1. Introduction
Globally, an estimated 50 million people are living with hepatitis C virus (HCV), with approximately one million new infections per year [
1]. Among infected people, over half will remain chronically infected unless treated with antiviral medication. Cirrhosis develops in approximately 10–20% of people after 20–30 years of chronic infection, and progression is often clinically silent; evidence of liver disease might not occur until late in the course of the disease [
2]. In Australia, hepatitis C is considered a significant public health issue, and in response to this, unrestricted access to direct acting antiviral (DAA) therapy through public health subsidy was introduced in 2016 with a goal to eliminate hepatitis C as a public health threat in Australia by 2030. At the end of 2022, an estimated 60% of all people living with hepatitis C between 2016 and 2022 had been treated, although this excluded pregnant and lactating women [
3].
Pregnancy is a time when women are uniquely engaged with the healthcare system and are often motivated to participate in activities directed toward improvement of their own health and ensuring the health of their unborn child. Women are recommended to attend at least eight antenatal care appointments during pregnancy [
4], which provides a unique opportunity for healthcare interventions such as treatment for HCV. In this setting, and with global calls for the elimination of HCV infection, it is time to accelerate research and consider how DAAs can be integrated into the existing healthcare infrastructure of antenatal care.
Two key studies have recently shifted the paradigm for HCV treatment during pregnancy. These include the Hepatitis In Pregnancy (HIP)-1 and -2 studies. HIP-1 examined the pharmacokinetics of ledipasvir/sofosbuvir during pregnancy and found no clinically significant changes in drug exposures [
5]. Treatment was started between 23 and 24 weeks’ gestation and was safe and effective with a 100% cure rate among nine women [
5]. However, half of screen failures in this study were genotype 2/3 highlighting the need to evaluate a pan-genotypic regimen.
HIP-2 then enrolled women at a similar gestational age to examine sofosbuvir/velpatasvir (SOF/VEL) pharmacokinetics [
6]. SOF/VEL is an orally administered, well-tolerated DAA regimen with pangenotypic activity. In published trials, a 12-week treatment course of SOF/VEL resulted in cure rates between 97 and 100% when given as a once-a-day oral pill [
7]. Results on ten participants reported SOF/VEL exposures that were not clinically different in pregnancy [
6]. VEL area under the curve (AUC) and SOF maximum concentration (Cmax) were similar to historical data in non-pregnant women. However, SOF area under the curve (AUC) was 38% higher while the AUC and Cmax of its major plasma metabolite, GS-331007, were 38% and 43% lower (similar to HIP-1), respectively. Despite these differences, all women with SVR12 data (n = 9) were cured, and no HCV infant transmission occurred (n = 8) [
6].
Pregnancy is a time of significant physiological changes [
8]. These changes can affect drug absorption, distribution, metabolism, and excretion, leading to changes in pharmacokinetics (PK). Understanding the need for dose adjustments due to these pregnancy-induced PK changes is a critical first step to ensure safe and effective administration of drugs to the mother. Here, we evaluate the PKs, safety, and efficacy of antenatal SOF/VEL treatment administered for 12 weeks during the second and third trimester in a population outside of the United States.
2. Materials and Methods
2.1. Study Design
This was a multi-site, prospective, open-label, collaborative PK study conducted at two large maternity hospitals in Melbourne, Australia. Monash Health and Sunshine Hospital are two hospitals averaging 12,000 and 6000 deliveries per year, respectively (Australian New Zealand Clinical Trial Registry 126619000054112). This study was approved by Monash Health Human Research Ethics Committee. All participants provided informed consent prior to participation.
2.2. Screening
As part of routine antenatal care, all pregnant women underwent a clinical review including medical history, drug and alcohol use, smoking status, and review of the laboratory investigations undertaken as per their standard medical care during pregnancy including HIV antibody testing, hepatitis B surface antigen, electrolytes, full blood examination, urinalysis, liver function tests, oral glucose tolerance test, and syphilis testing. Screening of routine booking lists identified those women who were hepatitis C antibody-positive, and these women were approached by the research team to confirm eligibility and consider participation in this study. All enrolled participants completed a baseline survey exploring their attitude to hepatitis C infection in pregnancy and to treatment for maternal and/or fetal benefit.
