Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation
Abstract
1. Introduction
2. Methodology
3. Inflammation and Cardiovascular Disease
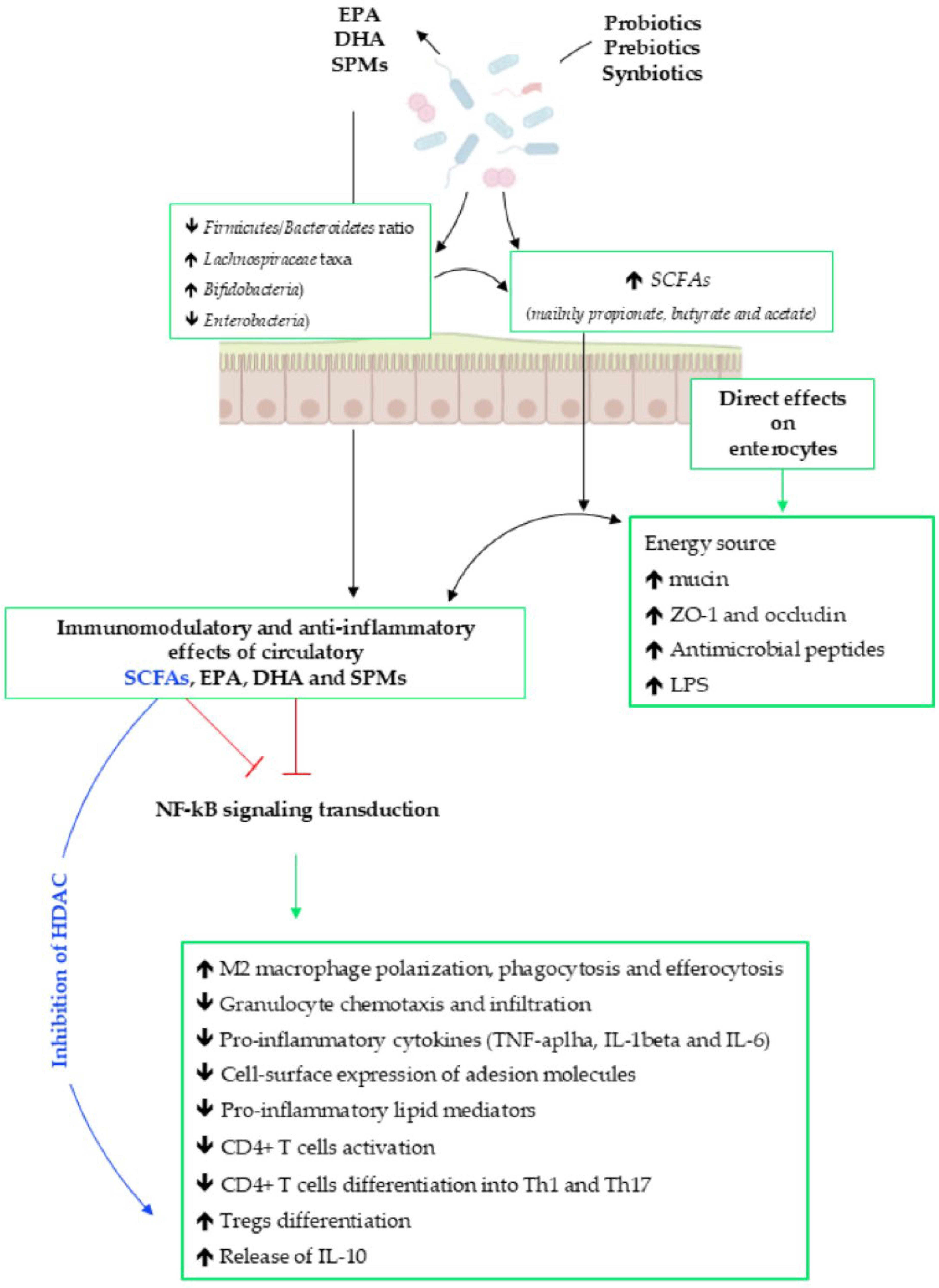
4. GM–Inflammation–Immunity Axis
5. EPA, DHA and SPMs
6. Evidence of the Interplay Among EPA, DHA, Prebiotics/Probiotics and GM
| Study Type | Intervention | Main Effects | Refs. |
|---|---|---|---|
| Experimental (rats) | Protection of intestinal barrier | ||
| Synbiotics | Prevention of dysbiosis Prevention of endothelial dysfunction Decrease in high blood pressure | [162] | |
| Experimental (rats) | Probiotics | Decrease in inflammatory response Increase in beneficial bacteria | [163] |
| Experimental (rats) | Reduction in the Firmicues/Bacteriodetes ratio and TMAO levels | ||
| Synbiotics | Increase in butyrate concentration and amount of Lactobacillus and Akkemansia muciniphila | [164] | |
| Reduction in the oxidative stress | |||
| Experimental (mice) | Prebiotics plus | Increase in Alloculum S24-7 and Akkemansia muciniphila | [166] |
| EPA + DHA | Reduction in Oscillospira and Ruminococcaceae |
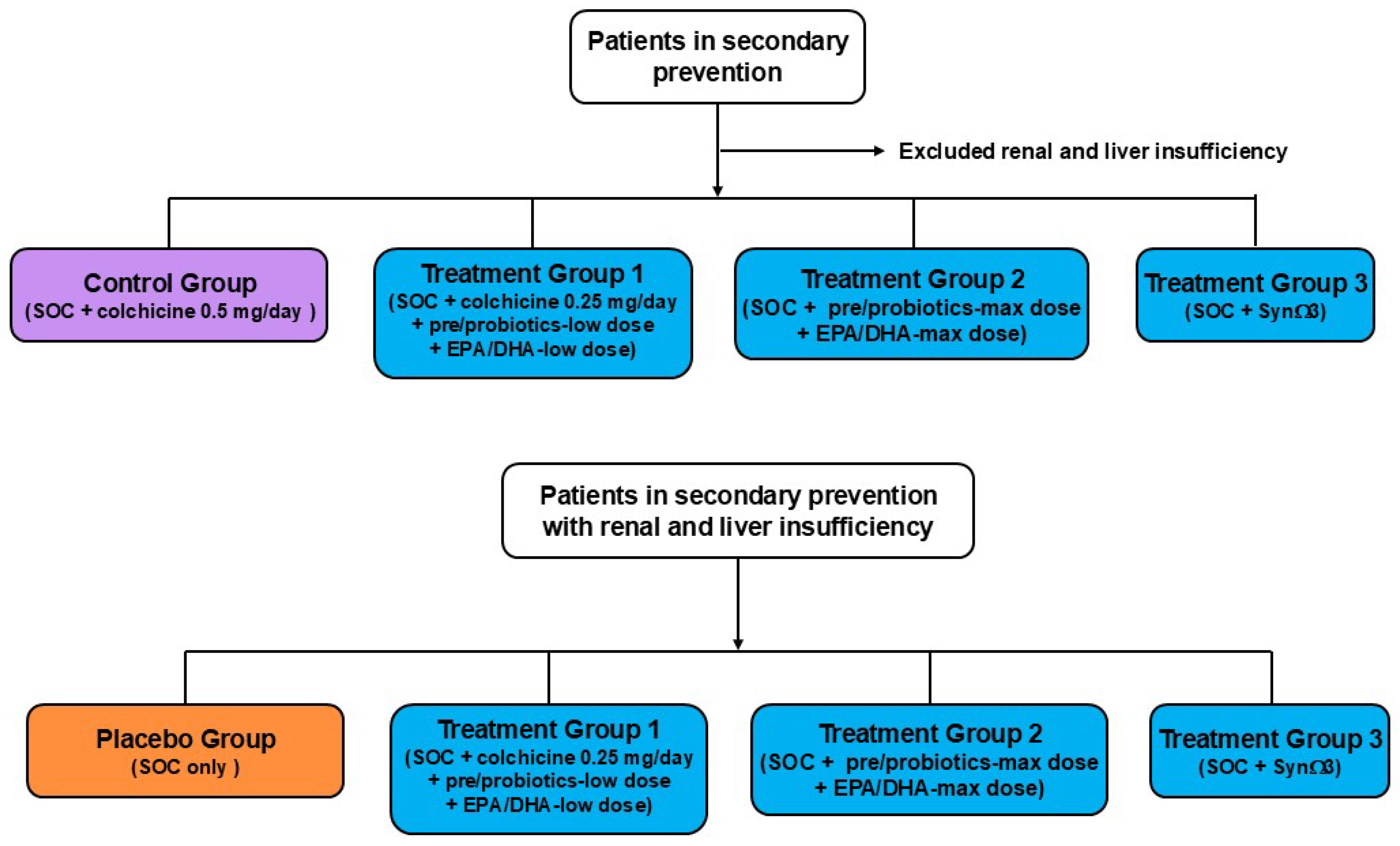
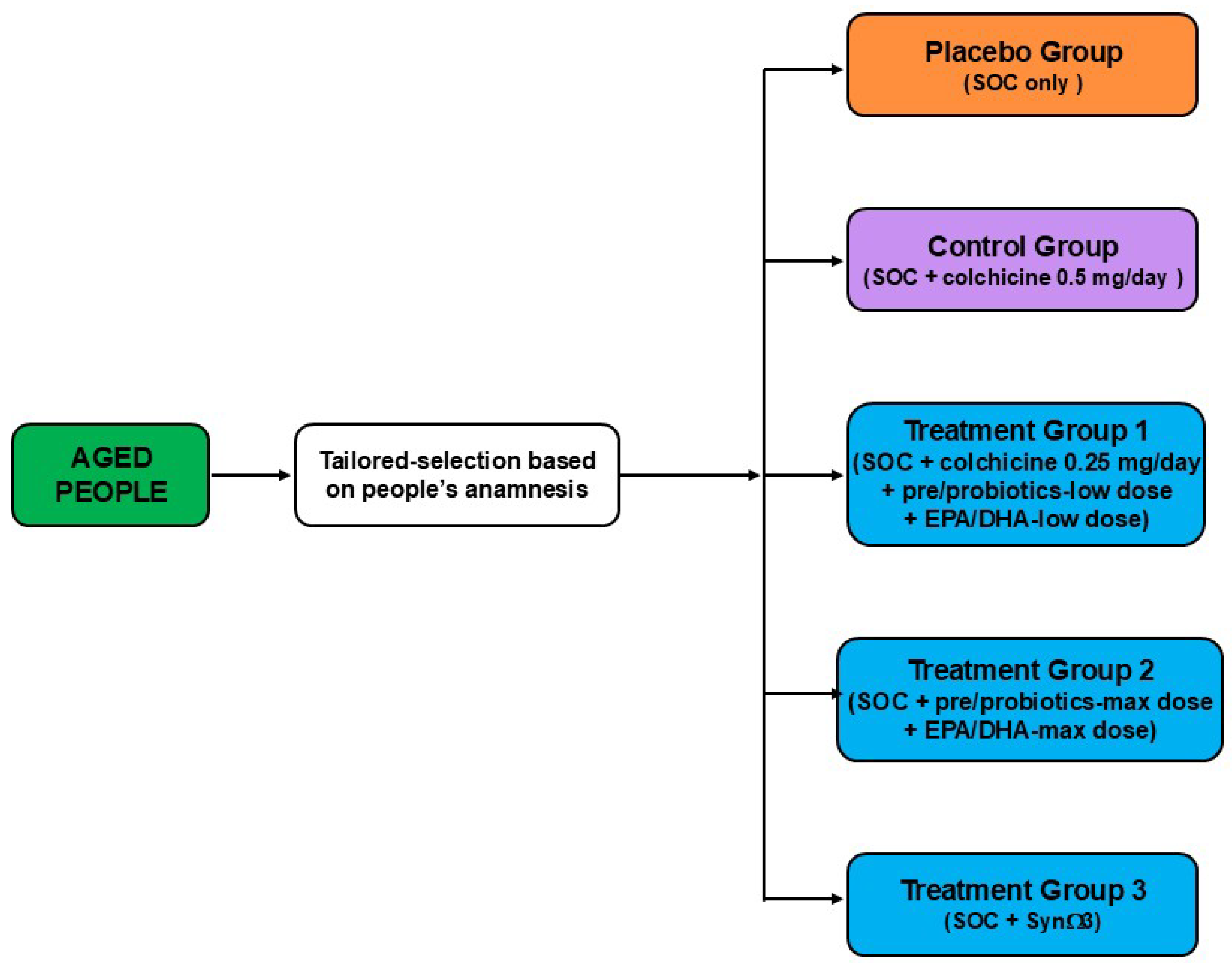
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Apo | Apolipoprotein |
| AA | Arachidonic acid |
| AhR | Aryl hydrocarbon receptor |
| CAVD | Calcific aortic valve disease |
| CV | Cardiovascular |
| CVD | CV disease |
| CRP | C-reactive protein |
| COX | Cyclooxygenase |
| CYP | Cytochrome 450 mixed-function oxidase |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FDA | U.S. Food and Drug Administration |
| FFAs | Free fatty acids |
| GPCR | G-protein-coupled receptor |
| HDL-C | High-density lipoprotein-cholesterol |
| IFN | Interferon |
| IL | Interleukin |
| LPS | Lipopolysaccharides |
| LOX | Lipoxygenase |
| lcFOS | Long-chain fructo-oligosaccharide |
| LDL-C | Low-density lipoprotein-cholesterol |
| MaRs | Marensins |
| NF-kB | Nuclear factor kappa B |
| NLRP3 | Nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family pyrin domain containing 3 |
| Tregs | Regulatory T cells |
| Rvs | Resolvins |
| 5-HT | Serotonin |
| scGOS | Short-chain galacto-oligosaccharide |
