Unlocking the Secrets of the Endometrium: Stem Cells, Niches and Modern Methodologies
Abstract
1. Introduction
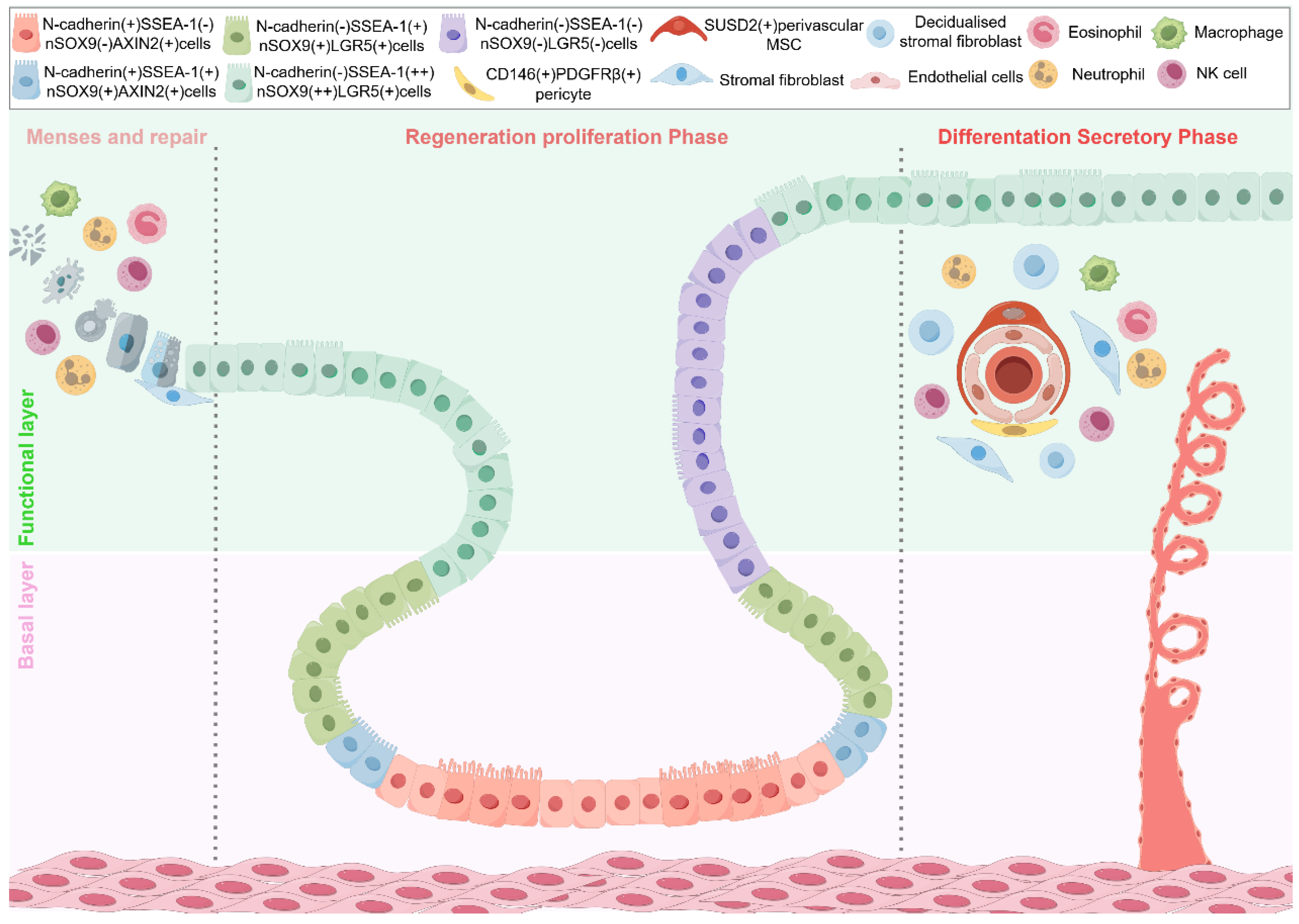
2. Endometrial Stem/Progenitor Cells in Endometrial Repair and Regeneration
2.1. Dynamic Changes of Epithelial, Stromal, and Immune Cells in Normal Endometrium and Related Diseases
2.2. Endometrial Epithelial Stem Cells
2.2.1. SSEA-1
2.2.2. N-Cadherin
2.2.3. AXIN2
2.2.4. SOX9 (nSOX9)
2.2.5. LGR5
2.2.6. “Junctional Zone” Stem Cell
2.2.7. The Hierarchical Structure of Endometrial Epithelial Stem Cells
2.3. Endometrial Mesenchymal Stem Cells (eMSCs)
2.3.1. SUSD2
2.3.2. PDGFRα
2.3.3. Perivascular eMSCs
2.3.4. Mesenchymal–Epithelial Transition (MET)
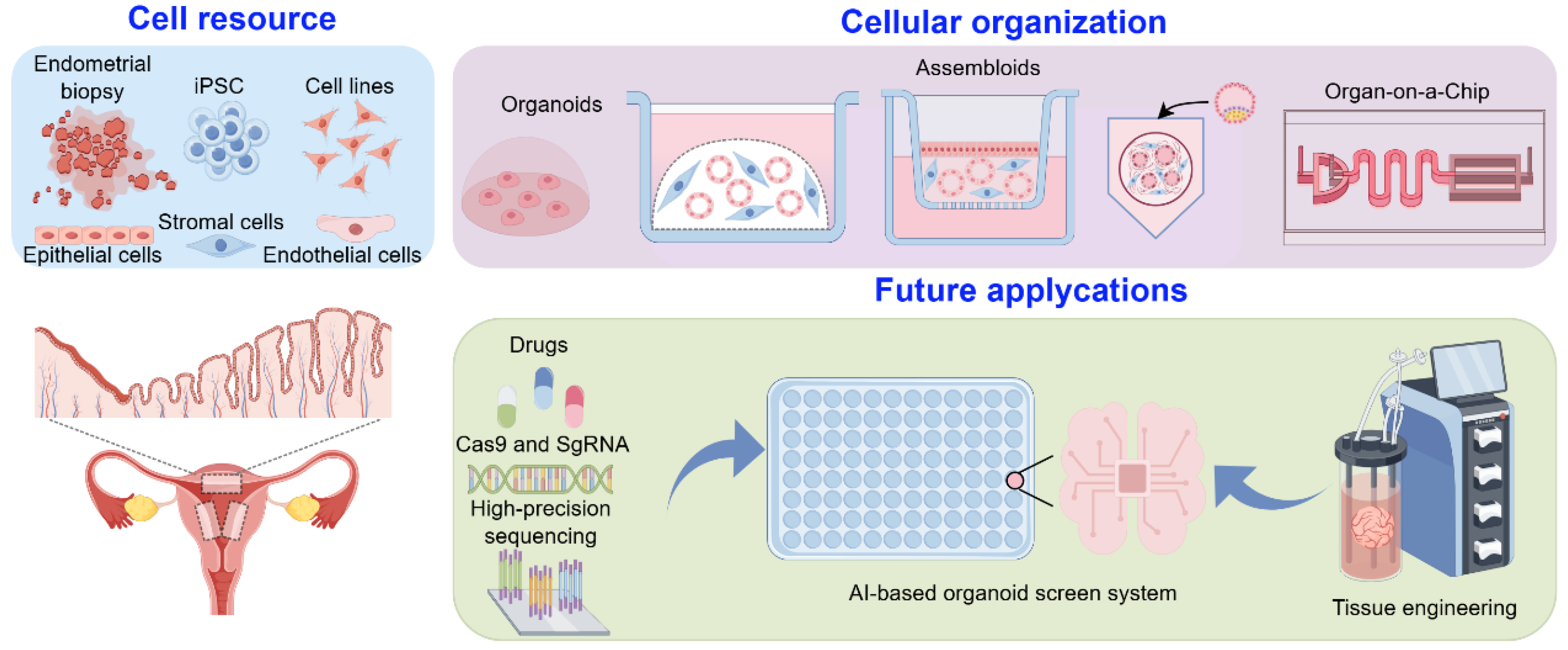
3. Modeling Endometrial Dynamics and Functionality In Vitro
3.1. Organoids
3.2. Assembloids
3.3. Microfluidic Systems (Organ-on-a-Chip)
4. Discussions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef]
- Li, S.-Y.; Song, Z.; Yan, Y.-P.; Li, B.; Song, M.-J.; Liu, Y.-F.; Yang, Z.-S.; Li, M.-Y.; Liu, A.-X.; Quan, S.; et al. Aldosterone from endometrial glands is benefit for human decidualization. Cell Death Dis. 2020, 11, 679. [Google Scholar] [CrossRef]
- Sun, B.L.; Cheng, X.; Wu, Q. The Endometrial Stem/Progenitor Cells and Their Niches. Stem Cell Rev. Rep. 2024, 20, 1273–1284. [Google Scholar] [CrossRef]
- Gargett, C.E. Stem Cells in Human Reproduction. Reprod. Sci. 2007, 14, 405–424. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S. Endometrial Stem Cells: Orchestrating Dynamic Regeneration of Endometrium and Their Implications in Diverse Endometrial Disorders. Int. J. Biol. Sci. 2024, 20, 864–879. [Google Scholar] [CrossRef]
- Murata, H.; Tanaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Garry, R. Pressure-Controlled Hysteroscopy During Menstruation. J. Minim. Invasive Gynecol. 2010, 17, 337–343. [Google Scholar] [CrossRef]
- Ludwig, H.; Metzger, H. The re-epithelization of endometrium after menstrual desquamation. Arch. Fr Gynkologie 1976, 221, 51–60. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Shynlova, O.; Tsui, P.; Jaffer, S.; Lye, S.J. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S2–S10. [Google Scholar] [CrossRef]
- Spooner, M.K.; Lenis, Y.Y.; Watson, R.; Jaimes, D.; Patterson, A.L. The role of stem cells in uterine involution. Reproduction 2021, 161, R61–R77. [Google Scholar] [CrossRef]
- Mulic-Lutvica, A.; Bekuretsion, M.; Bakos, O.; Axelsson, O. Ultrasonic evaluation of the uterus and uterine cavity after normal, vaginal delivery. Ultrasound Obstet. Gynecol. 2002, 18, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Kook, M.G.; Kim, S.-R.; Lee, J.W.; Park, C.H.; Oh, B.-C.; Jung, Y.; Hong, I.-S. Development of cell-laden multimodular Lego-like customizable endometrial tissue assembly for successful tissue regeneration. Biomater. Res. 2023, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Kim, S.-R.; Kim, S.-K.; Park, J.-R.; Hong, I.-S. A novel role of follicle-stimulating hormone (FSH) in various regeneration-related functions of endometrial stem cells. Exp. Mol. Med. 2022, 54, 1524–1535. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, S.-R.; Im, J.B.; Park, C.H.; Lee, H.-Y.; Hong, I.-S. 3D stem cell-laden artificial endometrium: Successful endometrial regeneration and pregnancy. Biofabrication 2021, 13, 45012. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Kim, S.-R.; Im, J.-B.; Lim, S.; Hong, I.-S. Tryptophanyl-tRNA Synthetase, a Novel Damage-Induced Cytokine, Significantly Increases the Therapeutic Effects of Endometrial Stem Cells. Mol. Ther. 2020, 28, 2458–2472. [Google Scholar] [CrossRef]