Treatment with SOF/VEL (sofosbuvir 400 mg/velpatasvir 100 mg (Gilead Sciences, Foster City, CA, USA) once daily) was initiated between 22 and 24 + 6 weeks’ gestation followed by three PK visits throughout the treatment course. Maternal HCV ribonucleic acid (RNA) was collected at the end of treatment and 12 weeks post treatment completion. Infants were followed through 12 months after birth.
2.3. Study Population
Pregnant women with HCV infection (any HCV subtype) between 18 and 45 years of age with a normal 18–22-week anomaly scan were eligible for the study. Women were excluded if they had multiple pregnancy; any abnormality on routine 18–22-week anomaly scan; negative HCV nucleic acid test; previous treatment for HCV with an NS5A inhibitor; any medications contraindicated with SOF/VEL; use of gastric acid modifiers in doses that exceeded the equivalent of 20 mg omeprazole daily or famotidine 40 mg twice daily; evidence of cirrhosis; any significant uncontrolled active or chronic cardiovascular, renal, or liver disease, or haematological, neurological, immunological or infectious disease (other than HCV); high risk of pre-term birth; co-infection with HIV-1; evidence of HBV infection (hepatitis B surface antigen (HBsAg)-positive); or severe intercurrent illness.
Participants were allowed to take proton pump inhibitors and other gastric acid modifiers during the study period if medically necessary but were recommended to not take these medications during the PK sampling visits.
2.4. PK Assessments
Women underwent PK sampling at 25 + 0 to 26 + 6 weeks’ (Visit 1), 29 + 0 to 31 + 6 weeks’ (Visit 2), and 33 + 0 to 34 + 6 weeks’ gestation (Visit 3). These visits were performed following an 8 h overnight fast (nothing but water). However, in the event food or beverages other than water had been consumed in the 8 h preceding study drug administration on the day of the intensive PK visit, the type and amount of food and beverage was recorded. The timing of the last SOF/VEL dose was also carefully recorded.
A pre-dose blood sample was obtained, followed by a standardised breakfast, observed dosing of SOF/VEL, and blood samples collected at 0.5, 1, 1.5, 2, 4, 5, and 24 h post dose. Participants had the option to participate in a longer, more intensive PK visit schedule which required collection of additional samples at 6 and 8 h post dose. For the first four hours post study drug administration, participants were asked to record all foods and beverages consumed other than water, but there were no restrictions after this time frame.
2.5. Analytical Methods and PK Analysis
PK blood samples were processed for plasma and analysed using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method to quantify SOF, GS-331007, and VEL [
6]. The linear range for each analyte ranged from 5.00 ng/mL to 1500 ng/mL, and each analyte had a lower limit of quantitation (LLOQ) of 5.00 ng/mL.
Plasma concentrations of SOF, GS-331007, and VEL were analysed by noncompartmental analysis (Phoenix Certara, version 8.4). The following PK parameters are reported for each analyte, unless indicated otherwise: maximum plasma concentration (Cmax), the predicted plasma concentration at 24 h post dose (Ctau), area under the curve over the 24 h dosing interval (AUCtau) or through the last measurable time point (AUClast), half-life (t1/2), apparent volume of distribution (V/F), and apparent oral clearance (CL/F).
AUCtau and AUClast were calculated using linear up-log down trapezoidal rule. Concentrations below the limit of quantitation (BLQ) were set to 0 when they occurred before Cmax, and when they occurred after Cmax, the first observation was set to half the LLOQ and then remaining time points were treated as missing. Cmax was based on direct observation of the data. Ctau was based on the measured concentration for samples collected exactly at 24 h post dose or predicted concentrations at 24 h based on partial AUC estimates. The elimination half-life was calculated as 0.693 divided by the elimination rate constant. V/F was calculated as CL/F divided by the elimination rate constant, and CL/F was calculated as the dose of SOF (400 mg) or VEL (100 mg) divided by the AUC.
2.6. Efficacy and Safety Assessment
Maternal HCV RNA was measured at the end of treatment and at 12 weeks after the end of treatment to evaluate the efficacy of SOF/VEL. HCV cure was defined as sustained viral response at 12 weeks after completion of therapy (SVR12). Neonatal HCV infection was evaluated by an HCV RNA test at ≥6 months of age. To assess the safety of SOF/VEL use during pregnancy, adverse obstetric events occurring during treatment and any major neonatal malformations were recorded.
2.7. Acceptability Assessment
Participants completed an investigator-designed questionnaire to determine their attitude to and acceptability of SOF/VEL treatment during pregnancy.