| SCFAs | Short chain fatty acids |
| SPMs | Specialized pro-resolving mediators |
| TMA | Trimethylamine |
| TMAO | TMA N-oxide |
| TNF | Tumour necrosis factor |
References
- Nurmohamed, N.S.; Navar, A.M.; Kastelein, J.J.P. New and Emerging Therapies for Reduction of LDL-Cholesterol and Apolipoprotein B: JACC Focus Seminar ¼. J. Am. Coll. Cardiol. 2021, 77, 1564–1575. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in Atherosclerosis-No Longer a Theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef]
- Parolini, C. Biotechnology Approaches for the Treatment of Dyslipidemia. Cardiovasc. Drugs Ther. 2021, 35, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Prominent, Reduce-It, and Strength Investigators. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef]
- Marchesi, M.; Parolini, C.; Caligari, S.; Gilio, D.; Manzini, S.; Busnelli, M.; Cinquanta, P.; Camera, M.; Brambilla, M.; Sirtori, C.R.; et al. Rosuvastatin does not affect human apolipoprotein A-I expression in genetically modified mice: A clue to the disputed effect of statins on HDL. Br. J. Pharmacol. 2011, 164, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Glynn, R.J.; Fruchart, J.-C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle Jr, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kstelein, J.J.P.; Koenig, W.; McGuire, D.M.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Vogel, B.; Sartori, S.; Bay, B.; Oliva, A.; Feng, Y.; Krishnan, P.; Sweeny, J.; Gitto, M.; Smith, K.; et al. Prognostic impact of residual inflammatory and triglyceride risk in statin-treated patients with well-controlled LDL cholesterol and atherosclerotic cardiovascular disease. Eur. J. Prev. Cardiol. 2025, in press. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Sirtori, C.R.; Chiesa, G.; Parolini, C. Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation. Nutrients 2018, 10, 1249. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Lamminpää, I.; Amedei, A.; Parolini, C. Effects of Marine-Derived Components on Cardiovascular Disease Risk Factors and Gut Microbiota Diversity. Mar. Drugs 2024, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. 2017, 198, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Mar. Drugs 2016, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Effects of Fish n-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef]