- He, W.; Zhu, X.; Xin, A.; Zhang, H.; Sun, Y.; Xu, H.; Li, H.; Yang, T.; Zhou, D.; Yan, H.; et al. Long-term maintenance of human endometrial epithelial stem cells and their therapeutic effects on intrauterine adhesion. Cell Biosci. 2022, 12, 175. [Google Scholar] [CrossRef]
- Fan, Y.; Lee, R.W.K.; Ng, X.W.; Gargett, C.E.; Chan, J.K.Y. Subtle changes in perivascular endometrial mesenchymal stem cells after local endometrial injury in recurrent implantation failure. Sci. Rep. 2023, 13, 225. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, H.; Wang, Y.; Zhao, L.; Huang, Y.; Wu, R. Current advances in understanding endometrial epithelial cell biology and therapeutic applications for intrauterine adhesion. Stem Cell Res. Ther. 2024, 15, 379. [Google Scholar] [CrossRef]
- Armingol, E.; Ashcroft, J.; Mareckova, M.; Prete, M.; Lorenzi, V.; Icoresi Mazzeo, C.; Lee, J.T.H.; Moullet, M.; Bayraktar, O.A.; Becker, C.; et al. Atlas-scale metabolic activities inferred from single-cell and spatial transcriptomics. biRxiv 2025, preprint. [Google Scholar] [CrossRef]
- Marecková, M.; Garcia-Alonso, L.; Moullet, M.; Lorenzi, V.; Petryszak, R.; Sancho-Serra, C.; Oszlanczi, A.; Mazzeo, C.I.; Wong, F.C.K.; Kelava, I.; et al. An integrated single-cell reference atlas of the human endometrium. Nat. Genet. 2024, 56, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, Y.L.; Xu, X.C.; Wang, J.Z.; Qiu, Z.R.; Yu, Y.Y.; Jiang, X.H.; Shao, W.Q.; Bai, D.D.; Wang, M.Z.; et al. Comprehensive transcriptional atlas of human adenomyosis deciphered by the integration of single-cell RNA-sequencing and spatial transcriptomics. Protein Cell 2024, 15, 530–546. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenge and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Endometriosis: An Immunologist’s Perspective. Int. J. Mol. Sci. 2025, 26, 5193. [Google Scholar] [CrossRef]
- Fonseca, M.A.S.; Haro, M.; Wright, K.N.; Lin, X.; Abbasi, F.; Sun, J.; Hernandez, L.; Orr, N.L.; Hong, J.; Choi-Kuaea, Y.; et al. Single-cell transcriptomic analysis of endometriosis. Nat. Genet. 2023, 55, 255–267. [Google Scholar] [CrossRef]
- Makoui, M.H.; Fekri, S.; Makoui, R.H.; Ansari, N.; Esmaeilzadeh, A. The Role of Mast Cells in the Development and Advancement of Endometriosis. Am. J. Reprod. Immunol. 2025, 93, e70019. [Google Scholar] [CrossRef]
- Ono, Y.; Tanaka, K.; Sato, E.; Ito, M.; Zhang, D.; Honda, M.; Ogawa, T.; Tagaya, H.; Fukuda, T.; Shinozaki, Y.; et al. Activated eosinophil plays a role in promoting fibrosis in endometriotic lesion. Sci. Rep. 2025, 15, 28015. [Google Scholar] [CrossRef]
- Syed, S.M.; Kumar, M.; Ghosh, A.; Tomasetig, F.; Ali, A.; Whan, R.M.; Alterman, D.; Tanwar, P.S. Endometrial Axin2+ Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation. Cell Stem Cell 2020, 26, 64–80.e13. [Google Scholar] [CrossRef]
- Youssef, K.K.; Nieto, M.A. Epithelial-mesenchymal transition in tissue repair and degeneration. Nat. Rev. Mol. Cell Biol. 2024, 25, 720–739. [Google Scholar] [CrossRef] [PubMed]
