Thymopentin Enhances Antitumor Immunity Through Thymic Rejuvenation and T Cell Functional Reprogramming
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Primary T Cell Isolation and Cell Culture
2.3. Experimental Animal Models and Therapeutic Interventions
2.3.1. Tumor Models
2.3.2. Cyclophosphamide-Induced Immunosuppressive Model
2.3.3. Pharmacological Treatments
2.3.4. Adoptive T Cell Therapy and Cytokine Detection by ELISA
2.4. Flow Cytometry Analysis
2.5. Cell Viability Assay
2.6. Statistical Analysis
3. Results
3.1. TP5 Demonstrates Broad-Spectrum Antitumor Efficacy Across Multiple Cancer Models
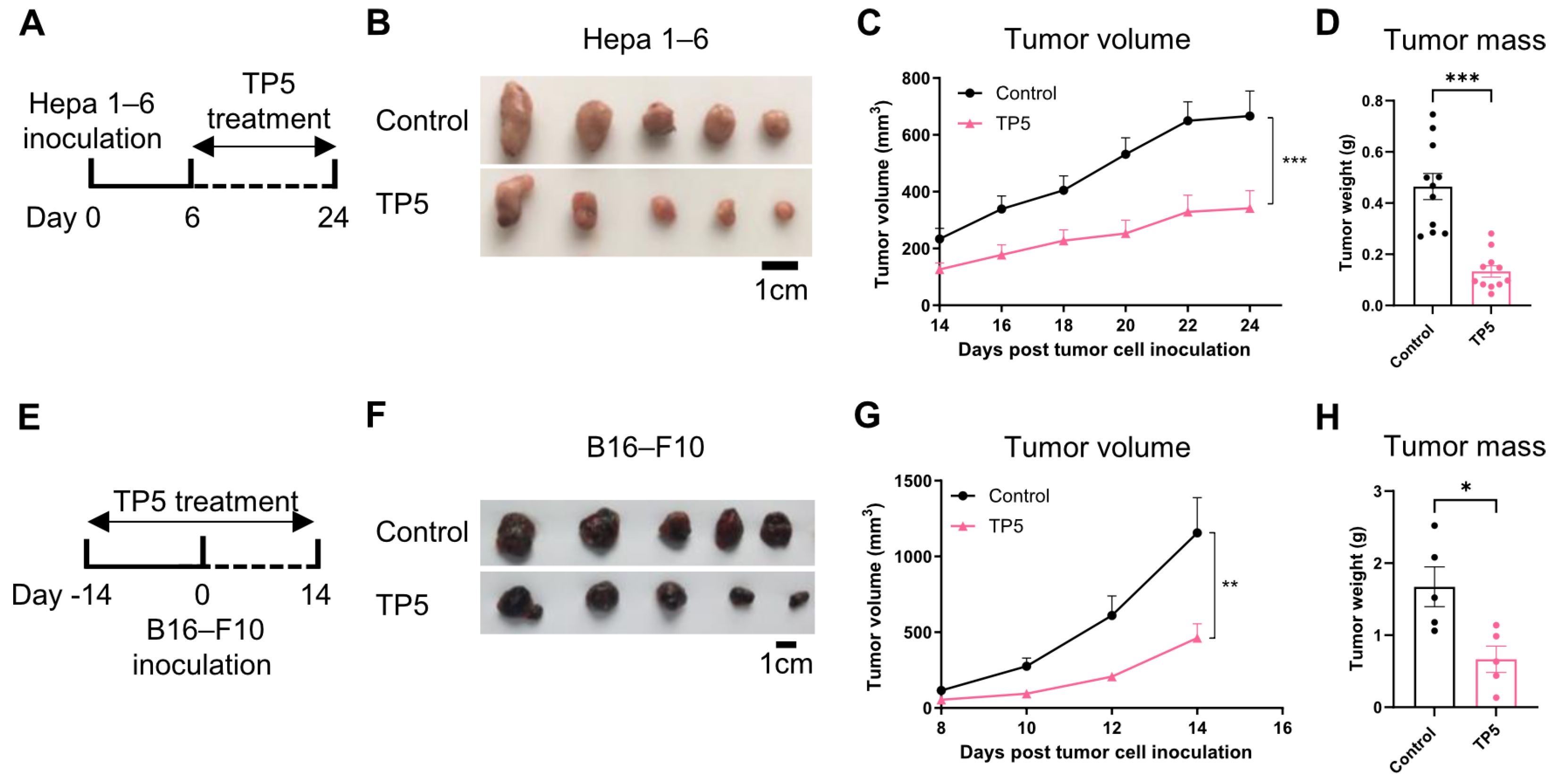
3.2. The Antitumor Activity of TP5 Relies on T Cell-Mediated Immunity
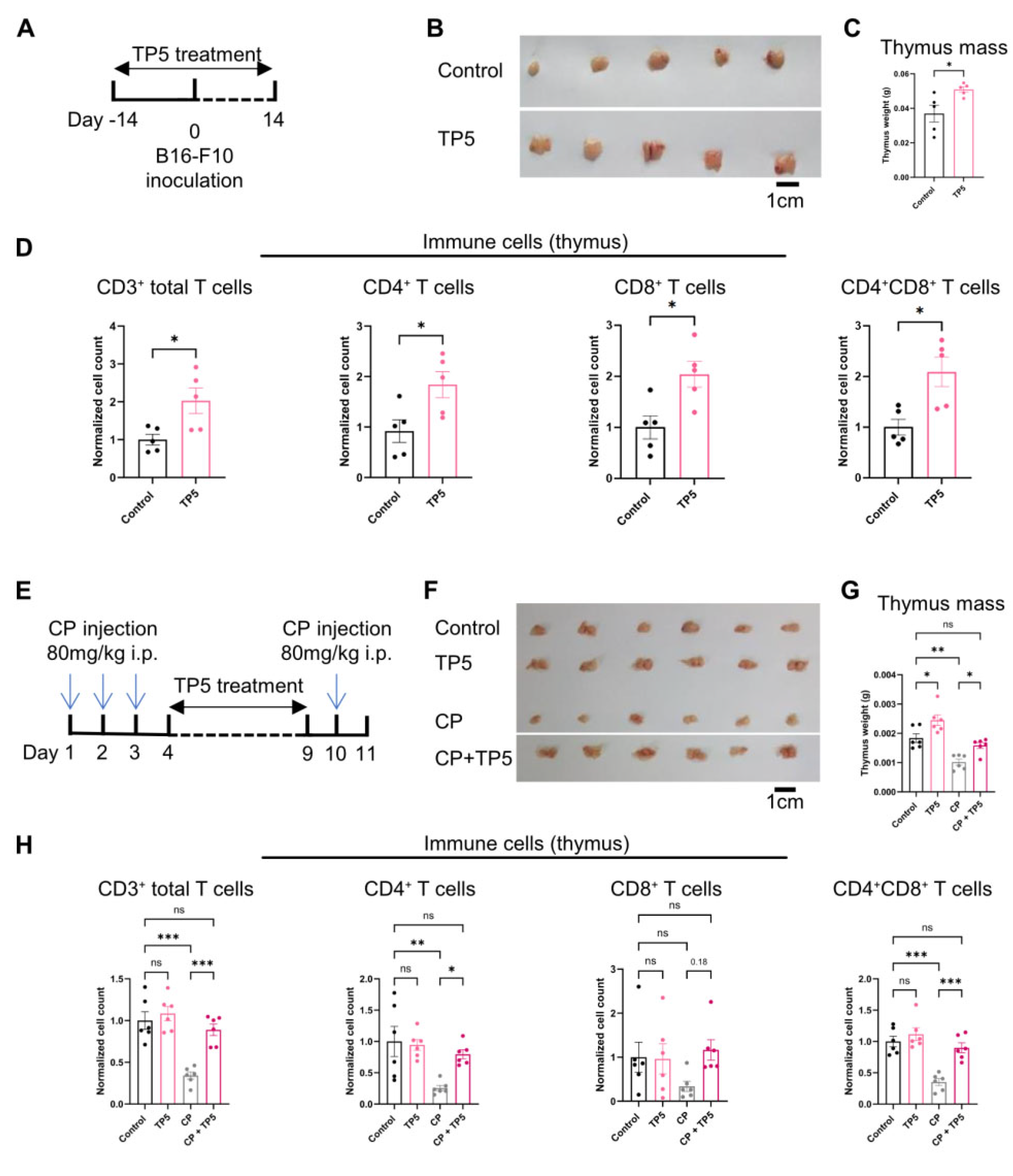
3.3. TP5 Promotes Thymus Rejuvenation and Counteracts Cancer-Associated Immunosuppression
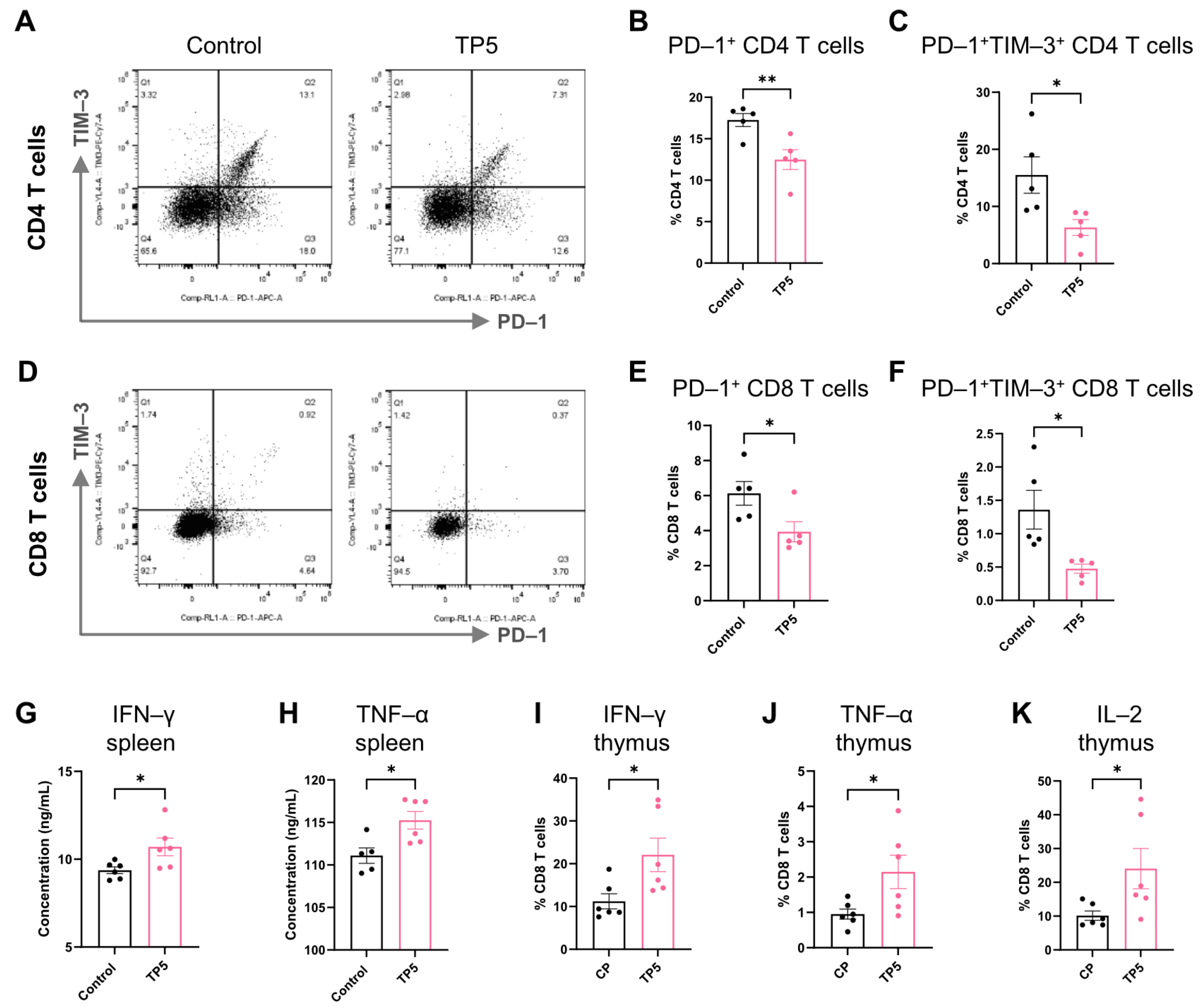
3.4. TP5 Protects Against T Cell Exhaustion and Restores Effector Function
3.5. TP5 Synergistically Enhances the Efficacy of Adoptive T Cell Therapy
4. Discussion
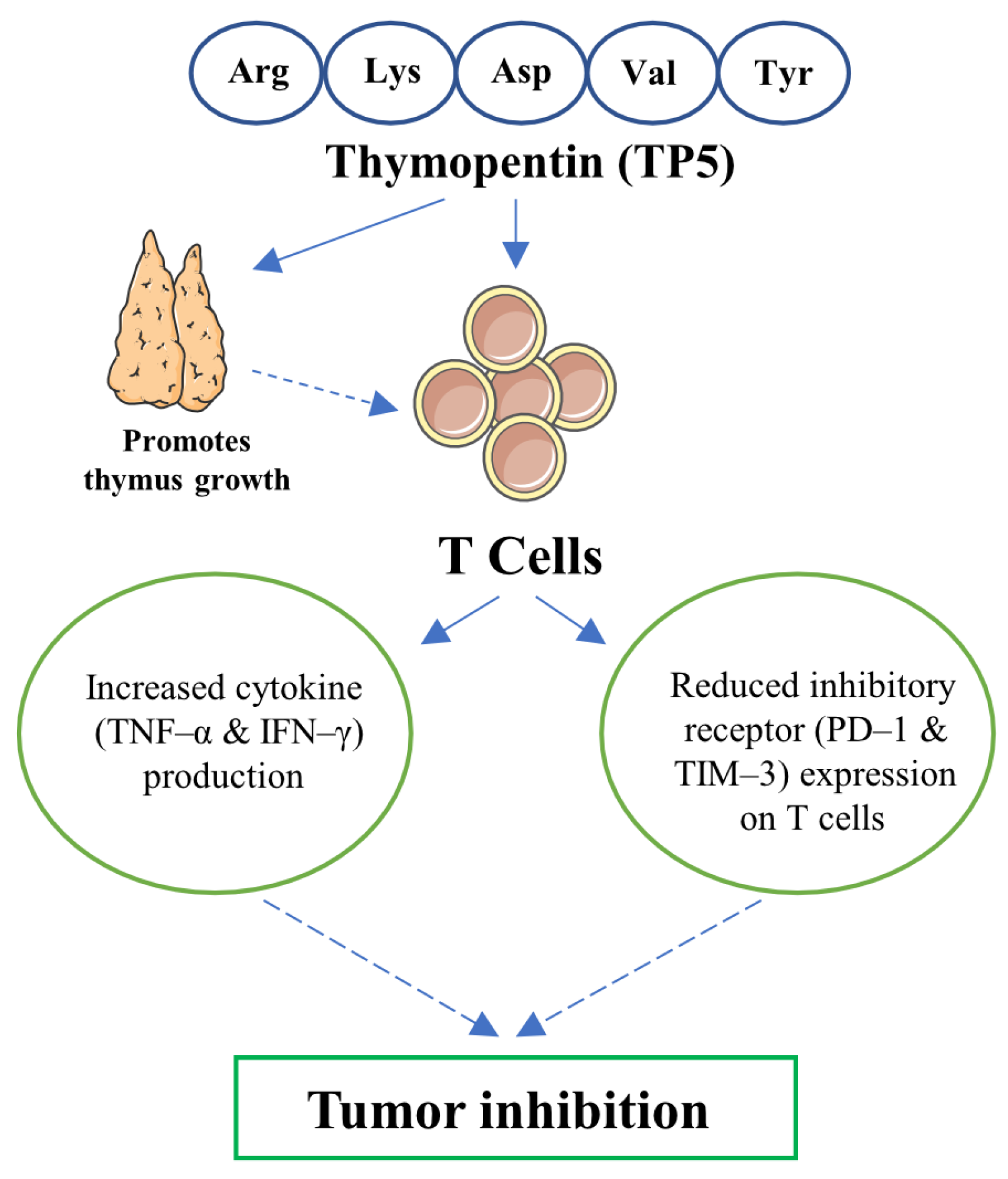
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TP5 | Thymopentin |
| ELISA | Directory of open access journals |
| TME | Tumor microenvironment |
| IL-2 | Interleukin 2 |
| IFN-γ | Interferon gamma |
| TNF-α | Tumor necrosis factor alpha |
| TILs | Tumor-infiltrating lymphocytes |
| SRB | Sulforhodamine-B |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824. [Google Scholar] [CrossRef]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef]
