Photobiomodulation Meets Mechanotransduction: Immune-Stromal Crosstalk in Orthodontic Remodeling
Abstract
1. Introduction
2. The Biological and Molecular Basis of Orthodontic Tooth Movement
| Transcription Factor | Mechanosensors/Receptors | Intermediary Molecules | Signaling Pathways | Specific Outcomes | References |
|---|---|---|---|---|---|
| Osteoclastogenesis | |||||
| NFATc1 (master regulator) | RANK (RANKL–RANK); ITAM-coupled receptors (OSCAR, TREM2/FcRγ); αvβ3 integrin; Piezo1/TRPV4; P2X7/P2Y | Oscillatory Ca2+ (SOCE); ATP; ROS (NOX2); NO | RANK → TRAF6 → NF-κB/MAPKs; ITAM receptors/Syk → PLCγ2 → CaN → NFATc1 (auto-amplification) | OC: Differentiation and fusion; activation of resorptive genes (CTSK, ACP5/TRAP) and others | [1,2,16,19,20] |
| AP-1 (c-Fos) | RANK; TNF receptors; integrins | ROS; Ca2+ | ERK1/2/JNK/p38 → AP-1; synergy with NFATc1 | Commitment to OC lineage; survival; priming of the resorptive programme | [1,16,18,21] |
| NF-κB (p65/p50) | RANK; TLRs (context) | ROS; ATP; NO | IKK → NF-κB activation; cross-talk with MAPKs and NFATc1 | Early differentiation; survival; inflammatory response | [1,16,19,20] |
| MITF | RANK; integrins; M-CSF (c-Fms) | Ca2+; ROS | ERK/PI3K–Akt; cooperation with NFATc1 | Lysosomal/acidification genes (CTSK, ACP5)↑ | [1,16,21] |
| CREB | GPCRs; c-Fms; RANK (indirect via cAMP) | cAMP↑; Ca2+ | PKA–CREB; CaMK–CREB | Survival, early proliferation of precursors; cooperation with NFATc1/c-Fos | [1,16,19,20] |
| HIF-1α | O2 tension; integrins | ROS/NO | PHD inhibition → HIF-1α; PI3K–Akt/ERK crosstalk | Glycolytic shift; enhances resorptive capacity under hypoxia | [16,21] |
| FoxO (context-dependent) | Growth factor receptors; oxidative stress sensors | ROS (activates) | Akt–FoxO; JNK–FoxO | Limits excessive ROS; restrains over-resorption (homeostatic brake) | [1,16,20] |
| Osteoblastogenesis | |||||
| RUNX2 | Integrins/FAK; Piezo1, TRPV4; LRP5/6–Frizzled (Wnt); BMP receptors | Ca2+ (SOCE via STIM1/ORAI1); ATP → P2Y; low ROS/NO; cAMP/PKA | Wnt/β-catenin; BMP–Smad1/5/8; ERK, p38; CaN–NFAT crosstalk | Lineage commitment; ALP↑; COL1A1↑; primes BGLAP/OCN | [1,2,10,11,12,13,25] |
| Osterix (SP7) | Integrins; BMP receptors; LRP5/6–Frizzled; Piezo1/TRPV4 | Ca2+; ROS; cAMP | BMP–Smad1/5/8; Wnt/β-catenin; ERK1/2; AP-1 support | Maturation; COL1A1/1A2; SPP1/OPN; mineralization genes | [1,10,12,25,27] |
| ATF4 | Integrins/FAK; PTH1R/GPCRs; amino-acid/ER-stress sensors | cAMP; Ca2+; ROS; metabolic cues | PKA–CREB/ATF4; PI3K–Akt–mTOR; PERK–eIF2α (ISR) | BGLAP/OCN↑; synthesis of collagen and other ECM components | [1,11,18,28] |
| NFAT (c1/c2 in osteoblasts) | Piezo1/TRPV4; GPCRs (P2Y); STIM1–ORAI1 (SOCE) | Ca2+ oscillations; ATP; NO; ROS → IP3R/SOCE) | PLC–IP3 → CaN–NFAT; crosstalk with ERK/p38 | ALP, COL1A1; OPG↑ | [1,2,10,11] |
| CREB | GPCRs (β-AR, PTH1R, P2Y); mechanosensitive tmACs | cAMP↑; Ca2+ (CaMKIV) | cAMP–PKA–CREB; Ca2+–CaMK–CREB; EPAC | Early proliferation/survival; primes matrix gene programs | [1,11,29] |
| BMPs/Smads; TGF-β/Smads; TGF-β/Non-smads | BMPR-I/II; TGFβR | ROS/NO fine-tune; Ca2+ minor role | BMP–Smad1/5/8 → Smad4; TGF-β–Smad2/3 → Smad4; non-Smad (TAK1–p38/JNK; PI3K–Akt) | BMP-Smads: RUNX2/SP7↑; osteogenic genes↑; TGF-β-Smads: COL1, CTGF, TIMP↑; OPG↑/RANKL↓ | [1,12,25,30] |
| AP-1 (c-Fos/c-Jun) | Integrins/FAK; cytokine receptors; stretch-activated channels | ROS (↑MAPKs); Ca2+ | ERK, JNK, p38 → AP-1 | Proliferation; MMPs (remodeling); COL1 regulation | [1,2,18,21] |
| HIF-1α | O2 tension sensors; integrins; mechanosensitive receptors → NO production | ROS/NO | PHD inhibition → HIF-1α; crosstalk with PI3K–Akt/ERK | VEGF↑ (angiogenesis); glycolysis; osteogenesis–angiogenesis coupling | [1,22,31] |
3. Photobiomodulation in Orthodontics: Focused Clinical Applications
4. Summary of Recent Systematic Reviews and Meta-Analyses on the Effects of PBM in Orthodontics
| References | Focus | n_studies | Key Result/Effect Size | Heterogeneity/Risk Bias | Bottom Line | Notes |
|---|---|---|---|---|---|---|
| Long et al. (2015) [53] | Acceleration (OTM) | 5 RCTs | Subgroup: 780 nm & ~5 J/cm2 showed larger effects. | High bias | Weak evidence for small acceleration | Wavelength 780 nm; ~20 mW; ~5 J/cm2 subgroup showed benefits; GRADE weak |
| Imani et al. (2018) [54] | Acceleration (OTM) (canine retraction) | 6 RCTs | Significant increase in rate of canine movement | Risk bias mixed (low, high, or undefined) | LLLT can accelerate canine distalization; | Various diode lasers (e.g., 808–980 nm); dosing heterogeneous |
| AlShahrani et al. (2019) [55] | Acceleration (OTM) | 12 (RCTs + CCTs) | MD 0.59 favoring PBM; I2~95% | Low risk bias or undefined | Possible benefit, but heterogeneity limits certainty | Included GaAlAs & OrthoPulse; urged protocol standardization |
| Bakdach & Hadad (2020) [44] | Acceleration (OTM) (canine retraction) | 25 RCTs | Statistically significant retraction acceleration reported | Variable protocols; risk bias is not clearly defined | Evidence graded low–very low; clinical significance uncertain | Recommends reporting total J/month rather than J/cm2 |
| Jedliński et al. (2020) [56] | Acceleration (OTM) Canine retraction; incisors alignment; intrusion | 6 RCTs; 8 meta analyses | PBM reduced treatment time | Risk bias is not clearly presented | Suggests benefit; protocol heterogeneity persists | Compared different lasers/parameters |
| Li et al. (2021) [57] | Acceleration (OTM) | 8 RCTs + 1 quasi-RCT | Month 1 & 3 not significant; some benefit at month 2 in subgroups | Risk bias variable | Insufficient evidence overall; more RCTs needed | Split-mouth & parallel RCTs; varying wavelengths/energies |
| Yavagal et al. (2021) [47] | Acceleration (children) | 14 (9 in meta-analysis) | Significant increase in movement | Risk bias heterogeneous | PBM optimal protocols unclear | Pediatric populations; diverse devices |
| Huang et al. (2023) [58] | Alignment-phase acceleration | 8 (5 RCTs + 3 CCTs) | Significantly increased rate and reduced duration of alignment | Large; improved after subgrouping; risk bias heterogeneous | PBM accelerates alignment; protocol optimization needed | Included lasers and LEDs |
| Grajales et al. (2023) [59] | Acceleration (OTM) | 18 RCTs (split-mouth only) | Trend toward faster movement; early (1 mo) effect not significant; | Present; Moderate risk bias | Evidence limited; more homogeneous trials required | Wavelengths ≤ 810 nm; ED ≤ 5.3 J/cm2 were associated with faster OTM. |
| Olmedo-Hernández et al. (2022) [60] | Acceleration (OTM) | 22 (RCTs + CCTs) | No supportive evidence for LLLT effect on OTM in pooled analysis; | Present; high bias risk | No significant benefit; emphasized need for quality RCTs | Focused on acceleration; stringent inclusion; LLLT or LED |
| Ren et al. (2015) [62] | Pain after OTM | 14 RCTs (659 pts) | LLLT reduced orthodontic pain by ~39% vs. placebo; | Present; High risk of bias in most RCTs | Analgesic effect probable; evidence quality low | Diode lasers; varied parameters |
| Shi et al. (2015) [63] | Pain (separator placement) | 6 (5 RCTs and 1 CCTs) | Analgesic benefit noted; reliability limited | Present; variable risk/bias | Suggests reduced pain; more robust trials needed | Focus on separators |
| Deana et al. (2017) [64] | Pain during early OTM | 20 RCTs | Reduced spontaneous & chewing pain at 24–72 h vs. placebo; | Present; high risk bias | LLLT likely reduces acute orthodontic pain; caution due to bias | Near-infrared LLLT subgroup |
| Li et al. (2015) [51] | Pain during OTM | 11 RCTs | Pain reduction significant | Significant; different levels of risk bias | Insufficient evidence for routine use purely for pain relief | Called for higher-quality trials; LLLT and LED |
| Costa et al. (2021) [65] | Miniscrew (TAD) stability | 6 (5 RCTs, 1 CCTs) | LLLT significantly increased OMI stability (e.g., Cohen’s d ~ 0.67) | Present; dominate low risk bias | Suggests stability benefit; more RCTs required | Early healing phases emphasized |
| Michelogiannakis et al. (2022) [66] | Miniscrew (MSI) stability | 6 RCTs | 2 RCTs positive; 1 no difference Increased stability in some subgroups after 2 mo | Low–moderate; insufficient data to analyze bias | Some evidence of improved stability; overall limited | LLLT parameters varied; called for more RCTs |
| Zheng et al. (2023) [50] | Miniscrew (MSI) stability | 3 RCTs | Two RCTs ↑ stability; one no difference | Low–moderate; Risk bias was not clearly defined | Inconclusive but promising; more trials needed | Low-intensity lasers; split-mouth and parallel designs |
| Michelogiannakis et al. (2019 [52]) | OIIRR during OTM | 9 (mixed designs) | Conflicting—some reduction/reparative effect; others potential increase | Moderate–high; study designs mixed; risk bias is not clearly defined | Overall impact on OIIRR remains inconclusive | Human & animal data referenced |