- Parolini, C. The Role of Marine n-3 Polyunsaturated Fatty Acids in Inflammatory-Based Disease: The Case of Rheumatoid Arthritis. Mar. Drugs 2023, 22, 17. [Google Scholar] [CrossRef]
- Parolini, C.; Adorni, M.P.; Busnelli, M.; Manzini, S.; Cipollari, E.; Favari, E.; Lorenzon, P.; Ganzetti, G.; Fingerle, J.; Bernini, F.; et al. Infusions of Large Synthetic HDL Containing Trimeric apoA-I Stabilize Atherosclerotic Plaques in Hypercholesterolemic Rabbits. Can. J. Cardiol. 2019, 35, 1400–1408. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Parolini, C.; Marchesi, M.; Chiesa, G. HDL therapy for the treatment of cardiovascular diseases. Curr. Vasc. Pharmacol. 2009, 7, 550–556. [Google Scholar] [CrossRef]
- Vergallo, R.; Crea, F. Atherosclerotic plaque healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef]
- Dellera, F.; Ganzetti, G.S.; Froio, A.; Manzini, S.; Busnelli, M.; Meinitzer, A.; Sirtori, C.R.; Chiesa, G.; Parolini, C. L-homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. Thromb. Haemost. 2016, 116, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Manzini, S.; Pinna, C.; Busnelli, M.; Cinquanta, P.; Rigamonti, E.; Ganzetti, G.S.; Dellera, F.; Sala, A.; Calabresi, L.; Franceschini, G.; et al. Beta2-adrenergic activity modulates vascular tone regulation in lecithin:cholesterol acyltransferase knockout mice. Vasc. Pharmacol. 2015, 74, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Sepsis and high-density lipoproteins: Pathophysiology and potential new therapeutic targets. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167761. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Parolini, C. Pathophysiology of bone remodelling cycle: Role of immune system and lipids. Biochem. Pharmacol. 2025, 235, 116844. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- Russo, E.; Cinci, L.; Di Gloria, L.; Baldi, S.; D’Ambrosio, M.; Nannini, G.; Bigagli, E.; Curini, L.; Pallecchi, M.; Arcese, D.A.; et al. Crohn’s disease recurrence updates: First surgery vs. surgical relapse patients display different profiles of ileal microbiota and systemic microbial-associated inflammatory factors. Front. Immunol. 2022, 13, 886468. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef]