- Hossain, M.A.; Liu, G.; Dai, B.; Si, Y.; Yang, Q.; Wazir, J.; Birnbaumer, L.; Yang, Y. Reinvigorating exhausted CD8+ cytotoxic T lymphocytes in the tumor microenvironment and current strategies in cancer immunotherapy. Med. Res. Rev. 2021, 41, 156–201. [Google Scholar] [CrossRef]
- Goldstein, G.; Audhya, T.K. Thymopoietin to thymopentin: Experimental studies. Surv. Immunol. Res. 1985, 4 (Suppl. S1), 1–10. [Google Scholar] [CrossRef]
- Goldstein, G.; Scheid, M.P.; Boyse, E.A.; Schlesinger, D.H.; Van Wauwe, J. A synthetic pentapeptide with biological activity characteristic of the thymic hormone thymopoietin. Science 1979, 204, 1309–1310. [Google Scholar] [CrossRef]
- Mascart-Lemone, F.; Huygen, K.; Clumeck, N.; Brenez, D.; Bolla, K.; Duchateau, J. Stimulation of cellular function by thymopentin (TP-5) in three AIDS patients. Lancet 1983, 2, 735–736. [Google Scholar] [CrossRef]
- Sundal, E.; Bertelletti, D. Thymopentin treatment of rheumatoid arthritis. Arzneim.-Forsch. 1994, 44, 1145–1149. [Google Scholar] [PubMed]
- Ranges, G.E.; Goldstein, G.; Boyse, E.A.; Schield, M.P. T cell development in normal and thymopentin-treated nude mice. J. Exp. Med. 1982, 156, 1057–1064. [Google Scholar] [CrossRef]
- Zhu, M.X.; Wan, W.L.; Li, H.S.; Wang, J.; Chen, G.A.; Ke, X.Y. Thymopentin enhances the generation of T-cell lineage derived from human embryonic stem cells in vitro. Exp. Cell Res. 2015, 331, 387–398. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, X.; Lin, Y.; Yue, C.; Wang, Y.; Quan, F.; Zhang, Z.; Liu, X.; Lu, Y.; Zhan, Y.; et al. Thymopentin ameliorates dextran sulfate sodium-induced colitis by triggering the production of IL-22 in both innate and adaptive lymphocytes. Theranostics 2019, 9, 7490–7505. [Google Scholar] [CrossRef]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef]