2.8. Statistical Analysis
Baseline demographics, PK, safety, and efficacy outcomes were summarised using descriptive statistics. The primary outcome was to determine if the PKs of SOF and VEL were similar in pregnancy compared to those in non-pregnant women. Linear mixed effects modelling was performed to determine if AUCtau, Cmax, and Ctau differed across PK visit for each analyte, and to allow for a single geometric mean to be calculated across the repeated measures within participants (R Version 4.4.1). AUCtau, Cmax, and Ctau during pregnancy were then compared to historical data in non-pregnant women to generate geometric mean ratios with 90% confidence intervals (CIs).
4. Discussion
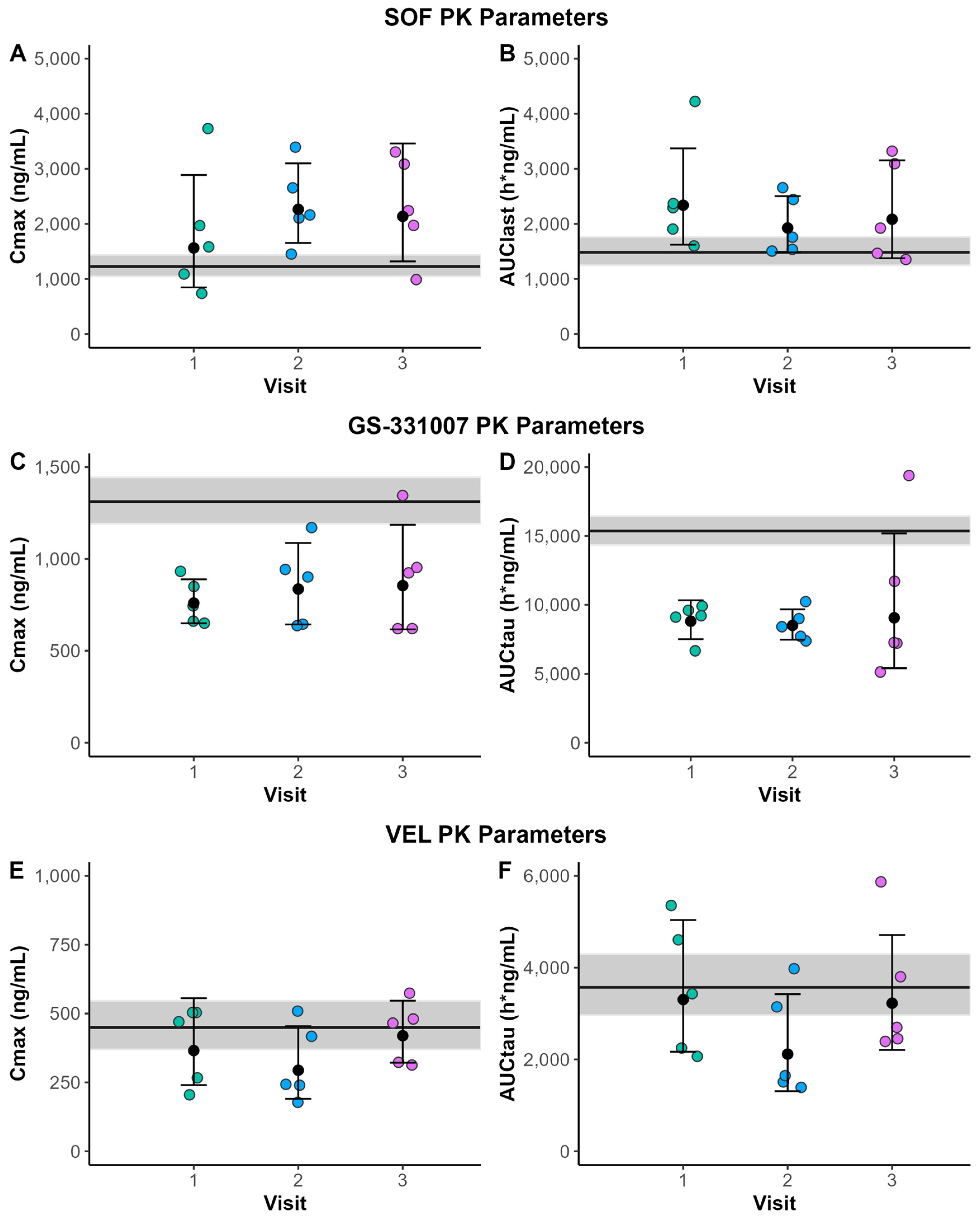
In the present study, SOF AUClast and Cmax were higher, whereas GS-331007 AUCtau and Cmax estimates were lower in pregnancy. VEL AUCtau and Cmax were comparable to non-pregnant women, whereas Ctau was slightly reduced. Despite these differences, 4/5 participants achieved SVR12 (with one participant achieving an end of treatment negative PCR but did not attend for follow-up testing at 12 weeks post completion of treatment). SOF/VEL was generally well tolerated, with no significant side effects reported.