| Domínguez Camacho et al. (2020) [67] | Effective wavelength range for acceleration | 9 RCTs | Suggests 730–830 nm range as potentially effective; average speed improvement 24% | Variable heterogeneity and variable risk bias | Parameter guidance tentative; evidence base limited | Non-comparative synthesis; calls for standardization |
| Cronshaw et al. (2019) [61] | PBM across orthodontic applications | 9 RCTs | Acceleration & post-op recovery (20–40% increase); evidence quality variable; better effects on pain reduction | High protocol variability | Encouraging but low–moderate certainty; protocol consensus needed | Overview (acceleration, pain, tissue healing); LLLT and LED |
5. Molecular Aspects of Photobiomodulation and the Integration of Mechanical and Photon-Induced Signals During Orthodontic Tooth Movement
6. Photobiomodulation and Cellular Crosstalk in Orthodontic Tooth Movement: Cellular Dynamics, Matrix Remodeling, and Vascular Adaptation
7. Apoptosis and Autophagy in Orthodontic Tooth Movement: Mechanosensitive Responses and Cellular Interplay Under Photobiomodulation
8. Immune Dynamics in Orthodontic Tooth Movement: Unresolved Mechanisms of Photobiomodulation
8.1. Innate Immunity
8.2. Adaptive Immunity
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Maltha, J.C.; Van ’t Hof, M.A.; Kuijpers-Jagtman, A.M. Optimum Force Magnitude for Orthodontic Tooth Movement: A Mathematic Model. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 71–77. [Google Scholar] [CrossRef] [PubMed]
- d’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar] [CrossRef] [PubMed]
- Al-Naoum, F.; Hajeer, M.Y.; Al-Jundi, A. Does alveolar corticotomy accelerate orthodontic tooth movement when retracting upper canines? A split-mouth design randomized controlled trial. J. Oral Maxillofac. Surg. 2014, 72, 1880–1889. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.J.; Yoon, H.J.; Lee, P.J.; Moon, W.; Park, Y.G. Effect of piezopuncture on tooth movement and bone remodeling in dogs. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 23–31. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs Light Emitting Diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Aljibrin, F.J.; Alqahtani, A.M.; Saklou, R.; Alhassan, I.A.; Alamer, A.H.; Al Ameer, M.H.; Hatami, M.S.; Dahhas, F.Y. Photobiomodulation in Orthodontics: Mechanisms and Clinical Efficacy for Faster Tooth Movement. Cureus 2024, 16, e59061. [Google Scholar] [CrossRef]
- Henneman, S.; Von den Hoff, J.W.; Maltha, J.C. Mechanobiology of Tooth Movement. Eur. J. Orthod. 2008, 30, 299–306. [Google Scholar] [CrossRef]
- Robling, A.G.; Turner, C.H. Mechanical Signaling for Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef]
- Maltha, J.C.; Kuijpers-Jagtman, A.M. Mechanobiology of Orthodontic Tooth Movement: An Update. J. World Fed. Orthod. 2023, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Heufelder, A.E. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 2001, 79, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Song, K.S.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Praneetpong, N.; Ono, W.; Ono, N. Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption. J. Bone Metab. 2023, 30, 297–310. [Google Scholar] [CrossRef]
- Ubuzima, P.; Nshimiyimana, E.; Mukeshimana, C.; Mazimpaka, P.; Mugabo, E.; Mbyayingabo, D.; Mohamed, A.S.; Habumugisha, J. Exploring Biological Mechanisms in Orthodontic Tooth Movement: Bridging the Gap between Basic Research Experiments and Clinical Applications—A Comprehensive Review. Ann. Anat. 2024, 255, 152286. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Peng, Y.; Liu, N.; Liu, Q. The Relationship between MAPK Signaling Pathways and Osteogenic Differentiation of Periodontal Ligament Stem Cells: A Literature Review. PeerJ 2025, 13, e19193. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamaño, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Lu, S.Y.; Li, M.; Lin, Y.L. Mitf regulates osteoclastogenesis by modulating NFATc1 activity. Exp. Cell Res. 2014, 328, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef] [PubMed]
- Marques-Carvalho, A.; Kim, H.N.; Almeida, M. The Role of Reactive Oxygen Species in Bone Cell Physiology and Pathophysiology. Bone Rep. 2023, 19, 101664. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, B.; Kosson, D.; Domaracka, K. Lights and Shadows of NSAIDs in Bone Healing: The Role of Prostaglandins in Bone Metabolism. Drug Des. Devel. Ther. 2018, 12, 1753–1758. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-Catenin Signaling Components and Mechanisms in Bone Formation, Homeostasis, and Disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Yang, X.; Matsuda, K.; Bialek, P.; Jacquot, S.; Masuoka, H.C.; Schinke, T.; Li, L.; Brancorsini, S.; Sassone-Corsi, P.; Townes, T.M.; et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: Implication for Coffin–Lowry syndrome. Cell 2004, 117, 387–398. [Google Scholar] [CrossRef]
- Zhang, R.; Edwards, J.R.; Ko, S.Y.; Dong, S.; Liu, H.; Oyajobi, B.O.; Papasian, C.; Deng, H.W.; Zhao, M. Transcriptional regulation of BMP2 expression by the PTH-CREB signaling pathway in osteoblasts. PLoS ONE 2011, 6, e20780. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017, 9, a022129. [Google Scholar] [CrossRef]
- Kirschneck, C.; Thuy, M.; Leikam, A.; Memmert, S.; Deschner, J.; Damanaki, A.; Spanier, G.; Proff, P.; Jantsch, J.; Schröder, A. Role and Regulation of Mechanotransductive HIF-1α Stabilisation in Periodontal Ligament Fibroblasts. Int. J. Mol. Sci. 2020, 21, 9530. [Google Scholar] [CrossRef]
- Di Palma, G.; Inchingolo, A.M.; Patano, A.; Palumbo, I.; Guglielmo, M.; Trilli, I.; Netti, A.; Ferrara, I.; Viapiano, F.; Inchingolo, A.D.; et al. Photobiomodulation and Growth Factors in Dentistry: A Systematic Review. Photonics 2023, 10, 1095. [Google Scholar] [CrossRef]
- Cunha, M.J.; Esper, L.A.; Sbrana, M.C.; de Oliveira, P.G.; do Valle, A.L.; de Almeida, A.L. Effect of Low-Level Laser on Bone Defects Treated with Bovine or Autogenous Bone Grafts: In Vivo Study in Rat Calvaria. BioMed Res. Int. 2014, 2014, 104230. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Tang, Q.; Zhang, Y.; Yin, Y.; Chen, L. Application of Antioxidant Compounds in Bone Defect Repair. Antioxidants 2024, 13, 789. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Hasan, S.; Saeed, S.; Khan, A.; Khan, M. Low-Level Laser Therapy in Temporomandibular Joint Disorders: A Systematic Review. J. Med. Life 2021, 14, 148–164. [Google Scholar] [CrossRef]
- Shukla, D.; Muthusekhar, M.R. Efficacy of low-level laser therapy in temporomandibular disorders: A systematic review. Natl. J. Maxillofac. Surg. 2016, 7, 62–66. [Google Scholar] [CrossRef]
- Turhani, D.; Scheriau, M.; Kapral, D.; Benesch, T.; Jonke, E.; Bantleon, H.P. Pain Relief by Single Low-Level Laser Irradiation in Orthodontic Patients Undergoing Fixed Appliance Therapy. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 371–377. [Google Scholar] [CrossRef]
- Furquim, R.D.; Pascotto, R.C.; Rino Neto, J.; Cardoso, J.R.; Ramos, A.L. Low-Level Laser Therapy Effects on Pain Perception Related to the Use of Orthodontic Elastomeric Separators. Dental Press J. Orthod. 2015, 20, 37–42. [Google Scholar] [CrossRef]
- Yassin, A.M.; Shehata, F.I.; Al Sawa, A.A.; Karam, S.S. Effect of Low Level Laser Therapy on Orthodontic Induced Inflammatory Root Resorption in Rats. Alex. Dent. J. 2020, 45, 62–67. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, K.; Liu, F.; Zhao, Y. The simultaneous protective effect of photobiomodulation on root resorption while accelerating orthodontic tooth movement in rats. Lasers Med. Sci. 2025, 40, 394. [Google Scholar] [CrossRef]
- Farid, K.A.; Eid, A.A.; Kaddah, M.A.; Elsharaby, F.A. The Effect of Combined Corticotomy and Low Level Laser Therapy on the Rate of Orthodontic Tooth Movement: Split Mouth Randomized Clinical Trial. Laser Ther. 2019, 28, 275–283. [Google Scholar] [CrossRef]
- Flieger, R.; Gedrange, T.; Grzech-Leśniak, K.; Dominiak, M.; Matys, J. Low-Level Laser Therapy with a 635 nm Diode Laser Affects Orthodontic Mini-Implants Stability: A Randomized Clinical Split-Mouth Trial. J. Clin. Med. 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Bakdach, W.M.M.; Hadad, R. Effectiveness of low-level laser therapy in accelerating the orthodontic tooth movement: A systematic review and meta-analysis. Dent. Med. Probl. 2020, 57, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The Effectiveness of Low-Level Laser Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Meta-Analysis. J. Periodontal Res. 2017, 52, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Del Vecchio, G.; Trilli, I.; Ferrante, L.; Dipalma, G.; Palermo, A.; Inchingolo, A.D. Low-Level Light Therapy in Orthodontic Treatment: A Systematic Review. Appl. Sci. 2023, 13, 10393. [Google Scholar] [CrossRef]