- Wang, W.; Zou, W. Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy. Mol. Cell 2020, 80, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Xu, Y.; Youn, J.I.; Cabrera, R.; Zhang, X.; Gabrilovich, D.; Nelson, D.R.; Liu, C. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab. Investig. 2011, 91, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.G.; Hutson, A.; Barbi, J.; Iyer, R.; Thanavala, Y. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight 2019, 4, e130116. [Google Scholar] [CrossRef]
- Majumdar, S.; Nandi, D. Thymic Atrophy: Experimental Studies and Therapeutic Interventions. Scand. J. Immunol. 2018, 87, 4–14. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kamphorst, A.O.; Im, S.J.; Kissick, H.T.; Pillai, R.N.; Ramalingam, S.S.; Araki, K.; Ahmed, R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 2018, 69, 301–318. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, R.; Wu, R.; Petitte, J.N.; Hou, Y.; Si, D.; Ahmad, B.; Guo, H.; Zhang, M.; et al. Targeting the TLR2 Receptor with a Novel Thymopentin-Derived Peptide Modulates Immune Responses. Front. Immunol. 2021, 12, 620494. [Google Scholar] [CrossRef]
- Fletcher, M.; Ramirez, M.E.; Sierra, R.A.; Raber, P.; Thevenot, P.; Al-Khami, A.A.; Sanchez-Pino, D.; Hernandez, C.; Wyczechowska, D.D.; Ochoa, A.C.; et al. l-Arginine depletion blunts antitumor T-cell responses by inducing myeloid-derived suppressor cells. Cancer Res. 2015, 75, 275–283. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e813. [Google Scholar] [CrossRef]
