. Biology of bone morphogenetic protein in bone repair and regeneration: A role for autologous blood coagulum as carrier. Bone 2020, 141, 115602. [Google Scholar] [CrossRef] [PubMed]
- Stokovic, N.; Ivanjko, N.; Maticic, D.; Luyten, F.P.; Vukicevic, S. Bone Morphogenetic Proteins, Carriers, and Animal Models in the Development of Novel Bone Regenerative Therapies. Materials 2021, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Daluiski, A.; Engstrand, T.; Bahamonde, M.E.; Gamer, L.W.; Agius, E.; Stevenson, S.L.; Cox, K.; Rosen, V.; Lyons, K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001, 27, 84–88. [Google Scholar] [CrossRef]
- Kokabu, S.; Gamer, L.; Cox, K.; Lowery, J.; Tsuji, K.; Raz, R.; Economides, A.; Katagiri, T.; Rosen, V. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol. Endocrinol. 2012, 26, 87–94. [Google Scholar] [CrossRef]
- Vukicevic, S.; Helder, M.N.; Luyten, F.P. Developing human lung and kidney are major sites for synthesis of bone morphogenetic protein-3 (osteogenin). J. Histochem. Cytochem. 1994, 42, 869–875. [Google Scholar] [CrossRef]
- Banovac, I.; Grgurevic, L.; Rumenovic, V.; Vukicevic, S.; Erjavec, I. BMP3 Affects Cortical and Trabecular Long Bone Development in Mice. Int. J. Mol. Sci. 2022, 23, 785. [Google Scholar] [CrossRef] [PubMed]
- Bahamonde, M.E.; Lyons, K.M. BMP3: To be or not to be a BMP. J. Bone Jt. Surg. Am. 2001, 83-A (Suppl. 1), S56–S62. [Google Scholar] [CrossRef]
- Rosen, V. BMP and BMP inhibitors in bone. Ann. N. Y. Acad. Sci. 2006, 1068, 19–25. [Google Scholar] [CrossRef]
- Gamer, L.W.; Cox, K.; Carlo, J.M.; Rosen, V. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Dev. Dyn. 2009, 238, 2374–2381. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Schmitt, G.; Speicher-Mentges, S.; Orth, P.; Madry, H.; Cucchiarini, M. Effects of Recombinant Adeno-Associated Virus-Mediated Overexpression of Bone Morphogenetic Protein 3 on the Chondrogenic Fate of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Hum. Gene Ther. 2022, 33, 950–958. [Google Scholar] [CrossRef]
- Wang, Z.; Storm, D.R. Extraction of DNA from mouse tails. Biotechniques 2006, 41, 410–412. [Google Scholar] [CrossRef]
- Lazic Mosler, E.; Lukac, N.; Flegar, D.; Fadljevic, M.; Radanovic, I.; Cvija, H.; Kelava, T.; Ivcevic, S.; Sucur, A.; Markotic, A.; et al. Fas receptor induces apoptosis of synovial bone and cartilage progenitor populations and promotes bone loss in antigen-induced arthritis. FASEB J. 2019, 33, 3330–3342. [Google Scholar] [CrossRef]
- Vukicevic, S.; Oppermann, H.; Verbanac, D.; Jankolija, M.; Popek, I.; Curak, J.; Brkljacic, J.; Pauk, M.; Erjavec, I.; Francetic, I.; et al. The clinical use of bone morphogenetic proteins revisited: A novel biocompatible carrier device OSTEOGROW for bone healing. Int. Orthop. 2014, 38, 635–647. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- Garcia-Alcalde, F.; Okonechnikov, K.; Carbonell, J.; Cruz, L.M.; Gotz, S.; Tarazona, S.; Dopazo, J.; Meyer, T.F.; Conesa, A. Qualimap: Evaluating next-generation sequencing alignment data. Bioinformatics 2012, 28, 2678–2679. [Google Scholar] [CrossRef]