- Yavagal, C.M.; Matondkar, S.P.; Yavagal, P.C. Efficacy of Laser Photobiomodulation in Accelerating Orthodontic Tooth Movement in Children: A Systematic Review with Meta-Analysis. Int. J. Clin. Pediatr. Dent. 2021, 14 (Suppl. S1), S94–S100. [Google Scholar] [CrossRef]
- El-Angbawi, A.; McIntyre, G.; Fleming, P.S.; Bearn, D. Non-Surgical Adjunctive Interventions for Accelerating Tooth Movement in Patients Undergoing Orthodontic Treatment. Cochrane Database Syst. Rev. 2023, 6, CD010887. [Google Scholar] [CrossRef]
- Elgadi, R.; Sedky, Y.; Franzen, R. The Effectiveness of Low-Level Laser Therapy on Orthodontic Tooth Movement: A Systematic Review. Lasers Dent. Sci. 2023, 7, 129–137. [Google Scholar] [CrossRef]
- Zheng, D.H.; Du, Y.Q.; Zhang, Q.Q.; Hou, F.C.; Niu, S.Q.; Zang, Y.J.; Li, B. Effect of low-level laser therapy on orthodontic dental alignment: A systematic review and meta-analysis. Lasers Med. Sci. 2023, 38, 184. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, J.Y.; Zeng, X.T.; Guo, Y. Low-Level Laser Therapy for Orthodontic Pain: A Systematic Review. Lasers Med. Sci. 2015, 30, 1789–1803. [Google Scholar] [CrossRef]
- Michelogiannakis, D.; Al-Shammery, D.; Akram, Z.; Rossouw, P.E.; Javed, F.; Romanos, G.E. Influence of Low-Level Laser Therapy on Orthodontically-Induced Inflammatory Root Resorption: A Systematic Review. Arch. Oral Biol. 2019, 100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhou, Y.; Xue, J.; Liao, L.; Ye, N.; Jian, F.; Wang, Y.; Lai, W. The Effectiveness of Low-Level Laser Therapy in Accelerating Orthodontic Tooth Movement: A Meta-Analysis. Lasers Med. Sci. 2015, 30, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Golshah, A.; Safari-Faramani, R.; Sadeghi, M. Effect of Low-Level Laser Therapy on Orthodontic Movement of Human Canine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Acta Inform. Med. 2018, 26, 139–143. [Google Scholar] [CrossRef] [PubMed]
- AlShahrani, I.; Togoo, R.A.; Hosmani, J.; Alhaizaey, A. Photobiomodulation in Acceleration of Orthodontic Tooth Movement: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2019, 47, 102220. [Google Scholar] [CrossRef]
- Jedliński, M.; Romeo, U.; Del Vecchio, A.; Palaia, G.; Galluccio, G. Comparison of the Effects of Photobiomodulation with Different Lasers on Orthodontic Movement and Reduction of the Treatment Time with Fixed Appliances in Novel Scientific Reports: A Systematic Review with Meta-Analysis. Photobiomodul. Photomed. Laser Surg. 2020, 38, 455–465. [Google Scholar] [CrossRef]
- Li, J.; Ge, X.; Guan, H.; Jia, L.; Chang, W.; Ma, W. The Effectiveness of Photobiomodulation on Accelerating Tooth Movement in Orthodontics: A Systematic Review and Meta-Analysis. Photobiomodul. Photomed. Laser Surg. 2021, 39, 232–244. [Google Scholar] [CrossRef]
- Huang, T.; Wang, Z.; Li, J. Efficiency of Photobiomodulation on Accelerating the Tooth Movement in the Alignment Phase of Orthodontic Treatment—A Systematic Review and Meta-Analysis. Heliyon 2023, 9, e13220. [Google Scholar] [CrossRef]
- Grajales, M.; Ríos-Osorio, N.; Jiménez-Peña, O.; Méndez-Sánchez, J.; Sanchez-Fajardo, K.; García-Perdomo, H.A. Effectiveness of Photobiomodulation with Low-Level Lasers on the Acceleration of Orthodontic Tooth Movement: A Systematic Review and Meta-Analysis of Split-Mouth Randomised Clinical Trials. Lasers Med. Sci. 2023, 38, 200. [Google Scholar] [CrossRef]
- Olmedo-Hernández, O.L.; Mota-Rodríguez, A.N.; Torres-Rosas, R.; Argueta-Figueroa, L. Effect of the Photobiomodulation for Acceleration of the Orthodontic Tooth Movement: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2022, 37, 2323–2341. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Lynch, E. Systematic Review of Orthodontic Treatment Management with Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 862–868. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Yang, Y. The Effectiveness of Low-Level Diode Laser Therapy on Orthodontic Pain Management: A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2015, 30, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, S.; Jia, F.; Xu, J. Does Low Level Laser Therapy Relieve the Pain Caused by the Placement of the Orthodontic Separators?—A Meta-Analysis. Head Face Med. 2015, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Deana, N.F.; Zaror, C.; Sandoval, P.; Alves, N. Effectiveness of Low-Level Laser Therapy in Reducing Orthodontic Pain: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2017, 2017, 8560652. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.F.; Maia, T.A.C.; de Barros Silva, P.G.; Abreu, L.G.; Gondim, D.V.; Santos, P.C.F. Effects of Low-Level Laser Therapy on the Orthodontic Mini-Implants Stability: A Systematic Review and Meta-Analysis. Prog. Orthod. 2021, 22, 6. [Google Scholar] [CrossRef]
- Michelogiannakis, D.; Jabr, L.; Barmak, A.B.; Rossouw, P.E.; Kotsailidi, E.A.; Javed, F. Influence of Low-Level-Laser Therapy on the Stability of Orthodontic Mini-Screw Implants: A Systematic Review and Meta-Analysis. Eur. J. Orthod. 2022, 44, 11–21. [Google Scholar] [CrossRef]
- Domínguez Camacho, A.; Montoya Guzmán, D.; Velásquez Cujar, S.A. Effective Wavelength Range in Photobiomodulation for Tooth Movement Acceleration in Orthodontics: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2020, 38, 581–590. [Google Scholar] [CrossRef]
- Shiva, S.; Gladwin, M.T. Shining a light on tissue NO stores: Near-infrared release of NO from nitrite and nitrosylated hemes. J. Mol. Cell. Cardiol. 2009, 46, 1–3. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Peng, J.; Song, W.; Zhao, J.; Chen, L. The Effects and Mechanisms of PBM Therapy in Accelerating Orthodontic Tooth Movement. Biomolecules 2023, 13, 1140. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, F.; Wei, Y.; Chen, W.R.; Chen, Q.; Xing, D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid. Redox Signal. 2014, 20, 733–746. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Chellini, F.; Sassoli, C.; Nosi, D.; Deledda, C.; Tonelli, P.; Zecchi-Orlandini, S.; Formigli, L.; Giannelli, M. Low Pulse Energy Nd:YAG Laser Irradiation Exerts a Biostimulative Effect on Different Cells of the Oral Microenvironment: An In Vitro Study. Lasers Surg. Med. 2010, 42, 527–539. [Google Scholar] [CrossRef]
- Solís-López, A.; Kriebs, U.; Marx, A.; Mannebach, S.; Liedtke, W.B.; Caterina, M.J.; Freichel, M.; Tsvilovskyy, V.V. Analysis of TRPV Channel Activation by Stimulation of FCεRI and MRGPR Receptors in Mouse Peritoneal Mast Cells. PLoS ONE 2017, 12, e0171366. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.H.; Teixeira, H.; Tsai, A. Mechanistic Insight into Orthodontic Tooth Movement Based on Animal Studies: A Critical Review. J. Clin. Med. 2021, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Joo, C.; Seong, J.; Vafabakhsh, R.; Botvinick, E.L.; Berns, M.W.; Palmer, A.E.; Wang, N.; Ha, T.; Jakobsson, E.; et al. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. eLife 2015, 4, e04876. [Google Scholar] [CrossRef] [PubMed]
- Chansaenroj, J.; Suwittayarak, R.; Egusa, H.; Samaranayake, L.P.; Osathanon, T. Mechanical force modulates inflammation and immunomodulation in periodontal ligament cells. Med. Rev. 2024, 4, 544–548. [Google Scholar] [CrossRef]
- Ward, C.W.; Prosser, B.L.; Lederer, W.J. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid. Redox Signal. 2014, 20, 929–936. [Google Scholar] [CrossRef]
- D’Angelo, D.; Rizzuto, R. The Mitochondrial Calcium Uniporter (MCU): Molecular Identity and Role in Human Diseases. Biomolecules 2023, 13, 1304. [Google Scholar] [CrossRef]
- Hofer, A.M. Interactions between calcium and cAMP signaling. Curr. Med. Chem. 2012, 19, 5768–5773. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Redondo, P.C.; Rosado, J.A. Store-operated Ca2+ entry. Adv. Exp. Med. Biol. 2012, 740, 349–382. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A Mutual Interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Deng, Y.; Li, Y.; Liu, S.; Yang, Y.; Qiu, Y.; Li, B.; Sheng, W.; Liu, J.; et al. Recent Advances of NFATc1 in Rheumatoid Arthritis-Related Bone Destruction: Mechanisms and Potential Therapeutic Targets. Mol. Med. 2024, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Rong, Y.; Tang, B.; Liu, X.; Chen, Y.; Zhou, L.; Song, J.; Zheng, L. The cytoskeleton dynamics-dependent LINC complex in periodontal ligament stem cells transmits mechanical stress to the nuclear envelope and promotes YAP nuclear translocation. Stem Cell Res. Ther. 2024, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, B.; Shin, S.; Kim, I. Low-level laser irradiation stimulates RANKL-induced osteoclastogenesis via the MAPK pathway in RAW 264.7 cells. Appl. Sci. 2021, 11, 5360. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef]