- Duchateau, J.; Servais, G.; Cooman, R.; Collet, H.; Bolla, K. In vitro influence of thymopentin on proliferative responses and phytohemagglutinin-induced interleukin 2 production in normal human lymphocyte cultures. Surv. Immunol. Res. 1985, 4 (Suppl. S1), 116–124. [Google Scholar] [CrossRef]
- Cillari, E.; Milano, S.; Dieli, M.; Arcoleo, F.; Perego, R.; Leoni, F.; Gromo, G.; Severn, A.; Liew, F.Y. Thymopentin reduces the susceptibility of aged mice to cutaneous leishmaniasis by modulating CD4 T-cell subsets. Immunology 1992, 76, 362–366. [Google Scholar] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, S.; Wang, S.; Zhang, Z.; Wang, X.; Chen, Z.; Wang, X.; Huang, S.; Zhang, D.; Wu, H. Tertiary lymphoid structures in diseases: Immune mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2024, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Percival, A.; Ganss, R. Therapeutic Induction of Tertiary Lymphoid Structures in Cancer Through Stromal Remodeling. Front. Immunol. 2021, 12, 674375. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, P.; Fu, J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett. 2007, 258, 31–37. [Google Scholar] [CrossRef]
- Chin, S.S.; Guillen, E.; Chorro, L.; Achar, S.; Ng, K.; Oberle, S.; Alfei, F.; Zehn, D.; Altan-Bonnet, G.; Delahaye, F.; et al. T cell receptor and IL-2 signaling strength control memory CD8+ T cell functional fitness via chromatin remodeling. Nat. Commun. 2022, 13, 2240. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Wherry, E.J.; Blattman, J.N.; Murali-Krishna, K.; van der Most, R.; Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003, 77, 4911–4927. [Google Scholar] [CrossRef]