- Jain, H.; Goyal, A.; Khan, A.T.M.A.; Khan, N.U.; Jain, J.; Chopra, S.; Sulaiman, S.A.; Reddy, M.M.; Patel, K.; Khullar, K.; et al. Insights into calcific aortic valve stenosis: A comprehensive overview of the disease and advancing treatment strategies. Ann. Med. Surg. 2024, 86, 3577–3590. [Google Scholar] [CrossRef]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Cinquetti, R.; Frascoli, M.; Parolini, C.; Chiesa, G.; Taramelli, R.; Acquati, F. Intracellular ANKRD1 protein levels are regulated by 26S proteasome-mediated degradation. FEBS Letter. 2009, 583, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yao, D.; Fan, Z.; Zhang, T.; Shen, Q.; Tong, F.; Qian, X.; Xu, L.; Jiang, C.; Dong, N. Beyond VICs: Shedding light on the over-looked VECs in calcific aortic valve disease. Biomed. Pharmacother. 2024, 178, 117143. [Google Scholar] [CrossRef] [PubMed]
- Kodali, S.K.; Velagapudi, P.; Hahn, R.T.; Abbott, D.; Leon, M.B. Valvular Heart Disease in Patients ≥80 Years of Age. J. Am. Coll. Cardiol. 2018, 71, 2058–2072. [Google Scholar] [CrossRef]
- Coffey, S.; Cairns, B.J.; Iung, B. The modern epidemiology of heart valve disease. Heart 2016, 102, 75–85. [Google Scholar] [CrossRef]
- Sherzad, A.G.; Shinwari, M.; Azimee, M.A.; Nemat, A.; Zeng, Q. Risk Factors for Calcific Aortic Valve Disease in Afghan Population. Vasc. Health Risk Manag. 2022, 18, 643–652. [Google Scholar] [CrossRef]
- Chen, H.Y.; Engert, J.C.; Thanassoulis, G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 96–102. [Google Scholar] [CrossRef]
- Huang, N.; Zhuang, Z.; Liu, Z.; Huang, T. Observational and Genetic Associations of Modifiable Risk Factors with Aortic Valve Stenosis: A Prospective Cohort Study of 0.5 Million Participants. Nutrients 2022, 14, 2273. [Google Scholar] [CrossRef]
- Parolini, C. A Compendium of the Biological Effects of Apolipoprotein A-IMilano. J. Pharmacol. Exp. Ther. 2020, 372, 54–62. [Google Scholar] [CrossRef]