- Navaridas, R.; Vidal-Sabanés, M.; Ruiz-Mitjana, A.; Altés, G.; Perramon-Güell, A.; Yeramian, A.; Egea, J.; Encinas, M.; Gatius, S.; Matias-Guiu, X.; et al. In Vivo Intra-Uterine Delivery of TAT-Fused Cre Recombinase and CRISPR/Cas9 Editing System in Mice Unveil Histopathology of Pten/p53-Deficient Endometrial Cancers. Adv. Sci. 2023, 10, e2303134. [Google Scholar] [CrossRef] [PubMed]
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel microarchitecture of human endometrial glands: Implications in endometrial regeneration and pathologies. Hum. Reprod. Update 2022, 28, 153–171. [Google Scholar] [CrossRef]
- Cooke, P.S.; Spencer, T.E.; Bartol, F.F.; Hayashi, K. Uterine glands: Development, function and experimental model systems. Mol. Hum. Reprod. 2013, 19, 547–558. [Google Scholar] [CrossRef]
- Potten, C.S.; Loeffler, M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 1990, 110, 1001–1020. [Google Scholar] [CrossRef] [PubMed]
- Tempest, N.; Jansen, M.; Baker, A.M.; Hill, C.J.; Hale, M.; Magee, D.; Treanor, D.; Wright, N.A.; Hapangama, D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020, 251, 440–451. [Google Scholar] [CrossRef]
- Ferenczy, A. Studies on the cytodynamics of human endometrial regeneration. Am. J. Obstet. Gynecol. 1976, 124, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.S.; Schwab, K.E.; Gargett, C.E. Clonogenicity of Human Endometrial Epithelial and Stromal Cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2006, 13, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, A.J.; Palial, K.; Al-lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef]
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Sprung, C.N.; Nguyen, H.P.T. Differential Expression of Wnt Signaling Molecules Between Pre- and Postmenopausal Endometrial Epithelial Cells Suggests a Population of Putative Epithelial Stem/Progenitor Cells Reside in the Basalis Layer. Endocrinology 2012, 153, 2870–2883. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Xiao, L.; Deane, J.A.; Tan, K.S.; Cousins, F.L.; Masuda, H.; Sprung, C.N.; Rosamilia, A.; Gargett, C.E. N-cadherin identifies human endometrial epithelial progenitor cells by stem cell assays. Hum. Reprod. 2017, 32, 2254–2268. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Cousins, F.L.; Pandoy, R.; Jin, S.; Gargett, C.E. The Elusive Endometrial Epithelial Stem/Progenitor Cells. Front. Cell Dev. Biol. 2021, 9, 640319. [Google Scholar] [CrossRef]
- Wang, J.; Wan, X.; Le, Q. Cross-regulation between SOX9 and the canonical Wnt signalling pathway in stem cells. Front. Mol. Biosci. 2023, 10, 1250530. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Hashimura, M.; Suzuki, E.; Yoshida, T.; Kuwata, T. Transcriptional Up-Regulation of Sox9 by NF-κB in Endometrial Carcinoma Cells, Modulating Cell Proliferation Through Alteration in the p14ARF/p53/p21WAF1 Pathway. Am. J. Pathol. 2012, 181, 684–692. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Drury, J.; Da Silva, L.; Al-Lamee, H.; Earp, A.; Valentijn, A.J.; Edirisinghe, D.P.; Murray, P.A.; Fazleabas, A.T.; Gargett, C.E. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum. Reprod. 2019, 34, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, L.; Handfield, L.-F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- van Kerkhof, P.; Kralj, T.; Spanevello, F.; van Bloois, L.; Jordens, I.; van der Vaart, J.; Jamieson, C.; Merenda, A.; Mastrobattista, E.; Maurice, M.M. RSPO3 Furin domain-conjugated liposomes for selective drug delivery to LGR5-high cells. J. Control. Release 2023, 356, 72–83. [Google Scholar] [CrossRef]