- Luis, T.C.; Luc, S.; Mizukami, T.; Boukarabila, H.; Thongjuea, S.; Woll, P.S.; Azzoni, E.; Giustacchini, A.; Lutteropp, M.; Bouriez-Jones, T.; et al. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nat. Immunol. 2016, 17, 1424–1435. [Google Scholar] [CrossRef]
- Bhandoola, A.; von Boehmer, H.; Petrie, H.T.; Zuniga-Pflucker, J.C. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: Plenty to choose from. Immunity 2007, 26, 678–689. [Google Scholar] [CrossRef]
- Cepeda, S.; Griffith, A.V. Thymic stromal cells: Roles in atrophy and age-associated dysfunction of the thymus. Exp. Gerontol. 2018, 105, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Savino, W. The thymus is a common target organ in infectious diseases. PLoS Pathog. 2006, 2, e62. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Wang, H.; Amin, N.; Jung, J.; Appay, R.; Cui, J.; Song, Q.; Cardone, A.; Park, D.M.; Gilbert, M.R.; et al. TP5, a Peptide Inhibitor of Aberrant and Hyperactive CDK5/p25: A Novel Therapeutic Approach against Glioblastoma. Cancers 2020, 12, 1935. [Google Scholar] [CrossRef]
- He, W.; Jiang, X.; Zhang, Z.R. Preparation and evaluation of poly-butylcyanoacrylate nanoparticles for oral delivery of thymopentin. J. Pharm. Sci. 2008, 97, 2250–2259. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, W.; Wu, C.; Pan, Z.; Yao, G.; Fang, L.; Su, W. Myristic acid-modified thymopentin for enhanced plasma stability and immune-modulating activity. Int. Immunopharmacol. 