- Roth, C.E.; Craveiro, R.B.; Niederau, C.; Malyaran, H.; Neuss, S.; Jankowski, J.; Wolf, M. Mechanical Compression by Simulating Orthodontic Tooth Movement in an In Vitro Model Modulates Phosphorylation of AKT and MAPKs via TLR4 in Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2022, 23, 8062. [Google Scholar] [CrossRef]
- Roberts, W.E.; Huja, S.S.; Roberts, J.A. Bone modeling: Biomechanics, molecular mechanisms, and clinical perspectives. Semin. Orthod. 2004, 10, 123–161. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, Z. Expression and regulation of the ERK1/2 and p38 MAPK signaling pathways in periodontal tissue remodeling of orthodontic tooth movement. Mol. Med. Rep. 2018, 17, 1499–1506. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, J.; Muhammed, F.K.; Zheng, B.; Zhang, Y.; Liu, Y. Effect of orthodontic force on the expression of PI3K, Akt, and p70S6K in the human periodontal ligament during orthodontic loading. Cell Biochem. Funct. 2017, 35, 372–377. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y. The microRNA-132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through the mTOR signaling pathway. Cell. Physiol. Biochem. 2014, 33, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, L.; Zhu, Y.; Liu, Y.; Dai, H.; Zhou, J.; Geng, D.; Wang, L.; Ji, Y. Tension force-induced bone formation in orthodontic tooth movement via modulation of the GSK-3β/β-catenin signaling pathway. J. Mol. Histol. 2018, 49, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yue, Y.; Xu, Z.; Guo, L.; Wu, C.; Zhang, D.; Luo, L.; Huang, W.; Chen, H.; Yang, D. mTORC1 signaling pathway regulates tooth repair. Int. J. Oral Sci. 2023, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Groeger, S.; Meyle, J.; Ruf, S. MAPK and β-catenin signaling: Implication and interplay in orthodontic tooth movement. Front. Biosci. (Landmark Ed.) 2022, 27, 54. [Google Scholar] [CrossRef]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wang, J.; Gong, D.X.; Gu, H.Y.; Hu, S.S.; Zhang, H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: A microarray analysis. Lasers Med. Sci. 2012, 27, 509–519. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Li, J.; Yue, Y.; Li, J.; Wang, M.; Wei, N.; Hao, L. Mechanisms of mechanical force in periodontal homeostasis: A review. Front. Immunol. 2024, 15, 1438726. [Google Scholar] [CrossRef]
- Amaroli, A.; Clemente Vargas, M.R.; Pasquale, C.; Raffetto, M.; Ravera, S. Photobiomodulation on isolated mitochondria at 810 nm: First results on the efficiency of the energy conversion process. Sci. Rep. 2024, 14, 11060. [Google Scholar] [CrossRef]
- Sasaki, K.; Takeshita, N.; Fukunaga, T.; Seiryu, M.; Sakamoto, M.; Oyanagi, T.; Maeda, T.; Takano-Yamamoto, T. Vibration accelerates orthodontic tooth movement by inducing osteoclastogenesis via transforming growth factor-β signalling in osteocytes. Eur. J. Orthod. 2022, 44, 698–704. [Google Scholar] [CrossRef]
- Kikuta, J.; Tsukada, M.; Takagi, K.; Shimizu, M.; Hikida, T.; Nakayama, E.; Kasai, K. TGF-β1 stimulates bone resorption during orthodontic tooth movement. Int. J. Oral-Med. Sci. 2020, 19, 193–199. [Google Scholar] [CrossRef]
- Kitaura, H.; Ohori, F.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; Narita, K.; Murakami, K.; Kanetaka, H. The role of cytokines in orthodontic tooth movement. Int. J. Mol. Sci. 2025, 26, 6688. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast–Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Asiry, M.A. Biological Aspects of Orthodontic Tooth Movement: A Review of Literature. Saudi J. Biol. Sci. 2018, 25, 1027–1032. [Google Scholar] [CrossRef]
- Lontos, K.; Adamik, J.; Tsagianni, A.; Galson, D.L.; Chirgwin, J.M.; Suvannasankha, A. The Role of Semaphorin 4D in Bone Remodeling and Cancer Metastasis. Front. Endocrinol. 2018, 9, 322. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; Barrena-Blázquez, S.; Jiménez-Álvarez, L.; Fraile-Martinez, O.; García-Montero, C.; López-González, L.; Torres-Carranza, D.; García-Puente, L.M.; Carranza, S.T.; Álvarez-Mon, M.Á.; et al. The RANK–RANKL–OPG System: A Multifaceted Regulator of Homeostasis, Immunity, and Cancer. Medicina 2023, 59, 1752. [Google Scholar] [CrossRef]
- Di Cicco, G.; Marzano, E.; Mastrostefano, A.; Pitocco, D.; Castilho, R.S.; Zambelli, R.; Mascio, A.; Greco, T.; Cinelli, V.; Comisi, C.; et al. The Pathogenetic Role of RANK/RANKL/OPG Signaling in Osteoarthritis and Related Targeted Therapies. Biomedicines 2024, 12, 2292. [Google Scholar] [CrossRef]
- Milligan, M.; Arudchelvan, Y.; Gong, S.-G. Effects of two wattages of low-level laser therapy on orthodontic tooth movement. Arch. Oral Biol. 2017, 80, 62–68. [Google Scholar] [CrossRef]
- Fujita, S.; Yamaguchi, M.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod. Craniofacial Res. 2008, 11, 143–155. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, K. Clinical research: Low-level laser therapy in accelerating orthodontic tooth movement. BMC Oral Health 2021, 21, 324. [Google Scholar] [CrossRef]
- Kasai, K.; Yamaguchi, M.; Fujita, S.; Yoshida, T.; Utsunomiya, T.; Yamamoto, H. Molecular effects of low-energy laser irradiation during orthodontic tooth movement. Semin. Orthod. 2015, 21, 203–209. [Google Scholar] [CrossRef]
- Domínguez, A.; Gómez, C.; Palma, J.C. Effects of Low-Level Laser Therapy on Orthodontics: Rate of Tooth Movement, Pain, and Release of RANKL and OPG in GCF. Lasers Med. Sci. 2015, 30, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, Y.; Katagiri, S.; Hirota, T.; Niimi, H.; Hatasa, M.; Watanabe, K.; Shimohira, T.; Mizutani, K.; Kitazawa, M.; Matsuzawa, A.; et al. Laser irradiation decreases sclerostin expression in bone and osteogenic cells. FASEB J. 2020, 34, 12877–12893. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.A.; Somaiah, S.; Muddaiah, S.; Shetty, B.; Reddy, G.; Roopa, S. A Comparative Evaluation of Interleukin-1 Beta and Prostaglandin E2 with and without Low-Level Laser Therapy during En Masse Retraction. Contemp. Clin. Dent. 2018, 9, 267–275. [Google Scholar] [CrossRef]
- Barekatain, N.; Farhad, S.Z.; Rafiei, M.; Sheikh, Y. The Effect of Low Laser Therapy on GCF Level of PGE2 During Orthodontic Treatment. J. Res. Dent. Sci. 2023, 20, 160–165. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs Expression Levels in the Periodontal Ligament during Orthodontic Tooth Movement: A Systematic Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.; Schechter, I.; Thomadakis, G.; Fourie, J.; Lemmer, J. Biological Events in Periodontal Ligament and Alveolar Bone Associated with Application of Orthodontic Forces. Sci. World J. 2015, 2015, 876509. [Google Scholar] [CrossRef]
- Jivrajani, S.J.; Bhad-Patil, W.A. Effect of low-intensity laser therapy (LILT) on MMP-9 expression in gingival crevicular fluid and rate of orthodontic tooth movement in patients undergoing canine retraction: A randomized controlled trial. Int. Orthod. 2020, 18, 330–339, Erratum in Int. Orthod. 2020, 18, 895–896. https://doi.org/10.1016/j.ortho.2020.01.008. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, K.-A.; Anderson, S.; Kang, Y.-G.; Kim, S.-J. Combined effect of photobiomodulation with a matrix metalloproteinase inhibitor on the rate of relapse in rats. Angle Orthod. 2016, 86, 206–213. [Google Scholar] [CrossRef]
- Bloemen, V.; Schoenmaker, T.; de Vries, T.J.; Everts, V. Direct Cell–Cell Contact between Periodontal Ligament Fibroblasts and Osteoclast Precursors Synergistically Increases the Expression of Genes Related to Osteoclastogenesis. J. Cell. Physiol. 2010, 222, 565–573. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van Oers, R.F.; Bakker, A.D.; Bacabac, R.G. Nitric Oxide Signaling in Mechanical Adaptation of Bone. Osteoporos. Int. 2014, 25, 1427–1437. [Google Scholar] [CrossRef]
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki, T.; Suda, T.; Matsuo, K. Bidirectional EphrinB2-EphB4 Signaling Controls Bone Homeostasis. Cell Metab. 2006, 4, 111–121. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Wu, F. Low-Level Laser Irradiation Enhances the Proliferation and Osteogenic Differentiation of PDLSCs via BMP Signaling. Lasers Med. Sci. 2022, 37, 941–948. [Google Scholar] [CrossRef]