- Kostic, L.; Leung, C.; Ahmad Murad, K.; Kancheva, S.; Perna, S.; Lee, B.; Barker, N. Lgr5 marks stem/progenitor cells contributing to epithelial and muscle development in the mouse esophagus. Nat. Commun. 2024, 15, 7145. [Google Scholar] [CrossRef]
- Ng, A.; Tan, S.; Singh, G.; Rizk, P.; Swathi, Y.; Tan, T.Z.; Huang, R.Y.-J.; Leushacke, M.; Barker, N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014, 16, 745–757. [Google Scholar] [CrossRef]
- Tempest, N.; Baker, A.M.; Wright, N.A.; Hapangama, D.K. Does human endometrial LGR5 gene expression suggest the existence of another hormonally regulated epithelial stem cell niche? Hum. Reprod. 2018, 33, 1052–1062. [Google Scholar] [CrossRef]
- Seishima, R.; Leung, C.; Yada, S.; Murad, K.B.A.; Tan, L.T.; Hajamohideen, A.; Tan, S.H.; Itoh, H.; Murakami, K.; Ishida, Y.; et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat. Commun. 2019, 10, 5378. [Google Scholar] [CrossRef]
- Jin, S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 6848–6857. [Google Scholar] [CrossRef]
- Cousins, F.L.; Filby, C.E.; Gargett, C.E. Endometrial Stem/Progenitor Cells–Their Role in Endometrial Repair and Regeneration. Front. Reprod. Health 2022, 3, 811537. [Google Scholar] [CrossRef]
- Masuda, H.; Anwar, S.S.; Bühring, H.-J.; Rao, J.R.; Gargett, C.E. A Novel Marker of Human Endometrial Mesenchymal Stem-Like Cells. Cell Transplant. 2012, 21, 2201–2214. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Patterson, A.L.; George, J.W.; Chatterjee, A.; Carpenter, T.J.; Wolfrum, E.; Chesla, D.W.; Teixeira, J.M. Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties. Hum. Reprod. 2020, 35, 44–57. [Google Scholar] [CrossRef]
- Spitzer, T.L.B.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype1. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Kirkwood, P.M.; Gibson, D.A.; Shaw, I.; Dobie, R.; Kelepouri, O.; Henderson, N.C.; Saunders, P.T.; Azziz, R. Single-cell RNA sequencing and lineage tracing confirm mesenchyme to epithelial transformation (MET) contributes to repair of the endometrium at menstruation. Elife 2022, 11, e77663. [Google Scholar] [CrossRef]
- Li, S.; Ding, L. Endometrial Perivascular Progenitor Cells and Uterus Regeneration. J. Pers. Med. 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Queckbörner, S.; Syk Lundberg, E.; Gemzell-Danielsson, K.; Davies, L.C. Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res. Ther. 2020, 11, 15. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, F.; Yan, G.; Hu, Y.; Sun, H.; Ding, L. Human endometrial perivascular stem cells exhibit a limited potential to regenerate endometrium after xenotransplantation. Hum. Reprod. 2020, 36, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Zhang, L.; Arango, N.A.; Teixeira, J.; Pru, J.K. Mesenchymal-to-Epithelial Transition Contributes to Endometrial Regeneration Following Natural and Artificial Decidualization. Stem Cells Dev. 2013, 22, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal–epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. Structural changes in endometrial basal glands during menstruation. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. A re-appraisal of the morphological changes within the endometrium during menstruation: A hysteroscopic, histological and scanning electron microscopic study. Hum. Reprod. 2009, 24, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Orvis, G.D.; Wang, Y.; Behringer, R.R. Stromal-to-Epithelial Transition during Postpartum Endometrial Regeneration. PLoS ONE 2012, 7, e44285. [Google Scholar] [CrossRef]