- Alushi, B.; Curini, L.; Christopher, M.R.; Grubitzch, H.; Landmesser, U.; Amedei, A.; Lauten, A. Calcific Aortic Valve Disease-Natural History and Future Therapeutic Strategies. Front. Pharmacol. 2020, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Skowasch, D.; Schrempf, S.; Preusse, C.J.; Likungu, J.A.; Welz, A.; Lüderitz, B.; Bauriedel, G. Tissue resident C reactive protein in degenerative aortic valves: Correlation with serum C reactive protein concentrations and modification by statins. Heart 2006, 92, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Zeller, J.; Loseff-Silver, J.; Khoshmanesh, K.; Baratchi, S.; Lai, A.; Nero, T.L.; Roy, A.; Watson, A.; Dayawansa, N.; Sharma, P.; et al. Shear-Sensing by C-Reactive Protein: Linking Aortic Stenosis and Inflammation. Circ. Res. 2024, 135, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef]
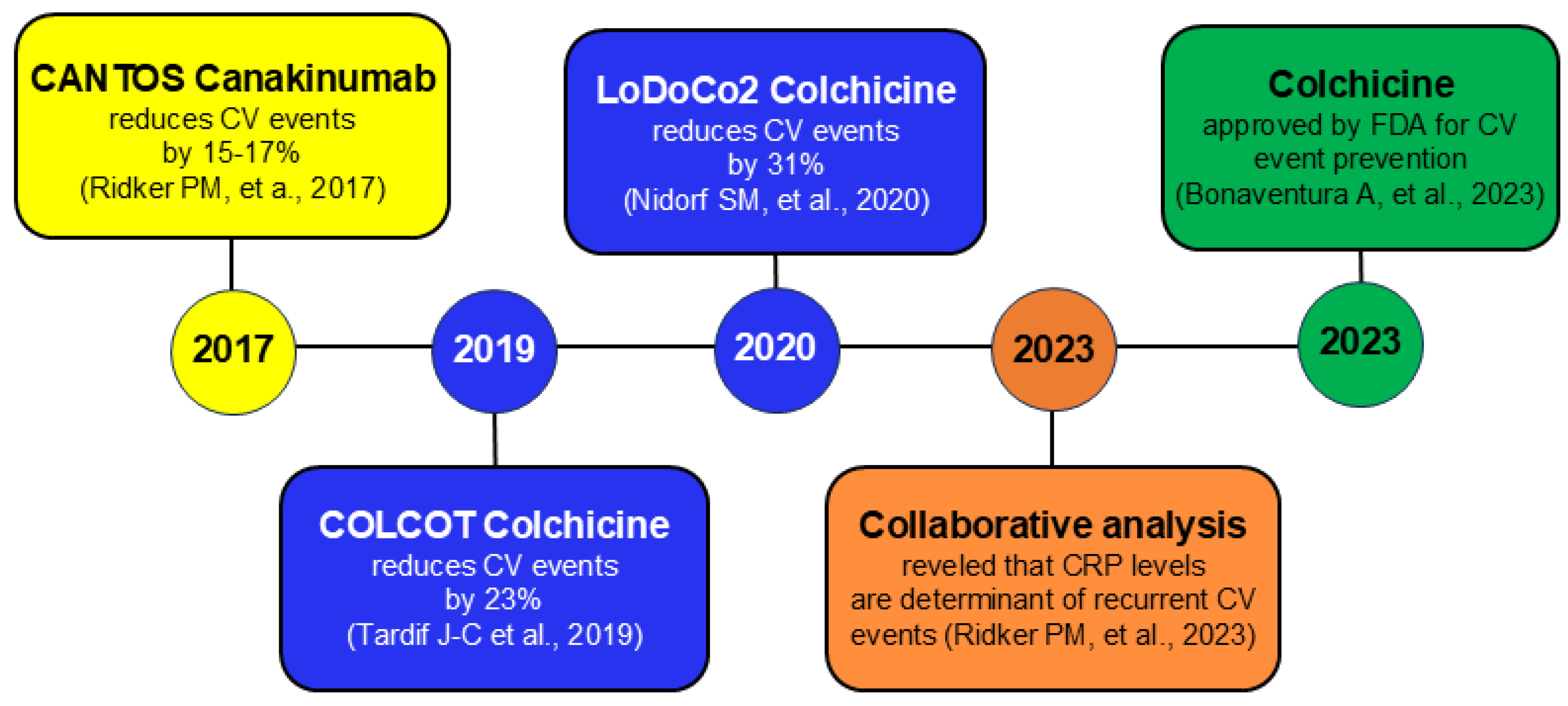
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Bonaventura, A.; Abbate, A. Colchicine for cardiovascular prevention: The dawn of a new era has finally come. Eur. Heart J. 2023, 44, 3303–3304. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Andreu, V.P.; Augustijn, H.E.; Chen, L.; Zhernakova, A.; Fu, J.; Fischbach, M.A.; Dodd, D.; Medema, M.H. gutSMASH predicts specialized primary metabolic pathways from the human gut microbiota. Nat. Biotechnol. 2023, 41, 1416–1423. [Google Scholar] [CrossRef]
- Rey, F.E.; Faith, J.J.; Bain, J.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; Gordon, J.I. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 2010, 285, 22082–22090. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.-D.; Flint, H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4349. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Louca, P.; Zhang, X.; Wells, P.M.; Steves, C.J.; Spector, T.D.; Falchi, M.; Valdes, A.M.; Menni, C. Circulating Levels of the Short-Chain Fatty Acid Acetate Mediate the Effect of the Gut Microbiome on Visceral Fat. Front. Microbiol. 2021, 12, 711359. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Innume Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu YHazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Constantino-Jonapa, L.A.; Espinoza-Palacios, Y.; Escalona-Montaño, A.R.; Hernández-Ruiz, P.; Amezcua-Guerra, L.M.; Amedei, A.; Aguirre-García, M.M. Contribution of Trimethylamine N-Oxide (TMAO) to Chronic Inflammatory and Degenerative Diseases. Biomedicines 2023, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Badley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.H.W.; Wu, Y.; Hazen, S.L.; et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Cir. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes. 2016, 124, 251–256. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Morbid Obesity Study Group; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Sci. Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Alkhahaf, L.M.; Ryan, K.S. Biosynthetic manipulation of tryptophan in bacteria: Pathways and mechanisms. Chem. Biol. 2015, 22, 317–328. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Spencer, N.J.; Keating, D.J. Role of 5-HT in the enteric nervous system and enteroendocrine cells. Br. J. Pharmacol. 2025, 182, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Reigstad, C.S.; Salmonson, C.E.; Rainey 3rd, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.M.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Tyler, K.; MacEachern, S.J.; Balemba, O.B.; Johnson, A.C.; Brooks, E.M.; Zhao, H.; Swain, G.M.; Moses, P.L.; Galligan, J.J.; et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenteroglogy 2012, 142, 844–854. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Baganz, N.L.; Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef]
- Parolini, C. Marine n-3 polyunsaturated fatty acids: Efficacy on inflammatory-based disorders. Life Sci. 2020, 263, 118591. [Google Scholar] [CrossRef]
- Parolini, C.; Caligari, S.; Gilio, D.; Manzini, S.; Busnelli, M.; Montagnani, M.; Locatelli, M.; Diani, E.; Giavarini, F.; Caruso, D.; et al. Reduced biliary sterol output with no change in total faecal excretion in mice expressing a human apolipoprotein A-I variant. Liver Int. 2012, 32, 1363–1371. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Dixit, V.D. Immunological complications of obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef]