- Wu, N.; Song, J.; Liu, X.; Ma, X.; Guo, X.; Liu, T.; Wu, M. Effect of a low-energy Nd:YAG laser on periodontal ligament stem cell homing through the SDF-1/CXCR4 signaling pathway. BMC Oral Health 2023, 23, 501. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Parsamanesh, G.; Shahabi, S.; Jazaeri, M.; Baghaei, K.; Fekrazad, R. The Effect of Laser Photobiomodulation on Periodontal Ligament Stem Cells. Photochem. Photobiol. 2021, 97, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Matos, A.A.; Santesso, M.R.; Tokuhara, C.K.; Leite, A.L.; Bagnato, V.S.; Machado, M.A.; Peres-Buzalaf, C.; Oliveira, R.C. Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J. Photochem. Photobiol. B 2016, 163, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mylona, V.; Anagnostaki, E.; Chiniforush, N.; Barikani, H.; Lynch, E.; Grootveld, M. Photobiomodulation Effects on Periodontal Ligament Stem Cells: A Systematic Review of In Vitro Studies. Curr. Stem Cell Res. Ther. 2024, 19, 544–558. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Han, K.; Wang, X.; Guo, Z.; Deng, Q.; Li, J.; Lv, S.; Yu, W. Effects of low-level laser on periodontal tissue remodeling in hPDLCs under tensile stress. Lasers Med. Sci. 2023, 38, 232. [Google Scholar] [CrossRef]
- Cury, V.; Moretti, A.I.; Assis, L.; Bossini, P.; de Souza Crusca, J.; Neto, C.B.; Fangel, R.; de Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low Level Laser Therapy Increases Angiogenesis in a Model of Ischemic Skin Flap in Rats Mediated by VEGF, HIF-1α and MMP-2. J. Photochem. Photobiol. B 2013, 125, 164–170. [Google Scholar] [CrossRef]
- Bai, J.; Li, L.; Kou, N.; Bai, Y.; Zhang, Y.; Lu, Y.; Gao, L.; Wang, F. Low Level Laser Therapy Promotes Bone Regeneration by Coupling Angiogenesis and Osteogenesis. Stem Cell Res. Ther. 2021, 12, 432. [Google Scholar] [CrossRef]
- Sokos, D.; Everts, V.; de Vries, T.J. Role of periodontal ligament fibroblasts in osteoclastogenesis: A review. J. Periodontal Res. 2015, 50, 152–159. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A.; Robling, A.G. Bone and skeletal muscle: Key players in mechanotransduction and potential overlapping mechanisms. Bone 2015, 80, 24–36. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, X.; Ruan, Y.; Huang, Y. Photobiomodulation therapy’s impact on angiogenesis and osteogenesis in orthodontic tooth movement: In vitro and in vivo study. BMC Oral Health 2024, 24, 147. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Shi, M.; Gan, P.; Huang, Q.; Wang, A.; Tan, G.; Fang, Y.; Liao, H. Low-Level Laser Therapy Induces Human Umbilical Vascular Endothelial Cell Proliferation, Migration and Tube Formation through Activating the PI3K/Akt Signaling Pathway. Microvasc. Res. 2020, 129, 103959. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef] [PubMed]
- Szymczyszyn, A.; Doroszko, A.; Szahidewicz-Krupska, E.; Rola, P.; Gutherc, R.; Jasiczek, J.; Mazur, G.; Derkacz, A. Effect of the Transdermal Low-Level Laser Therapy on Endothelial Function. Lasers Med. Sci. 2016, 31, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; He, H. Autophagy in orthodontic tooth movement: Advances, challenges, and future perspectives. Mol. Med. 2025, 31, 245. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Lin, F.; Zheng, Q.; Xu, X.; Mei, L. Dynamic study into autophagy and apoptosis during orthodontic tooth movement. Exp. Ther. Med. 2021, 21, 430. [Google Scholar] [CrossRef]
- Li, Y.; Jacox, L.A.; Coats, S.; Kwon, J.; Xue, P.; Tang, N.; Rui, Z.; Wang, X.; Kim, Y.I.; Wu, T.J.; et al. Roles of autophagy in orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2021, 159, 582–593. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, B.; Yang, K. Autophagy regulates osteogenic differentiation of human periodontal ligament stem cells induced by orthodontic tension. Stem Cells Int. 2022, 2022, 2983862. [Google Scholar] [CrossRef]
- Küng, C.; Lazarou, M.; Nguyen, T.N. Advances in mitophagy initiation mechanisms. Curr. Opin. Cell Biol. 2025, 94, 102493. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, S.; Fu, Y.; Wang, J.; Liu, J.; Wei, F. Mechanical force induces mitophagy-mediated anaerobic oxidation in periodontal ligament stem cells. Cell. Mol. Biol. Lett. 2023, 28, 57. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, H.; Yan, J.; Ma, S.; Tan, J. Age-related mitophagy regulates orthodontic tooth movement by affecting PDLSCs mitochondrial function and RANKL/OPG. FASEB J. 2024, 38, e23865. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wei, X.; Ju, C.; Wang, X.; Zhang, Z.; Ma, Y.; Zhu, Z.; Li, X.; Song, Z.; Luo, L.; et al. Protective effect of photobiomodulation against hydrogen peroxide-induced oxidative damage by promoting autophagy through inhibition of PI3K/AKT/mTOR pathway in MC3T3-E1 cells. Oxid. Med. Cell Longev. 2022, 2022, 7223353. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, W.; Zhu, B.; Li, M.; Zhang, X. Photobiomodulation therapy at 650 nm enhances osteogenic differentiation of osteoporotic bone marrow mesenchymal stem cells through modulating autophagy. Photodiagnosis Photodyn. Ther. 2024, 50, 104389. [Google Scholar] [CrossRef]
- Silva, H.N.M.D.; Fernandes, E.M.; Pereira, V.A.; Mizobuti, D.S.; Covatti, C.; Rocha, G.L.D.; Minatel, E. LEDT and idebenone treatment modulate autophagy and improve regenerative capacity in the dystrophic muscle through an AMPK-pathway. PLoS ONE 2024, 19, e0300006. [Google Scholar] [CrossRef]
- Wider, J.M.; Gruley, E.; Morse, P.T.; Wan, J.; Lee, I.; Anzell, A.R.; Fogo, G.M.; Mathieu, J.; Hish, G.; O’Neil, B.; et al. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest. Crit. Care 2023, 27, 491. [Google Scholar] [CrossRef]
- Chen, H.; Li, N.; Liu, N.; Zhu, H.; Ma, C.; Ye, Y.; Shi, X.; Luo, G.; Dong, X.; Tan, T.; et al. Photobiomodulation modulates mitochondrial energy metabolism and ameliorates neurological damage in an APP/PS1 mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 72. [Google Scholar] [CrossRef]
- da Silva Neto Trajano, L.A.; Siqueira, P.B.; de Sousa Rodrigues, M.M.; Barreto Pires, B.R.; de Souza da Fonseca, A.; Mencalha, A.L. Does photobiomodulation alter mitochondrial dynamics? Photochem. Photobiol. 2025, 101, 21–37. [Google Scholar] [CrossRef]
- Kaplan, M.; Kalajzic, Z.; Choi, T.; Maleeh, I.; Ricupero, C.L.; Skelton, M.N.; Daily, M.L.; Chen, J.; Wadhwa, S. The role of inhibition of osteocyte apoptosis in mediating orthodontic tooth movement and periodontal remodeling: A pilot study. Prog. Orthod. 2021, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Jiang, L.; Lu, Z.; Zeng, G.; Yi, S.; Zhou, H.; Xie, R.; Zhang, H.; Chen, J.; Lin, C. Osteocyte apoptosis: The roles and key molecular mechanisms. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jin, C.; Wang, G.; Li, J.; Liu, J.; Li, S. How osteocyte death promotes osteoclast formation. Front. Immunol. 2025, 16, 1551542. [Google Scholar] [CrossRef] [PubMed]
- Moin, S.; Kalajzic, Z.; Utreja, A.; Nihara, J.; Wadhwa, S.; Uribe, F.; Nanda, R. Osteocyte death during orthodontic tooth movement in mice. Angle Orthod. 2014, 84, 1086–1092. [Google Scholar] [CrossRef]
- Hatai, T.; Yokozeki, M.; Funato, N.; Baba, Y.; Moriyama, K.; Ichijo, H.; Kuroda, T. Apoptosis of Periodontal Ligament Cells Induced by Mechanical Stress during Tooth Movement. Oral Dis. 2001, 7, 287–290. [Google Scholar] [CrossRef]
- Duggal, R.; Singh, N. Detection of apoptosis in human periodontal ligament during orthodontic tooth movement. J. Dent. Spec. 2015, 3, 217. [Google Scholar] [CrossRef]
- Wu, Y.; Zhuang, J.; Zhao, D.; Xu, C. Interaction between caspase-3 and caspase-5 in the stretch-induced programmed cell death in the human periodontal ligament cells. J. Cell Physiol. 2019, 234, 13571–13581. [Google Scholar] [CrossRef]
- Yu, K.W.; Yao, C.C.; Jeng, J.H.; Shieh, H.Y.; Chen, Y.J. Periostin inhibits mechanical stretch-induced apoptosis in osteoblast-like MG-63 cells. J. Formos. Med. Assoc. 2018, 117, 292–300. [Google Scholar] [CrossRef]
- Goga, Y.; Chiba, M.; Shimizu, Y.; Mitani, H. Compressive Force Induces Osteoblast Apoptosis via Caspase-8. J. Dent. Res. 2006, 85, 240–244. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, Y.; Huang, Y.; Yang, Y.; Zhao, Y.; Chen, S.; Deng, J.; Li, W.; Han, B. Periodontal ligament cell apoptosis activates Lepr+ osteoprogenitors in orthodontics. J. Dent. Res. 2024, 103, 937–947. [Google Scholar] [CrossRef]
- Shen, X.; Wu, W.; Ying, Y.; Zhou, L.; Zhu, H. A regulatory role of Piezo1 in apoptosis of periodontal tissue and periodontal ligament fibroblasts during orthodontic tooth movement. Aust. Endod. J. 2023, 49 (Suppl. S1), 228–237. [Google Scholar] [CrossRef]
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schröder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M. In vitro compression model for orthodontic tooth movement modulates human periodontal ligament fibroblast proliferation, apoptosis and cell cycle. Biomolecules 2021, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Çifter, M.; Çekici, A.; Olgaç, V.; İşsever, H.; Işık, G. Effects of orthodontic force magnitude on cell apoptosis and RANKL-induced osteoclastogenesis: Studies in a rat model. J. Orofac. Orthop. 2020, 81, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xu, C.; Sun, S.Y.; Zhang, F.Q. Cyclic stretching force induces apoptosis in human periodontal ligament cells via caspase-9. Arch. Oral Biol. 2009, 54, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Xing, D. LPLI Inhibits Apoptosis Upstream of Bax Translocation via a GSK-3β–Inactivation Mechanism. J. Cell. Physiol. 2010, 224, 218–228. [Google Scholar] [CrossRef]