2017, 47, 88–94. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.Y.; Cao, X.; Li, W.H.; Gong, T.; Zhang, Z.R. Thymopentin-loaded phospholipid-based phase separation gel with long-lasting immunomodulatory effects: In vitro and in vivo studies. Acta Pharmacol. Sin. 2019, 40, 514–521. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.A.; Zhang, Y.; Ji, L.; Chen, Y.; Luan, Y.; Si, Y.; Fang, Y.; Qiu, J.; Wang, Z.; Liu, G. Thymopentin Enhances Antitumor Immunity Through Thymic Rejuvenation and T Cell Functional Reprogramming. Biomedicines 2025, 13, 2494. https://doi.org/10.3390/biomedicines13102494
Hossain MA, Zhang Y, Ji L, Chen Y, Luan Y, Si Y, Fang Y, Qiu J, Wang Z, Liu G. Thymopentin Enhances Antitumor Immunity Through Thymic Rejuvenation and T Cell Functional Reprogramming. Biomedicines. 2025; 13(10):2494. https://doi.org/10.3390/biomedicines13102494
Chicago/Turabian StyleHossain, Md Amir, Ye Zhang, Li Ji, Yumei Chen, Yue Luan, Yaxuan Si, Yuqing Fang, Junlan Qiu, Zhuo Wang, and Guilai Liu. 2025. "Thymopentin Enhances Antitumor Immunity Through Thymic Rejuvenation and T Cell Functional Reprogramming" Biomedicines 13, no. 10: 2494. https://doi.org/10.3390/biomedicines13102494
APA StyleHossain, M. A., Zhang, Y., Ji, L., Chen, Y., Luan, Y., Si, Y., Fang, Y., Qiu, J., Wang, Z., & Liu, G. (2025). Thymopentin Enhances Antitumor Immunity Through Thymic Rejuvenation and T Cell Functional Reprogramming. Biomedicines, 13(10), 2494. https://doi.org/10.3390/biomedicines13102494