- López-Vicario, C.; Titos, E.; Walker, M.E.; Alcaraz-Quiles, J.; Casulleras, M.; Durán-Güell, M.; Flores-Costa, R.; Pérez-Romero, N.; Forné, M.; Dalli, J.; et al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 2019, 33, 7072–7083. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.K. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediators Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Polyunsaturated fatty acids and metabolic health: Novel insights. Curr. Opin. Clin. Nutr. Metab. Care. 2022, 25, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Omega-3 (n-3) Fatty Acid-Statin Interaction: Evidence for a Novel Therapeutic Strategy for Atherosclerotic Cardiovascular Disease. Nutrients 2024, 16, 962. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Bhat, S.; Sarkar, S.; Zaffar, D.; Dandona, P.; Kalyani, R.R. Omega-3 Fatty Acids in Cardiovascular Disease and Diabetes: A Review of Recent Evidence. Curr. Cardiol. Rep. 2023, 25, 51–65. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Schiele, R.; Schneider, S.; Diller, F.; Victor, N.; Gohlke, H.; Gottwik, M.; Steinbeck, G.; Del Castillo, U.; Sack, R.; et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010, 122, 2152–2159. [Google Scholar] [CrossRef]
- Galan, P.; Kesse-Guyot, E.; Czernichow, S.; Briancon, S.; Blacher, J.; Hercberg, S.; SUFOLOM3 Collaborative Group. Effects of Bvitamins omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ 2010, 341, c6273. [Google Scholar] [CrossRef]
- ORIGIN Trial Investigators; Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Díaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef]
- Risk Prevention Study Collaborative Group; Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzonsa, I.; Milani, V.; Silletta, M.G.; et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef]