The PK results in this study largely mirrored the previously reported results of SOF/VEL use in pregnancy from Chappell et al. (2024) [
6]. The changes in exposure to SOF (~40% increase) and GS-331007 (~40% decrease) in the present study were comparable to previous reports [
6]. In both the present study and previous results, VEL AUC
tau and Cmax were similar between pregnant and non-pregnant women, but we identified lower C
tau values.
Numerous physiological changes occur in pregnancy that can affect drug PK [
8]. Increases in gastric pH (becomes less acidic) during pregnancy may contribute to the 20% reduction in systemic exposure to VEL since the solubility of VEL decreases with increasing pH [
9]. None of the participants were prescribed gastric acid modifying medications. Furthermore, the slightly reduced VEL exposure in this study could also be due to increases in CYP3A4 activity during pregnancy, which is a minor metabolising enzyme of VEL [
9,
10]. CYP2B6 and CYP2C8 also play a minor role in VEL metabolism; however, changes in these enzymes during pregnancy less characterised.
Increased SOF exposure in pregnancy may be due to multiple pregnancy-related physiological changes. These include delayed gastric emptying, reduced intestinal motility, and increased intestinal blood flow [
7], all of which may increase oral absorption of SOF. Decreased expression of carboxylesterase 1 (CES1) is also possible, but there are conflicting reports on CES1 expression and activity in the presence of pregnancy-related hormones. Wu et al. [
11] found that 17β-estradiol reduced both expression and activity of CES1 in human and mouse hepatocytes, while Fashe et al. [
12] reported increased CES1 protein concentrations in human hepatocytes in the presence of pregnancy-related hormones. A separate clinical PK study, however, identified no differences in the PK of oseltamivir, another CES1 substrate, during pregnancy, but the renally eliminated carboxylate metabolite was significantly lower [
13]. Further studies are needed to clarify CES1 changes in pregnancy. GS-331007 is a metabolite of sofosbuvir and is predominantly eliminated by glomerular filtration and tubular secretion [
9,
10]. The lower systemic exposure to GS-331007 in pregnancy in our study may be explained by increases in glomerular filtration rates.
Whilst PK changes were observed for SOF/VEL in this study, all five pregnant participants achieved either an end of treatment or 12-week post treatment negative HCV nucleic test, supporting the use of this regimen in pregnancy without dose adjustment. Our data are reassuring that across different settings and populations, similar PK results are found. Furthermore, the medication was well tolerated in pregnancy, as has been reported in pivotal phase three trials outside of pregnancy [
13]. In terms of safety in pregnancy, this small study is inadequately powered to draw any conclusions, but no safety concerns were apparent.
There are several limitations of this study. Firstly, the small numbers recruited were fewer than originally planned. Recruitment was impacted by the COVID-19 pandemic as both antenatal sites converted to predominantly telehealth appointments rather than face to face. In addition, three of the five women did not attend arranged paediatric follow-up despite multiple attempts at contact and rescheduling of appointments. In relation to the PK analysis, a further limitation was that the AUCtau values tended to be higher in participants without the additional samples.
These results add to the limited published experience of prescribing antivirals in pregnancy and provide further support for a larger ongoing prospective study [
14] and other efforts to support HCV treatment in pregnancy [
15]. SOF/VEL was safe and well tolerated. For two of the participants, the HCV was newly diagnosed based on antenatal screening. This highlights the unique opportunity in pregnancy where women are routinely tested and may be identified at an early stage in the disease prior to liver damage occurring. This may also be an opportune time for treatment given pregnant women are generally more engaged in their healthcare during pregnancy. When surveyed about motivations to participate in this study, most were driven by a desire to be cured and to protect the baby. This highlights additional drivers that may be leveraged in pregnancy to support adherence and completion of treatment.