- Tian, Y.; Kim, H.; Kang, H.-W. In vitro anti-tumor effect of high-fluence low-power laser light on apoptosis of human colorectal cancer cells. Lasers Med. Sci. 2021, 36, 513–520. [Google Scholar] [CrossRef]
- Pansani, T.N.; Basso, F.G.; Turirioni, A.P.; Kurachi, C.; Hebling, J.; de Souza Costa, C.A. Effects of low-level laser therapy on the proliferation and apoptosis of gingival fibroblasts treated with zoledronic acid. Int. J. Oral Maxillofac. Surg. 2014, 43, 1030–1034. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chen, S.Y.; Hsieh, Y.L.; Teng, Y.H.; Cheng, Y.J. Low-level laser therapy prevents endothelial cells from TNF-α/cycloheximide-induced apoptosis. Lasers Med. Sci. 2018, 33, 279–286. [Google Scholar] [CrossRef]
- de Souza Oliveira, C.; de Oliveira, H.A.; Teixeira, I.L.A.; Antonio, E.L.; Silva, F.A.; Sunemi, S.; Leal-Junior, E.C.; de Tarso Camillo de Carvalho, P.; Tucci, P.J.F.; Serra, A.J. Low-level laser therapy prevents muscle apoptosis induced by a high-intensity resistance exercise in a dose-dependent manner. Lasers Med. Sci. 2020, 35, 1867–1870. [Google Scholar] [CrossRef]
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J. Dent. Res. 2022, 101, 11–20. [Google Scholar] [CrossRef]
- Wan Hassan, W.N.; Waddington, R.J. Immunology of tooth movement and root resorption in orthodontics. In Immunology for Dentistry; Wiley: Hoboken, NJ, USA, 2023; pp. 134–155. [Google Scholar] [CrossRef]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontol. 2000 2020, 82, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.M.; Amato, P.A.; Leão, F.V.; Gerlach, R.F.; Ferreira, J.T. Myeloperoxidase activity is increased in gingival crevicular fluid and whole saliva after fixed orthodontic appliance activation. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 613–616. [Google Scholar] [CrossRef]
- Cerdeira, C.D.; Lima Brigagão, M.R.; Carli, M.L.; de Souza Ferreira, C.; de Oliveira Isac Moraes, G.; Hadad, H.; Costa Hanemann, J.A.; Hamblin, M.R.; Sperandio, F.F. Low-level laser therapy stimulates the oxidative burst in human neutrophils and increases their fungicidal capacity. J. Biophotonics 2016, 9, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Wasik, M.; Gorska, E.; Modzelewska, M.; Nowicki, K.; Jakubczak, B.; Demkow, U. The influence of low-power helium-neon laser irradiation on function of selected peripheral blood cells. J. Physiol. Pharmacol. 2007, 58 Pt 2 (Suppl. S5), 729–737. [Google Scholar] [PubMed]
- Kujawa, J.; Agier, J.; Kozłowska, E. Impact of photobiomodulation therapy on pro-inflammation functionality of human peripheral blood mononuclear cells—A preliminary study. Sci. Rep. 2024, 14, 23111. [Google Scholar] [CrossRef]
- Zeng, M.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Zhang, J.; Yan, Y.; Liu, F.; He, D.; et al. Orthodontic force induces systemic inflammatory monocyte responses. J. Dent. Res. 2015, 94, 1295–1302. [Google Scholar] [CrossRef]
- He, D.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Luo, Q.; Song, Y.; Liu, F.; Yan, Y.; Gan, Y.; et al. M1-like macrophage polarization promotes orthodontic tooth movement. J. Dent. Res. 2015, 94, 1286–1294. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, S.; Sathe, A.A.; Lin, Y.; Zhang, Q.; Xu, L.; Yang, P.; Zhong, W.; Wang, Y.; Qian, Y. CCR2+ macrophages promote orthodontic tooth movement and alveolar bone remodeling. Front. Immunol. 2022, 13, 835986. [Google Scholar] [CrossRef]
- Schroder, A.; Kappler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of compressive and tensile strain on macrophages during simulated orthodontic tooth movement. Mediat. Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Translat. 2017, 10, 86–93. [Google Scholar] [CrossRef]
- Wang, G.; Peng, C.; Tang, M.; Wang, Y.; Li, J.; Chen, H.; Chang, X.; Shu, Z.; He, N.; Guo, J.; et al. Simultaneously Boosting Inflammation Resolution and Osteogenic Differentiation in Periodontitis Using Folic Acid-Modified Liposome–Thermosensitive Hydrogel Composites. Mater. Des. 2023, 234, 112314. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Sun, W.; Wang, Y.; Ren, L.; Wei, W.; Bai, D. Macrophages mediate corticotomy-accelerated orthodontic tooth movement. Sci. Rep. 2018, 8, 16788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Groeger, S.; Yong, J.; Ruf, S. Orthodontic Compression Enhances Macrophage M2 Polarization via Histone H3 Hyperacetylation. Int. J. Mol. Sci. 2023, 24, 3117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.Z.; Wang, Y.H.; Liao, W.T.; Chen, Y.J.; Kuo, C.H.; Kuo, H.F.; Hung, C.H. Effects of low-level laser therapy on M1-related cytokine expression in monocytes via histone modification. Mediat. Inflamm. 2014, 2014, 625048. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Chen, L.; Xu, W.; Wu, B. Photobiomodulation activates undifferentiated macrophages and promotes M1/M2 macrophage polarization via PI3K/AKT/mTOR signaling pathway. Lasers Med. Sci. 2023, 38, 86. [Google Scholar] [CrossRef]
- Fernandes, K.P.S.; Souza, N.H.C.; Mesquita-Ferrari, R.A.; de Fatima Teixeira da Silva, D.; Rocha, L.A.; Alves, A.N.; de Brito Sousa, K.; Kalil Bussadori, S.; Hamblin, M.R.; Daumas Nunes, F. Photobiomodulation with 660-nm and 780-nm laser on activated J774 macrophage-like cells: Effect on M1 inflammatory markers. J. Photochem. Photobiol. B Biol. 2015, 153, 344–351. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Zheng, Q.; Hu, X.; Wang, Z.; Liang, Z.; Li, K.; Song, J.; Ding, T.; Shen, X.; et al. Low-level laser therapy 810-nm up-regulates macrophage secretion of neurotrophic factors via PKA-CREB and promotes neuronal axon regeneration in vitro. J. Cell. Mol. Med. 2020, 24, 476–486. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Yu, S.; Yao, F.; Luo, Y.; Liu, Y.; Tian, D.; Cheng, L.; Jing, J. M2 Macrophages Promote PDGFRβ+ Pericytes Migration after Spinal Cord Injury in Mice via PDGFB/PDGFRβ Pathway. Front. Pharmacol. 2021, 12, 670813. [Google Scholar] [CrossRef]
- Keskiner, I.; Lutfioğlu, M.; Aydogdu, A.; Saygun, N.I.; Serdar, M.A. Effect of Photobiomodulation on Transforming Growth Factor-β1, Platelet-Derived Growth Factor-BB, and Interleukin-8 Release in Palatal Wounds after Free Gingival Graft Harvesting: A Randomized Clinical Study. Photomed. Laser Surg. 2016, 34, 263–271, Erratum in Photomed. Laser Surg. 2018, 36, 58. https://doi.org/10.1089/pho.2016.4094.correx.. [Google Scholar] [CrossRef]
- Prathapan Santhakumari, P.; Varma Raja, V.; Joseph, J.; Devaraj, A.; John, E.; Oommen Thomas, N. Impact of low-level laser therapy on orthodontic tooth movement and various cytokines in gingival crevicular fluid: A split-mouth randomized study. Cureus 2023, 15, e42809. [Google Scholar] [CrossRef]
- Yanaguizawa, M.S.; Suzuki, S.S.; Martinez, E.F.; Suzuki, H.; Pelegrin, M.C.; Garcez, A.S. Effects of Low-Level Laser Therapy in Orthodontic Patients on Immediate Inflammatory Response after Mini-Implants Insertion: A Preliminary Report. Photomed. Laser Surg. 2017, 35, 57–63. [Google Scholar] [CrossRef]
- Fernandes, M.R.U.; Suzuki, S.S.; Suzuki, H.; Martinez, E.F.; Garcez, A.S. Photobiomodulation increases intrusion tooth movement and modulates IL-6, IL-8 and IL-1β expression during orthodontically bone remodeling. J. Biophotonics 2019, 12, e201800311. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Souza Furtado, T.C.; Mendes, W.D.; Matsumoto, M.A.N.; Alves, S.Y.F.; Stuani, M.B.S.; Borsatto, M.C.; Corona, S.A.M. Photobiomodulation impacts the levels of inflammatory mediators during orthodontic tooth movement? A systematic review with meta-analysis. Lasers Med. Sci. 2022, 37, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Lin, T.; Chen, S.H.; Liao, Y.W.; Liu, C.M.; Yu, C.C. Er:YAG laser suppresses pro-inflammatory cytokines expression and inflammasome in human periodontal ligament fibroblasts with Porphyromonas gingivalis-lipopolysaccharide stimulation. J. Dent. Sci. 2024, 19, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Pastille, E.; Konermann, A. Exploring the Role of Innate Lymphoid Cells in the Periodontium: Insights into Immunological Dynamics during Orthodontic Tooth Movement. Front. Immunol. 2024, 15, 1428059. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, J.; Kim, B.Y.; Kim, Y.; Kim, N.G.; Shin, Y.I. Photobiomodulation ameliorates inflammatory parameters in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis. Front. Immunol. 2023, 14, 1122581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chiang, M.H.; Chen, P.H.; Ho, M.L.; Lee, H.E.; Wang, Y.H. Anti-inflammatory effects of low-level laser therapy on human periodontal ligament cells: In vitro study. Lasers Med. Sci. 2018, 33, 469–477. [Google Scholar] [CrossRef]