- Cousins, F.L.; Murray, A.; Esnal, A.; Gibson, D.A.; Critchley, H.O.D.; Saunders, P.T.K. Evidence from a Mouse Model That Epithelial Cell Migration and Mesenchymal-Epithelial Transition Contribute to Rapid Restoration of Uterine Tissue Integrity during Menstruation. PLoS ONE 2014, 9, e86378. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhou, H.J.; Lin, C.; Long, L.; Yang, X.; Zhang, H.; Taylor, H.; Min, W. CD34+KLF4+ Stromal Stem Cells Contribute to Endometrial Regeneration and Repair. Cell Rep. 2019, 27, 2709–2724.e2703. [Google Scholar] [CrossRef]
- Ghosh, A.; Syed, S.M.; Kumar, M.; Carpenter, T.J.; Teixeira, J.M.; Houairia, N.; Negi, S.; Tanwar, P.S. In Vivo Cell Fate Tracing Provides No Evidence for Mesenchymal to Epithelial Transition in Adult Fallopian Tube and Uterus. Cell Rep. 2020, 31, 107631. [Google Scholar] [CrossRef]
- Spooner-Harris, M.; Kerns, K.; Zigo, M.; Sutovsky, P.; Balboula, A.; Patterson, A.L. A re-appraisal of mesenchymal-epithelial transition (MET) in endometrial epithelial remodeling. Cell Tissue Res. 2022, 391, 393–408. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal Stromal Cells as Critical Contributors to Tissue Regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Cassier, P.A.; Navaridas, R.; Bellina, M.; Rama, N.; Ducarouge, B.; Hernandez-Vargas, H.; Delord, J.P.; Lengrand, J.; Paradisi, A.; Fattet, L.; et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 2023, 620, 409. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, W.Z.; Kong, D.S.; Liu, Z.M.; Shi, Z.Z.; Ma, X.H.; Li, Y.M.; Jiang, J. DACH1 suppresses epithelial to mesenchymal transition (EMT) through Notch1 pathway and reverses progestin resistance in endometrial carcinoma. Cancer Med. 2019, 8, 4380–4388. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Nie, N.; Shen, X.; Jiang, W.; Liu, Y.; Gong, L.; An, C.; Zhao, K.; Yao, X.; et al. SFRP4+ stromal cell subpopulation with IGF1 signaling in human endometrial regeneration. Cell Discov. 2022, 8, 95. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Lash, G.E.; Anderson, G.M.; Girling, J.E. Modelling menstruation in the common mouse: A narrative review. Reprod. Fertil. Dev. 2025, 37, RD25055. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Gómez, L.; López-Martínez, S.; Francés-Herrero, E.; Rodríguez-Eguren, A.; Pellicer, A.; Cervelló, I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Nalaboff, K.M.; Pellerito, J.S.; Ben-Levi, E. Imaging the Endometrium: Disease and Normal Variants. RadioGraphics 2001, 21, 1409–1424. [Google Scholar] [CrossRef]
- Kuramoto, H.; Tamura, S.; Notake, Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am. J. Obstet. Gynecol. 1972, 114, 1012–1019. [Google Scholar] [CrossRef]
- Kyo, S.; Nakamura, M.; Kiyono, T.; Maida, Y.; Kanaya, T.; Tanaka, M.; Yatabe, N.; Inoue, M. Successful Immortalization of Endometrial Glandular Cells with Normal Structural and Functional Characteristics. Am. J. Pathol. 2003, 163, 2259–2269. [Google Scholar] [CrossRef]