- Daley, T.; Smith, A.D. Predicting the molecular complexity of sequencing libraries. Nat. Methods 2013, 10, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Sayols, S.; Scherzinger, D.; Klein, H. dupRadar: A Bioconductor package for the assessment of PCR artifacts in RNA-Seq data. BMC Bioinform. 2016, 17, 428. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532-4–536-7. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Yoshizaki, K.; Saito, K.; Ikeuchi, T.; Iwamoto, T.; Rhodes, C.; Nakamura, T.; de Vega, S.; Morell, R.J.; Boger, E.T.; et al. G protein-coupled receptor Gpr115 (Adgrf4) is required for enamel mineralization mediated by ameloblasts. J. Biol. Chem. 2020, 295, 15328–15341. [Google Scholar] [CrossRef]
- Aspenberg, P.; Basic, N.; Tagil, M.; Vukicevic, S. Reduced expression of BMP-3 due to mechanical loading: A link between mechanical stimuli and tissue differentiation. Acta Orthop. Scand. 2000, 71, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Ewa, B.; Tomasz, L. Evolution of bone tissue based on angiogenesis as a crucial factor: New mathematical attempt. Math. Mech. Solids 2021, 27, 976–988. [Google Scholar] [CrossRef]
- Pitchford, S.C.; Furze, R.C.; Jones, C.P.; Wengner, A.M.; Rankin, S.M. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 2009, 4, 62–72. [Google Scholar] [CrossRef]
- Guo, Z.; Li, H.; Li, X.; Yu, X.; Wang, H.; Tang, P.; Mao, N. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells 2006, 24, 992–1000. [Google Scholar] [CrossRef]
- Bonyadi, M.; Waldman, S.D.; Liu, D.; Aubin, J.E.; Grynpas, M.D.; Stanford, W.L. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA 2003, 100, 5840–5845. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.H.; Vehof, J.W.; Spauwen, P.H.; Jansen, J.A. Ectopic bone formation in rats: The importance of the carrier. Biomaterials 2005, 26, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Ivanjko, N.; Stokovic, N.; Milesevic, M.; Rumenovic, V.; Windhager, R.; Sampath, K.T.; Kovacic, N.; Grcevic, D.; Vukicevic, S. rhBMP6 in autologous blood coagulum is a preferred osteoinductive device to rhBMP2 on bovine collagen sponge in the rat ectopic bone formation assay. Biomed. Pharmacother. 2023, 169, 115844. [Google Scholar] [CrossRef]
- Mizrahi, O.; Sheyn, D.; Tawackoli, W.; Kallai, I.; Oh, A.; Su, S.; Da, X.; Zarrini, P.; Cook-Wiens, G.; Gazit, D.; et al. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther. 2013, 20, 370–377. [Google Scholar] [CrossRef]
- Chiari, C.; Grgurevic, L.; Bordukalo-Niksic, T.; Oppermann, H.; Valentinitsch, A.; Nemecek, E.; Staats, K.; Schreiner, M.; Trost, C.; Kolb, A.; et al. Recombinant Human BMP6 Applied Within Autologous Blood Coagulum Accelerates Bone Healing: Randomized Controlled Trial in High Tibial Osteotomy Patients. J. Bone Min. Res. 2020, 35, 1893–1903. [Google Scholar] [CrossRef]
- Wozney, J.M. The bone morphogenetic protein family and osteogenesis. Mol. Reprod. Dev. 1992, 32, 160–167. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chen, W.; Luo, Y.; Wu, J.; Zhang, Y.; McVicar, A.; McConnell, M.; Liu, Y.; Zhou, H.D.; Li, Y.P. Runx1 up-regulates chondrocyte to osteoblast lineage commitment and promotes bone formation by enhancing both chondrogenesis and osteogenesis. Biochem. J. 2020, 477, 2421–2438. [Google Scholar] [CrossRef]