- Wada, N.; Tomokiyo, A.; Gronthos, S.; Bartold, P.M. Immunomodulatory properties of PDLSC and relevance to periodontal regeneration. Curr. Oral Health Rep. 2015, 2, 245–251. [Google Scholar] [CrossRef]
- Oner, F.; Kantarci, A. Periodontal Response to Nonsurgical Accelerated Orthodontic Tooth Movement. Periodontol. 2000 2025, Epub ahead of print. [Google Scholar] [CrossRef]
- Gao, Y.; Grassi, F.; Ryan, M.R.; Terauchi, M.; Page, K.; Yang, X.; Weitzmann, M.N.; Pacifici, R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007, 117, 122–132. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Z.; Quan, H.; Xiao, L.; Zhao, J.; Jiang, C.; Wang, Y.; Liu, J.; Gou, Y.; An, S.; et al. Osteoimmunology in orthodontic tooth movement. Oral Dis. 2015, 21, 694–704. [Google Scholar] [CrossRef]
- Miron, R.J.; Bohner, M.; Zhang, Y.; Bosshardt, D.D. Osteoinduction and Osteoimmunology: Emerging Concepts. Periodontol. 2000 2024, 94, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wong, P.; Li, J.; Lv, Z.; Xu, L.; Zhu, G.; He, M.; Luo, Y. Osteoimmunology: The correlation between osteoclasts and the Th17/Treg balance in osteoporosis. J. Cell. Mol. Med. 2022, 26, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, L.; Zheng, W.; Zhang, B. Matrix metalloproteinases and Th17 cytokines in the gingival crevicular fluid during orthodontic tooth movement. Eur. J. Paediatr. Dent. 2021, 22, 135–138. [Google Scholar] [PubMed]
- Tang, M.; Lu, L.; Yu, X. Interleukin-17A Interweaves the Skeletal and Immune Systems. Front. Immunol. 2021, 11, 625034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Wang, L.; Liu, A.; Zhou, H.; Liang, X.; Kang, N. The IL-17 level in gingival crevicular fluid as an indicator of orthodontically induced inflammatory root resorption. J. Orofac. Orthop. 2025, 86, 48–58. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Shi, Z.; Xu, D.; Luo, S.; Siegal, G.P.; Feng, X.; Wei, S. Interleukin-4 inhibits RANKL-induced NFATc1 expression via STAT6: A novel mechanism mediating its blockade of osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3385–3392. [Google Scholar] [CrossRef]
- te Velde, A.A.; Huijbens, R.J.; Heije, K.; de Vries, J.E.; Figdor, C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.S.; Kiesel, J.R.; DiPaolo, R.; Pagadala, M.S.; Aurora, R. Osteoclast-Activated FoxP3+ CD8+ T Cells Suppress Bone Resorption In Vitro. PLoS ONE 2012, 7, e38199. [Google Scholar] [CrossRef]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-γ-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Behm, C.; Zhao, Z.; Andrukhov, O. Immunomodulatory activities of periodontal ligament stem cells in orthodontic forces-induced inflammatory processes: Current views and future perspectives. Front. Oral Health 2022, 3, 877348. [Google Scholar] [CrossRef]
- Settem, R.P.; Honma, K.; Chinthamani, S.; Kawai, T.; Sharma, A. B-cell RANKL contributes to pathogen-induced alveolar bone loss in an experimental periodontitis mouse model. Front. Physiol. 2021, 12, 722859. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Han, X.; Yamashita, K.; Kitami, M.; Owaki, T.; Huang, G.T.; Sasaki, K.; Kurihara, H. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J. Periodontal Res. 2006, 41, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Terauchi, M.; Vikulina, T.; Roser-Page, S.; Weitzmann, M.N. B Cell Production of Both OPG and RANKL Is Significantly Increased in Aged Mice. Open Bone J. 2014, 6, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Stadler, I.; Evans, R.; Kolb, B.; Naim, J.O.; Narayan, V.; Buehner, N.; Lanzafame, R.J. In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg. Med. 2000, 27, 255–261. [Google Scholar] [CrossRef]
- Gulsoy, M.; Ozer, G.H.; Bozkulak, O.; Tabakoglu, H.O.; Aktas, E.; Deniz, G.; Ertan, C. The biological effects of 632.8-nm low energy He-Ne laser on peripheral blood mononuclear cells in vitro. J. Photochem. Photobiol. B 2006, 82, 199–202. [Google Scholar] [CrossRef]
- Bagheri, H.S.; Rasta, S.H.; Mohammadi, S.M.; Rahimi, A.A.R.; Movassaghpour, A.; Charoudeh, H.N. Low-Level Laser Irradiation Modulated Viability of Normal and Tumor Human Lymphocytes In Vitro. J. Lasers Med. Sci. 2020, 11, 174–180. [Google Scholar] [CrossRef]
- Al Musawi, M.S.; Jaafar, M.S.; Al-Gailani, B.; Ahmed, N.M.; Suhaimi, F.M.; Suardi, N. Effects of low-level laser irradiation on human blood lymphocytes in vitro. Lasers Med. Sci. 2017, 32, 405–411. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Q.; Chang, H.; Li, J.; Xing, D. Promoted CD4+ T cell-derived IFN-γ/IL-10 by photobiomodulation therapy modulates neurogenesis to ameliorate cognitive deficits in APP/PS1 and 3xTg-AD mice. J. Neuroinflammation 2022, 19, 253. [Google Scholar] [CrossRef]
- Kawakubo, M.; Cunningham, T.J.; Demehri, S.; Manstein, D. Fractional laser releases tumor-associated antigens in poorly immunogenic tumor and induces systemic immunity. Sci. Rep. 2017, 7, 12751. [Google Scholar] [CrossRef]
- Figueiredo Braga, L.T.; Ribeiro, I.M.; Barroso, S.; Kampke, E.H.; Santos Neves, L.N.; Andrade, S.C.; Barbosa, G.H.; Porto, M.L.; Meyrelles, S.S. Modulatory effects of photobiomodulation on oxidative and inflammatory responses in a murine model of periodontitis. Antioxidants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Mistry, A.; Pereira, R.; Kini, V.; Padhye, A. Effect of combined therapy using diode laser and photodynamic therapy on levels of IL-17 in gingival crevicular fluid in patients with chronic periodontitis. J. Lasers Med. Sci. 2016, 7, 250–255. [Google Scholar] [CrossRef]
- Sumida, T.S.; Cheru, N.T.; Hafler, D.A. The Regulation and Differentiation of Regulatory T Cells and Their Dysfunction in Autoimmune Diseases. Nat. Rev. Immunol. 2024, 24, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Lu, S.; Lv, H. Metabolic Disturbance and Th17/Treg Imbalance Are Associated with Progression of Gingivitis. Front. Immunol. 2021, 12, 670178. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, L. Osteoimmunology: The Crosstalk between T Cells, B Cells, and Osteoclasts in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 25, 2688. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Peng, J.; Yu, L.; Huang, S.; Xu, L.; Su, Y.; Chen, L. Orthodontic Treatment Induces Th17/Treg Cells to Regulate Tooth Movement in Rats with Periodontitis. Iran. J. Basic Med. Sci. 2020, 23, 1315–1322. [Google Scholar] [CrossRef]
- Lin, J.; Huang, J.; Zhang, Z.; Yu, X.; Cai, X.; Liu, C. Periodontal Ligament Cells under Mechanical Force Regulate Local Immune Homeostasis by Modulating Th17/Treg Cell Differentiation. Clin. Oral Investig. 2022, 26, 3747–3764. [Google Scholar] [CrossRef]
- de Brito, A.A.; Gonçalves Santos, T.; Herculano, K.Z.; Estefano-Alves, C.; de Alvarenga Nascimento, C.R.; Rigonato-Oliveira, N.C.; Chavantes, M.C.; Aimbire, F.; da Palma, R.K.; Ligeiro de Oliveira, A.P. Photobiomodulation therapy restores IL-10 secretion in a murine model of chronic asthma: Relevance to the population of CD4+CD25+Foxp3+ cells in lung. Front. Immunol. 2022, 12, 789426. [Google Scholar] [CrossRef]
- Dos Anjos, L.M.J.; Salvador, P.A.; de Souza, Á.C.; de Souza da Fonseca, A.; de Paoli, F.; Gameiro, J. Modulation of immune response to induced-arthritis by low-level laser therapy. J. Biophotonics 2019, 12, e201800120. [Google Scholar] [CrossRef]
- Choi, D.H.; Lim, J.H.; Lee, K.H.; Kim, M.Y.; Kim, H.Y.; Shin, C.Y.; Han, S.H.; Lee, J. Effect of 710-nm visible light irradiation on neuroprotection and immune function after stroke. Neuroimmunomodulation 2012, 19, 267–276. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef]
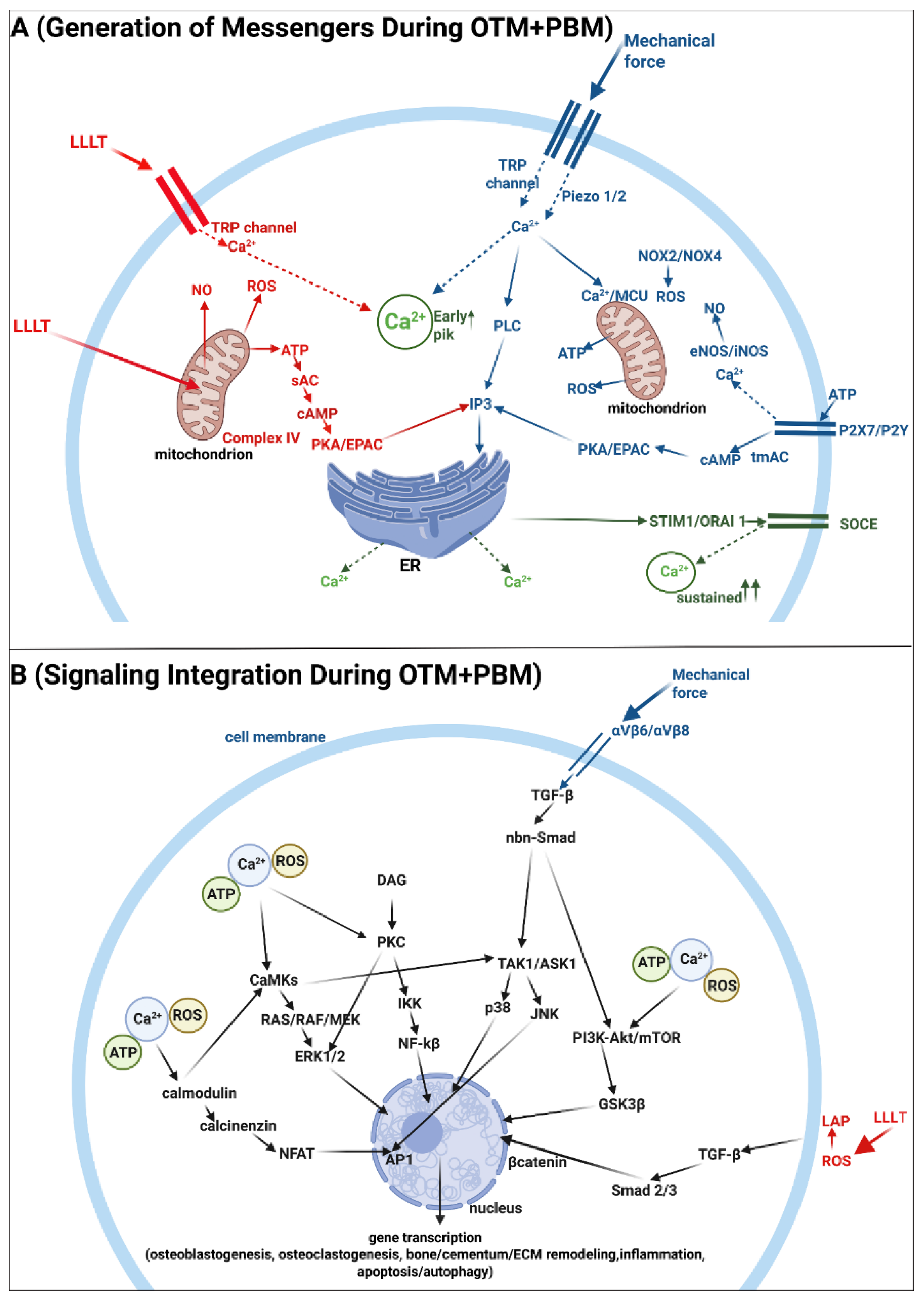

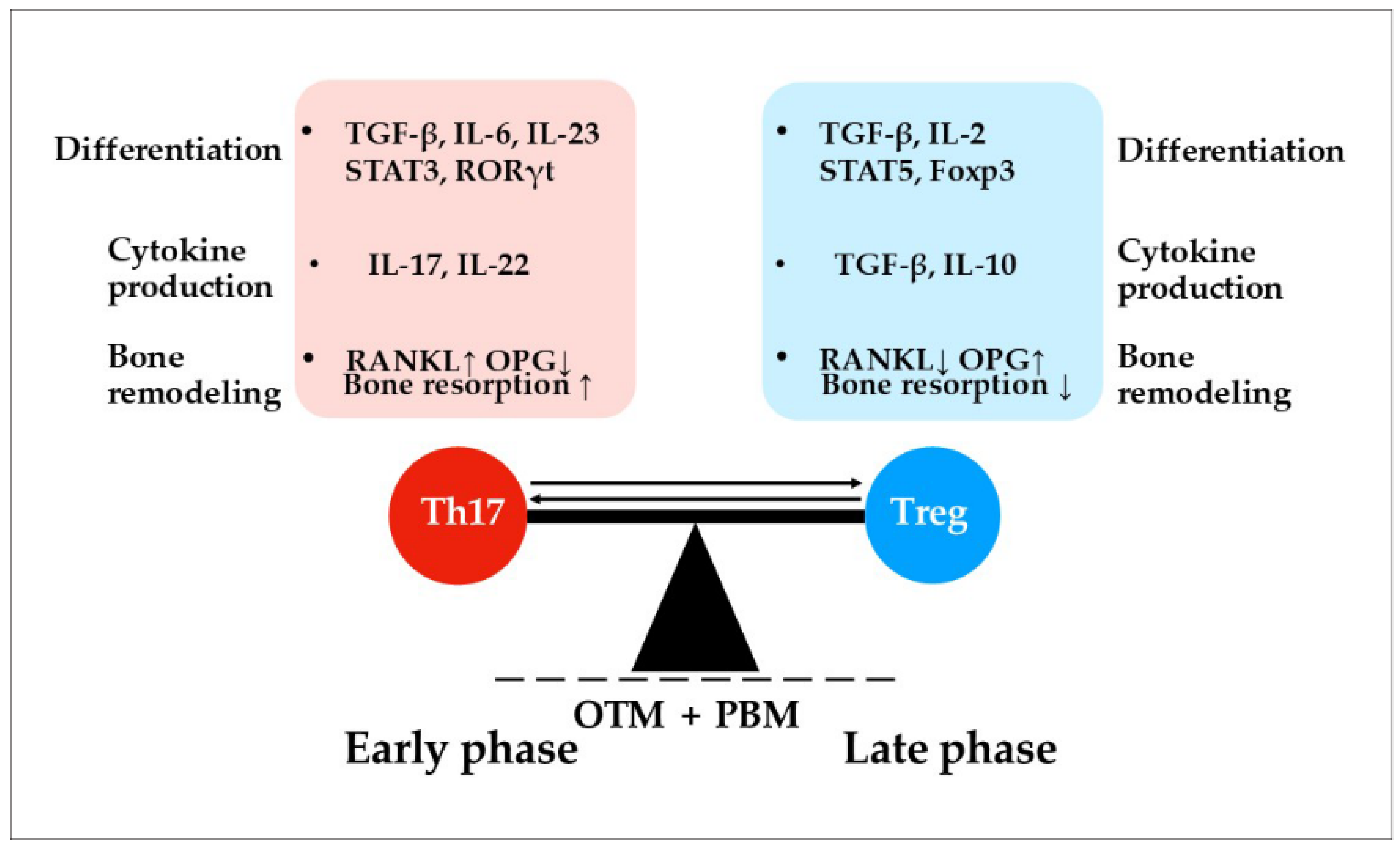
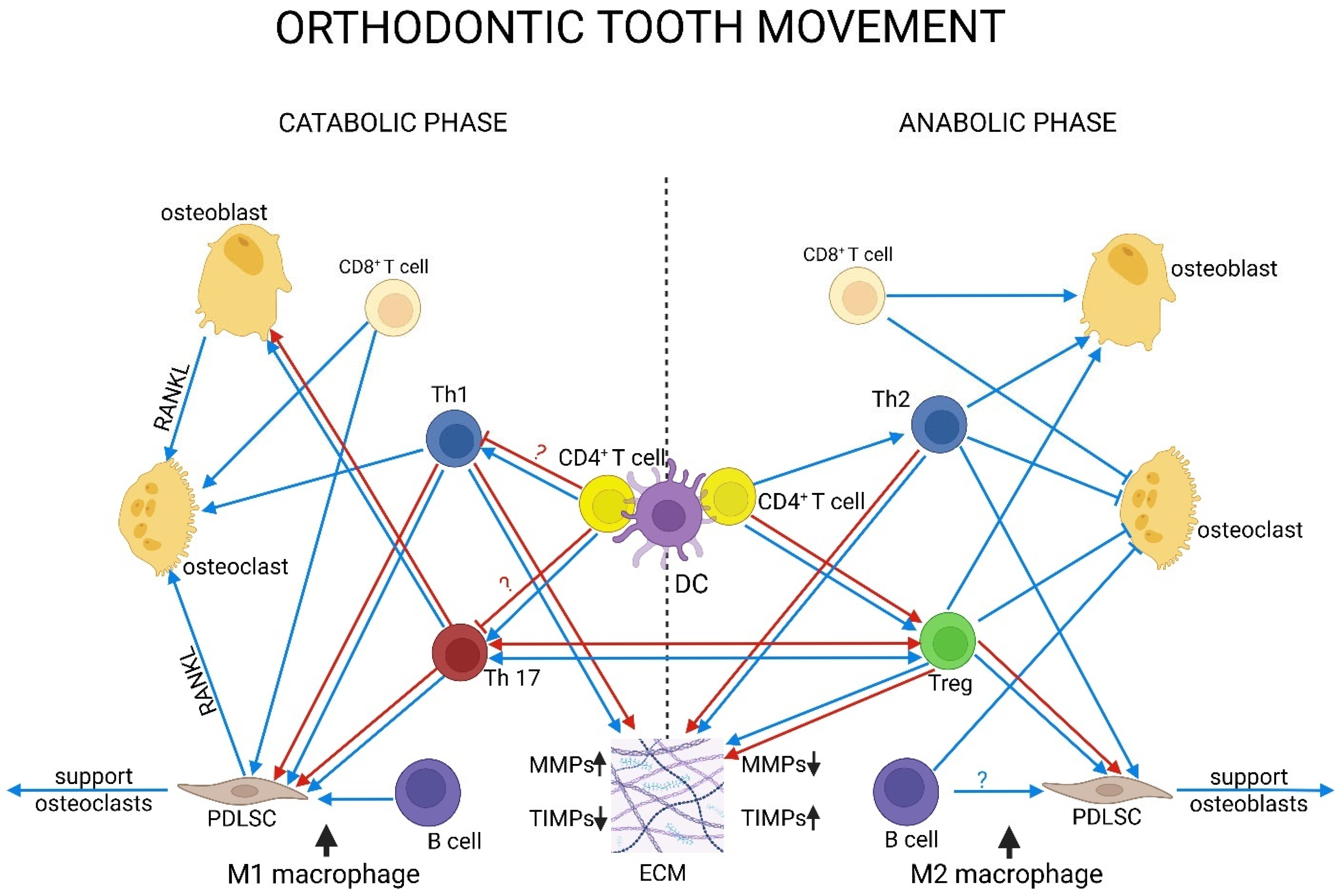
| References | Laser Types | Wavelengths (nm) | Main Indications Studied | Energy Density/Dosage (J/cm2) |
|---|---|---|---|---|
| Bakdach and Hadad (2020) [44] | Diode lasers (GaAlAs, InGaAsP) | 630–940 | Accelerating OTM (esp. canine retraction) | 2.5–8 |
| Inchingolo et al. (2023) [46] | Diode lasers, LED devices | 630–980 | Acceleration of OTM, pain reduction, miniscrew stability; root resorption | 1–25 |
| Yavagal et al. (2021) [47] | Diode lasers | 670–940 | Acceleration of tooth movement in children | 5–25 |
| El-Angbawi et al. (2023) [48] | Diode lasers, LED | 630–940 | Adjuncts for OTM, alignment, pain, treatment duration | 2–20 |
| Elgadi et al. (2023) [49] | Diode lasers | 670–980 | Acceleration of OTM, treatment duration reduction | 0.05–108 |
| Zheng et al. (2023) [50] | Diode lasers, intraoral/extraoral LED | 635–980 | Dental alignment | 6–150 (cumulative dose) |
| Li et al. (2015) [51] | Diode lasers | 630–980 | Pain reduction in orthodontics | 2–25 (most studies <10 J/cm2) |