- Yuhki, M.; Kajitani, T.; Mizuno, T.; Aoki, Y.; Maruyama, T. Establishment of an immortalized human endometrial stromal cell line with functional responses to ovarian stimuli. Reprod. Biol. Endocrinol. 2011, 9, 104. [Google Scholar] [CrossRef]
- Park, Y.; Jung, J.-G.; Yu, Z.-C.; Asaka, R.; Shen, W.; Wang, Y.; Jung, W.-H.; Tomaszewski, A.; Shimberg, G.; Chen, Y.; et al. A novel human endometrial epithelial cell line for modeling gynecological diseases and for drug screening. Lab. Investig. 2021, 101, 1505–1512. [Google Scholar] [CrossRef]
- Michalski, S.A.; Chadchan, S.B.; Jungheim, E.S.; Kommagani, R. Isolation of Human Endometrial Stromal Cells for In Vitro Decidualization. J. Vis. Exp. 2018, 139, e57684. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T.E. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Cloke, B.; Huhtinen, K.; Fusi, L.; Kajihara, T.; Yliheikkilae, M.; Ho, K.K.; Teklenburg, G.; Lavery, S.; Jones, M.C.; Trew, G.; et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 2008, 149, 4462–4474. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Bläuer, M.; Heinonen, P.K.; Martikainen, P.M.; Tomás, E.; Ylikomi, T. A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum. Reprod. 2005, 20, 864–871. [Google Scholar] [CrossRef]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Yang, L.; Semmes, E.C.; Ovies, C.; Megli, C.; Permar, S.; Gilner, J.B.; Coyne, C.B. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal-fetal interface. Elife 2022, 11, e79794. [Google Scholar] [CrossRef]
- Cheung, V.C.; Peng, C.-Y.; Marinić, M.; Sakabe, N.J.; Aneas, I.; Lynch, V.J.; Ober, C.; Nobrega, M.A.; Kessler, J.A. Pluripotent stem cell-derived endometrial stromal fibroblasts in a cyclic, hormone-responsive, coculture model of human decidua. Cell Rep. 2021, 35, 109138. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Dickson, M.J.; Edwards, N.; Hampton, K.; Garantziotis, S.; DeMayo, F.J. From cup to dish: How to make and use endometrial organoid and stromal cultures derived from menstrual fluid. Front. Endocrinol. 2023, 14, 1220622. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, Y.e.; Li, R.; Ma, Y.; Ma, S.; Chen, X.; Li, B.; Li, B.; Qi, X.; Ha, C. Construction organoid model of ovarian endometriosis and the function of estrogen and progesterone in the model. Sci. Rep. 2025, 15, 6636. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Dhakal, P.; Behura, S.K.; Schust, D.J.; Spencer, T.E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 23132–23142. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, W.; Sacco, M.; Downing, P.; Dimitriadis, E.; Zhao, F. Using organoids to investigate human endometrial receptivity. Front. Endocrinol. 2023, 14, 1158515. [Google Scholar] [CrossRef]
- Jiang, Y.; Palomares, A.R.; Munoz, P.; Nalvarte, I.; Acharya, G.; Inzunza, J.; Varshney, M.; Rodriguez-Wallberg, K.A. Proof-of-Concept for Long-Term Human Endometrial Epithelial Organoids in Modeling Menstrual Cycle Responses. Cells 2024, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Onesto, M.M.; Kim, J.-i.; Pasca, S.P. Assembloid models of cell-cell interaction to study tissue and disease biology. Cell Stem Cell 2024, 31, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Ban, Z.; Knöspel, F.; Schneider, M.R. Shedding light into the black box: Advances in in vitro systems for studying implantation. Dev. Biol. 2020, 463, 1–10. [Google Scholar] [CrossRef]