- Zhou, G.; Zheng, Q.; Engin, F.; Munivez, E.; Chen, Y.; Sebald, E.; Krakow, D.; Lee, B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19004–19009. [Google Scholar] [CrossRef]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Czekanska, E.M.; Bruderer, M.; Salzmann, G.; Alini, M.; Stoddart, M.J. In vitro osteogenic potential of human mesenchymal stem cells is predicted by Runx2/Sox9 ratio. Tissue Eng. Part A 2015, 21, 115–123. [Google Scholar] [CrossRef]
- Stoeger, T.; Proetzel, G.E.; Welzel, H.; Papadimitriou, A.; Dony, C.; Balling, R.; Hofmann, C. In situ gene expression analysis during BMP2-induced ectopic bone formation in mice shows simultaneous endochondral and intramembranous ossification. Growth Factors 2002, 20, 197–210. [Google Scholar] [CrossRef]
- Shi, S.; de Gorter, D.J.; Hoogaars, W.M.; t Hoen, P.A.; ten Dijke, P. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol. Life Sci. 2013, 70, 407–423. [Google Scholar] [CrossRef]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, I.R.; Vitale, M.; Elson, A.; Hoyland, J.A.; Bella, J. Role of OSCAR Signaling in Osteoclastogenesis and Bone Disease. Front. Cell Dev. Biol. 2021, 9, 641162. [Google Scholar] [CrossRef]
- Leatherdale, A.; Parker, D.; Tasneem, S.; Wang, Y.; Bihan, D.; Bonna, A.; Hamaia, S.W.; Gross, P.L.; Ni, H.; Doble, B.W.; et al. Multimerin 1 supports platelet function in vivo and binds to specific GPAGPOGPX motifs in fibrillar collagens that enhance platelet adhesion. J. Thromb. Haemost. 2021, 19, 547–561. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal 2020, 32, 957–981. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.; Seo, D.Y.; Lee, H.; Horton, J.D.; Park, J.; Scherer, P.E. The impact of endotrophin on the progression of chronic liver disease. Exp. Mol. Med. 2020, 52, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.I.; Hankenson, K.D. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone 2006, 38, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, Y.H.; Kim, S.H.; Han, S.H.; Hahn, S.B. Chondrogenic differentiation of mesenchymal stem cells and its clinical applications. Yonsei Med. J. 2004, 45, S41–S47. [Google Scholar] [CrossRef]

| Primer | 5′-3′ | 3′-5′ |
|---|---|---|
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| Runx2 | GAGGGACTATGGCGTCAAACA | GGATCCCAAAAGAAGCTTTGC |
| Sox9 | CGTCAACGGCTCCAGCA | TGCGCCCACACCATGA |
| Aggrecan | CCTGCTACTTCATCGACCCC | AGATGCTGTTGACTCGAACCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumenović, V.; Ivanjko, N.; Kovačić, N.; Vukičević, S.; Erjavec, I. BMP3 Deficiency Accelerates Cartilage-to-Bone Transition in Ectopic Bone. Biomedicines 2025, 13, 2508. https://doi.org/10.3390/biomedicines13102508
Rumenović V, Ivanjko N, Kovačić N, Vukičević S, Erjavec I. BMP3 Deficiency Accelerates Cartilage-to-Bone Transition in Ectopic Bone. Biomedicines. 2025; 13(10):2508. https://doi.org/10.3390/biomedicines13102508
Chicago/Turabian StyleRumenović, Viktorija, Natalia Ivanjko, Nataša Kovačić, Slobodan Vukičević, and Igor Erjavec. 2025. "BMP3 Deficiency Accelerates Cartilage-to-Bone Transition in Ectopic Bone" Biomedicines 13, no. 10: 2508. https://doi.org/10.3390/biomedicines13102508
APA StyleRumenović, V., Ivanjko, N., Kovačić, N., Vukičević, S., & Erjavec, I. (2025). BMP3 Deficiency Accelerates Cartilage-to-Bone Transition in Ectopic Bone. Biomedicines, 13(10), 2508. https://doi.org/10.3390/biomedicines13102508