| Ahmad et al. (2021) [36] | Diode, HeNe, Nd:YAG | 632.8–1064 | Temporomandibular joint disorders (pain, function) | 1.5–112.5 |
| Michelogiannakis et al. (2019) [52] | Diode lasers (GaAlAs) | 635–830 | OIIRR (root resorption) | 0.01–54 |
| Farid et al. (2019) [42] | Diode laser (InGaAs) | 940 | Corticotomy | 5 |
| Direction/Module | Key Mediators/Receptors | Effect in OTM (Side) | PBM Overlap/Modulation | References |
|---|---|---|---|---|
| Osteoblast → Osteoclast | RANKL, M-CSF, WNT5A | ↑ Osteoclastogenesis (compression) | ↑ RANKL, ↑ M-CSF; accelerated OTM | [16,74,103,108,109,110,111,112] |
| Osteoblast → Osteoclast (inhibitory) | SEMA3A, on osteoblasts → NRP1, on osteoclasts) | ↓ RANKL-mediated osteoclastogenesis | PBM effect unclear | [105] |
| Osteoclast → Osteoblast | EFNB2 ↔ EPHB4; S1P, CTHRC1, C3; matrix-released TGF-β, IGF-1 | ↑ Osteoblast differentiation/activation (coupling; tension/reconstruction) | Indirectly supported via PBM effects on repair | [16,103,122] |
| PDLSC → Osteoclast | RANKL, PGE2 via EP4, IL-6 | ↑ Osteoclastogenesis (compression) | ↑ RANKL; PGE2 mixed (↑ in one RCT; ↓ in another) | [16,74,114,115] |
| PDLSC → Osteoblast/angiogenesis | BMP/Smad, SDF-1/CXCR4, VEGF | ↑ Osteogenic markers; ↑ vascular support (tension) | PBM ↑ BMP, ↑ SDF-1/CXCR4, ↑ VEGF; proliferation sometimes unchanged | [123,124,125,126,127] |
| ROsteocyte → Osteoclast/Osteoblast | RANKL, SOST, PGE2 | SOST: ↑ RANKL (pro-OC) + ↓ Wnt (anti-OB); PGE2: pro-OC (compression) | PBM ↓ SOST (pro-OB on tension); PGE2 heterogeneous | [74,113,114,115] |
| ECM degradation | MMP-1/8 (fibrillar collagens), MMP-2/9/13, cathepsin K; control by TIMP-1/2 | Compression: ↑ MMPs, transient ↓ TIMPs → ↑ MMP/TIMP → ↑ migration of osteoclasts/immune/endothelial cells | PBM often ↑ MMPs early; under tension, PBM may ↑ TIMPs (hypothesis: net collagen gain) | [111,116,117,118,119,128] |
| Adhesion/leukocyte entry | ICAM-1, VCAM-1 | ↑ Adhesion and transmigration of leukocytes into PDL (early, compression) | PBM may modulate via NO/VEGF; direct OTM- data limited | [120,121,122] |
| NO signaling | eNOS → NO | Vasodilation; modulation of osteoclasts and immune functions | PBM can ↑ eNOS/NO (mainly non-OTM models) | [120,121,122,131,132] |
| Angiogenesis | VEGF, HIF-1α, ERK, PI3K/Akt/mTOR | Neovascularization (tension); VEGF also ↑ MMPs (links ECM and vasculature) | PBM ↑ VEGF, ↑ HIF-1α/ERK; ↑ HUVEC proliferation/migration; ↑ vascularization in OTM model | [129,130,131,132,133,134,135] |
| M1/M2 | Target | Key Mediators | Dominant Paths in Target | Functional Effects | OTM Net Effect | References |
|---|---|---|---|---|---|---|
| M1 | Osteoblasts | TNF-α, IL-1β, IL-6, IFN-γ; NO/ROS; EV (miR-155) | ↑NF-κB, p38/JNK; ↓BMP/Smad; ↓Wnt/β-catn (↑DKK1/SOST) | ↓RUNX2/OSX, ↓ALP, ↓COL1A1/OCN, ↓mineralization; ↑RANKL, ↓OPG. PBM: early transient M1 priming; later shift to M2. | Inhibits bone formation; shifts toward resorption | [89,171,172]; PBM: [185,186,187,188] |
| M1 | Osteoclasts (via stroma) | TNF-α, IL-1β, PGE2; induces RANKL in osteoblasts/PDLSCs | ↑RANK–RANKL–NF-κB in precursors | ↑Differentiation and resorptive activity. PBM: may add to early RANKL-driven OC-genesis. | Boosts resorption (early compression side) | [172,177,191,192,193,194]; PBM: [186,187,188] |
| M1 | PDLSCs | TNF-α, IL-1β, IL-6; NO/ROS; EV (miR-155) | ↑NF-κB/p38; ↓BMP/Smad; ↓Wnt/β-catn | ↓Proliferation/migration; ↓osteogenesis; ↑RANKL/↓OPG. PBM: can down-tune sustained M1 signaling over time. | Pro-resorptive, anti-repair microenvironment | [180,182,194]; PBM: [186,187,188] |
| M1 | ECM/vasculature | TNF-α, IL-1β; ↑MMP-1/3/9; ↓TIMPs; early ↓VEGF | — | Matrix degradation > deposition; delayed angiogenesis. PBM: context-dependent MMP/TIMP–VEGF effects. | Fast breakdown; slower consolidation | [116,117,118,119,129,130,131,132,133,134,135,194,195] |
| M2 | Osteoblasts | IL-10, TGF-β, IGF-1, PDGF; pro-osteogenic EV/miRNA | ↓NF-κB; ↓MAPK; ↑BMP/Smad; ↑PI3K–Akt/ERK; ↑Wnt/β-catenin; ↑NFATc | ↑RUNX2/OSX, ↑ALP, ↑COL1A1/OCN, ↑mineralization; ↓RANKL, ↑OPG. PBM: supports M2 polarization. | Promotes bone formation (repair phase) | [89,171,182,183]; PBM: [186,187,188,189,190] |
| M2 | Osteoclasts (via stroma) | IL-10, TGF-β; ↑OPG/↓RANKL in stroma | ↓NF-κB; ↓MAPK; ↑NFATc; weakened RANK–RANKL signaling | ↓Osteoclastogenesis and activity. PBM: M2 shift restrains resorption. | Restrains resorption; favors balance | [172,182,183]; PBM: [186,187,188] |
| M2 | PDLSCs | IL-10, TGF-β, VEGF, PDGF; reparative EV/exosomes | ↓NF-κB; ↑BMP/Smad; ↑PI3K–Akt/ERK; ↑Wnt/β-catn. | ↑Proliferation/migration; ↑osteogenesis & mineralization; ↑OPG/↓RANKL. PBM: pro-repair setting (tension). | Pro-repair, pro-osteogenic setting (tension side) | [182]; PBM/angiogenesis: [123,124,125,126,127,129,130,131,132,133,134,135] |
| M2 | ECM/vasculature | TGF-β; early ↑MMP-2/9 then ↑TIMPs; ↑VEGF | — | Controlled early remodeling; later ↑collagen I; enhanced angiogenesis. PBM: ↑VEGF; rebalances MMP/TIMP. | Organized remodeling and stable consolidation | [129,130,131,132,133,134,135,194]; PBM angiogenesis: [129,130,131,132,133,134,135,188,189,190] |
| Cell Type | Site | Shared Signals (PBM+OTM) | Main Targets | Functional Effects (Combined) | Parameters | References |
|---|---|---|---|---|---|---|
| Th1 | Compress. | IFN-γ, TNF; stromal RANKL/OPG; NF-κB | Osteoclast precursors; PDL stroma | ↑RANKL/↓OPG → ↑osteoclasts; ↑ECM degradation (MMP-9↑/TIMP↓). PBM may act synergistically early via inflammatory cues; context-dependent bone effects via IFN-γ. | RANKL/OPG,TRAF6–NF-κB, MMP-9, TRAP; PBM: JAK2/STAT4/STAT5 | [2,184,185,186,187,188,189,200,201,202] |
| Th1 | Tension | IL-10 (resolution), pro-repair angiogenic (VEGF) | Osteoblast lineage; microvasculature | ↑Osteogenesis (↑RUNX2/ALP); ↑angiogenesis (VEGF↑); matrix deposition. | VEGF, RUNX2, ALP, OCN; PBM: JAK2/STAT4/STAT5 | [201,202] |
| Th17 | Compress. | IL-17A → RANKL/OPG; NF-κB | Osteoclast precursors; stroma | OTM: ↑RANKL/↓OPG → ↑osteoclasts; MMP-9↑/TIMP↓. PBM (general) may later temper excessive inflammation. | RANKL/OPG, NF-κB, MMP-9, TRAP | [203,204,205,206,207] |
| Th17 | Tension | Reduced Th17 tone; pro-repair VEGF | Osteoblasts; endothelium | ↑Osteogenesis (↑RUNX2/ALP); ↑angiogenesis (VEGF↑). | VEGF, RUNX2, ALP | [203,205] |
| Th2 | Compress. | IL-10; lowered NF-κB; tempered RANKL/OPG drive | Stroma; osteoclast lineage | ↓RANKL signaling; restrained osteoclasts; MMP-9↓/TIMP↑. | IL-10, NF-κB, MMP-9, TRAP | [208,209] |
| Th2 | Tension | IL-10, VEGF | Osteoblasts; vessels | ↑Osteogenesis (↑RUNX2/ALP); ↑angiogenesis (VEGF↑); organized ECM. | VEGF, RUNX2, ALP | [208,209] |
| Tregs | Compress. | IL-10, TGF-β; NF-κB suppression | Osteoclast precursors; stroma; macrophages | ↓RANKL/↑OPG; restrain osteoclasts; limit MMPs; facilitate resolution. | IL-10, RANKL/OPG, NF-κB, TRAP↓ | [222,223,224,225,226] |
| Tregs | Tension | IL-10; repair cues incl. VEGF | Osteoblasts; endothelium | ↑Osteogenesis (↑RUNX2/ALP); ↑angiogenesis (VEGF↑). PBM likely promotes Treg bias in later OTM stages. | VEGF, RUNX2, ALP | [222,223,224,225,226,230,231] |
| CD8+ | Compress. | IFN-γ, RANKL/OPG, NF-κB | Osteoclast precursors; stroma | ↑RANKL/↓OPG → ↑osteoclasts; MMP-9↑/TIMP↓; OIIRR risk. FoxP3+ CD8+ can counter via IFN-γ/CTLA-4. | IFN-γ, RANKL/OPG, NF-κB, MMP-9 | [210,211] |
| CD8+ | Tension | Regulatory skew (IL-10), pro-repair VEGF | Osteoblasts; vessels | ↑Osteogenesis (↑RUNX2/ALP); ↑angiogenesis (VEGF↑). | VEGF, RUNX2, ALP | [210,211] |
| B cells | Compress. | Activated B → RANKL; Bregs → OPG, IL-10; NF-κB balance | Osteoclast precursors; stroma | Context-dependent: ↑RANKL (activation) vs. ↑OPG/IL-10 (restraint); MMP-9 tempered with Breg bias. | RANKL/OPG, IL-10, MMP-9, TRAP | [212,213,214,215] |
| B cells | Tension | IL-10/OPG support; VEGF | Osteoblasts; endothelium | Matrix deposition; ↑angiogenesis (VEGF↑); consolidation. | OPG, VEGF, RUNX2, ALP | [214,215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, J.; Čolić, M. Photobiomodulation Meets Mechanotransduction: Immune-Stromal Crosstalk in Orthodontic Remodeling. Biomedicines 2025, 13, 2495. https://doi.org/10.3390/biomedicines13102495
Marković J, Čolić M. Photobiomodulation Meets Mechanotransduction: Immune-Stromal Crosstalk in Orthodontic Remodeling. Biomedicines. 2025; 13(10):2495. https://doi.org/10.3390/biomedicines13102495
Chicago/Turabian StyleMarković, Jovan, and Miodrag Čolić. 2025. "Photobiomodulation Meets Mechanotransduction: Immune-Stromal Crosstalk in Orthodontic Remodeling" Biomedicines 13, no. 10: 2495. https://doi.org/10.3390/biomedicines13102495
APA StyleMarković, J., & Čolić, M. (2025). Photobiomodulation Meets Mechanotransduction: Immune-Stromal Crosstalk in Orthodontic Remodeling. Biomedicines, 13(10), 2495. https://doi.org/10.3390/biomedicines13102495