- Zambuto, S.G.; Clancy, K.B.H.; Harley, B.A.C. A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 2019, 9, 20190016. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. Elife 2021, 10, e69603. [Google Scholar] [CrossRef]
- Tian, J.; Yang, J.; Chen, T.; Yin, Y.; Li, N.; Li, Y.; Luo, X.; Dong, E.; Tan, H.; Ma, Y.; et al. Generation of Human Endometrial Assembloids with a Luminal Epithelium using Air–Liquid Interface Culture Methods. Adv. Sci. 2023, 10, e2301868. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Choi, Y.S.; Lee, M.J.; Kim, M.; Seo, S.J.; Kwak, S.M.; Park, S.; Cho, S.W.; Jin, Y. Uterus-Mimetic Extracellular Microenvironment for Engineering Female Reproductive System. Adv. Funct. Mater. 2025, 35, 2415149. [Google Scholar] [CrossRef]
- Shibata, S.; Endo, S.; Nagai, L.A.E.; Kobayashi, E.H.; Oike, A.; Kobayashi, N.; Kitamura, A.; Hori, T.; Nashimoto, Y.; Nakato, R.; et al. Modeling embryo-endometrial interface recapitulating human embryo implantation. Sci. Adv. 2024, 10, eadi4819. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Tamura, I.; Yoshimura, A.; Yoneda, T.; Takasaki, H.; Shiroshita, A.; Shirafuta, Y.; Sato, S.; Sugino, N. Establishment of an in vitro implantation model using a newly developed mouse endometrial organoid. Development 2025, 152, dev204461. [Google Scholar] [CrossRef]
- Hiraoka, T.; Aikawa, S.; Mashiko, D.; Nakagawa, T.; Shirai, H.; Hirota, Y.; Kimura, H.; Ikawa, M. An ex vivo uterine system captures implantation, embryogenesis, and trophoblast invasion via maternal-embryonic signaling. Nat. Commun. 2025, 16, 5755. [Google Scholar] [CrossRef]
- Deng, Z.M.; Dai, F.F.; Wang, R.Q.; Deng, H.B.; Yin, T.L.; Cheng, Y.X.; Chen, G.T. Organ-on-a-chip: Future of female reproductive pathophysiological models. J. Nanobiotechnol. 2024, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.E.; Hunziker, R.; Wikswo, J.P. Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Exp. Biol. Med. 2017, 242, 1559–1572. [Google Scholar] [CrossRef]
- Gnecco, J.S.; Pensabene, V.; Li, D.J.; Ding, T.B.; Hui, E.E.; Bruner-Tran, K.L.; Osteen, K.G. Compartmentalized Culture of Perivascular Stroma and Endothelial Cells in a Microfluidic Model of the Human Endometrium. Ann. Biomed. Eng. 2017, 45, 1758–1769. [Google Scholar] [CrossRef]
- Ahn, J.; Yoon, M.-J.; Hong, S.-H.; Cha, H.; Lee, D.; Koo, H.S.; Ko, J.-E.; Lee, J.; Oh, S.; Jeon, N.L.; et al. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum. Reprod. 2021, 36, 2720–2731. [Google Scholar] [CrossRef]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, S.-R.; Lee, J.W.; Park, C.H.; Yu, W.-J.; Lee, S.-J.; Chon, S.J.; Lee, D.H.; Hong, I.-S. Development of a novel dual reproductive organ on a chip: Recapitulating bidirectional endocrine crosstalk between the uterine endometrium and the ovary. Biofabrication 2020, 13, 15001. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Alvarez, M.; Bueno-Fernandez, C.; Rodriguez-Eguren, A.; Frances-Herrero, E.; Agustina-Hernandez, M.; Faus, A.; Gisbert Roca, F.; Martinez-Ramos, C.; Galan, A.; Pellicer, A.; et al. Hybrid Endometrial-Derived Hydrogels: Human Organoid Culture Models and In Vivo Perspectives. Adv. Health Mater. 2024, 13, e2303838. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, F.; Chen, Q.; Zeng, B.; Gu, W.; Zhang, Y.; Sun, F.; Wang, X.; Lin, X.; Liu, N.; et al. Microfluidic chip-integrated vascularized endometrial complexes: Mitochondrial function and paracrine crosstalk enhance regenerative potential. Bioact. Mater. 2025, 54, 551–569. [Google Scholar] [CrossRef]
- Lv, X.; Niu, W.; Zhang, B.; Chen, J.; Yang, S.; Xue, Y.; Dong, Y.; Yuan, P.; Pan, Y.; Tan, J.; et al. Self-Assembled Peptide Hydrogels Loaded with Umbilical Cord-Derived Mesenchymal Stem Cells Repairing Injured Endometrium and Restoring Fertility. Adv. Health Mater. 2024, 13, e2400524. [Google Scholar] [CrossRef]
- Quan, Q.; Gu, H.; Wang, Y.; Yu, M. Immune micro-environment analysis and drug screening for ovarian endometriosis. Genes Genom. 2024, 46, 803–815. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhang, Y.; Yang, R.; Dai, Y.; Lin, Y.; Sun, K.; Xu, H.; Tao, K. Photoacoustic Imaging Endometriosis Lesions with Nanoparticulate Polydopamine as a Contrast Agent. Adv. Health Mater. 2024, 13, e2302175. [Google Scholar] [CrossRef]
- Tan, Y.; Flynn, W.F.; Sivajothi, S.; Luo, D.; Bozal, S.B.; Dave, M.; Luciano, A.A.; Robson, P.; Luciano, D.E.; Courtois, E.T. Single-cell analysis of endometriosis reveals a coordinated transcriptional programme driving immunotolerance and angiogenesis across eutopic and ectopic tissues. Nat. Cell Biol. 2022, 24, 1306–1318. [Google Scholar] [CrossRef]
- Yamada, R.G.; Ueda, H.R. The circadian clock ticks in organoids. EMBO J. 2022, 41, e110157. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Sen, A.; Hoffmann, H.M. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol. Cell Endocrinol. 2020, 501, 110655. [Google Scholar] [CrossRef] [PubMed]
- De Bem, T.H.C.; Tinning, H.; Vasconcelos, E.J.R.; Wang, D.; Forde, N. Endometrium On-a-Chip Reveals Insulin- and Glucose-induced Alterations in the Transcriptome and Proteomic Secretome. Endocrinology 2021, 162, bqab054. [Google Scholar] [CrossRef]
- Frances-Herrero, E.; Lopez, R.; Hellstrom, M.; de Miguel-Gomez, L.; Herraiz, S.; Brannstrom, M.; Pellicer, A.; Cervello, I. Bioengineering trends in female reproduction: A systematic review. Hum. Reprod. Update 2022, 28, 798–837. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell. Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, M.M.A.; Coppes, R.P.; Beghin, A.; De Coppi, P.; Gerli, M.F.M.; de Graeff, N.; Pan, Q.; Saito, Y.; Shi, S.; Zadpoor, A.A.; et al. Clinical applications of human organoids. Nat. Med. 2025, 31, 409–421. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Ou, M.; Chen, D.; Zhang, S. Unlocking the Secrets of the Endometrium: Stem Cells, Niches and Modern Methodologies. Biomedicines 2025, 13, 2435. https://doi.org/10.3390/biomedicines13102435
Huang L, Ou M, Chen D, Zhang S. Unlocking the Secrets of the Endometrium: Stem Cells, Niches and Modern Methodologies. Biomedicines. 2025; 13(10):2435. https://doi.org/10.3390/biomedicines13102435
Chicago/Turabian StyleHuang, Lijun, Miaoxian Ou, Dunjin Chen, and Shuang Zhang. 2025. "Unlocking the Secrets of the Endometrium: Stem Cells, Niches and Modern Methodologies" Biomedicines 13, no. 10: 2435. https://doi.org/10.3390/biomedicines13102435
APA StyleHuang, L., Ou, M., Chen, D., & Zhang, S. (2025). Unlocking the Secrets of the Endometrium: Stem Cells, Niches and Modern Methodologies. Biomedicines, 13(10), 2435. https://doi.org/10.3390/biomedicines13102435