- Bowman, L.; Mafham, M.; Stevens, W.; Haynes, R.; Aung, T.; Chen, F.; Buck, G.; Collins, R.; Armitage, J.; ASCEND Study Collaborative Group. ASCEND: A Study of Cardiovascular Events in Diabetes: Characteristics of a randomized trial of aspirin of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 2018, 198, 135–144. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Approval Package—Omacor. 2004. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-654_Omacor.cfm (accessed on 24 July 2025).
- FDA Vascepa Prescribing Information and Indications. 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf (accessed on 24 July 2025).
- Miyauchi, K.; Iwata, H.; Nishizaki, Y.; Inoue, T.; Hirayama, A.; Kimura, K.; Ozai, Y.; Murohara, T.; Ueshima, K.; Kuwabara, Y.; et al. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid (RESPECT-EPA). Circulation 2024, 150, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef]
- Ameur, A.; Enroth, S.; Johansson, A.; Zaboli, G.; Igl, W.; Johansson, A.C.V.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Salazar, J.; Pirela, D.; Nava, M.; Castro, A.; Angarita, L.; Parra, H.; Durán-Agüero, S.; Rojas-Gómez, D.M.; Galbán, N.; Añez, R.; et al. Specialized Proresolving Lipid Mediators: A Potential Therapeutic Target for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3133. [Google Scholar] [CrossRef]
- Ganzetti, G.S.; Parolini, C. Microarray analysis identifies human apoA-IMilano and apoA-II as determinants of the liver gene expression related to lipid and energy metabolism. Exp. Cell Res. 2023, 433, 113626. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Katsiki, N.; Santos, R.D.; Mikhailidis, D.P.; Mantzoros, C.S.; Sahebkar, A. Effects of statins on specialized pro-resolving mediators: An additional pathway leading to resolution of inflammation. Metabolism 2022, 132, 155211. [Google Scholar] [CrossRef]
- Wang, I.E.; Yi, S.; Block, R.C.; Mousa, S.A. Aspirin and omega-3 polyunsaturated fatty acid use and their interaction in cardiovascular diseases and colorectal adenomas. Nutr. Res. Rev. 2022, 35, 295–307. [Google Scholar] [CrossRef]
- Arboleda, V.; Hackworth, A.; Bonnice, S.; Gonzalez, V.; Cabrera, D.; Colletti, C.; Baxter, C.; Aleman Oliva, C.; Kabir, S.; Huang, J.; et al. The role of aspirin, statins, colchicine, and IL-1 inhibitors in prevention of cardiovascular events: A systematic integrative review. J. Osteopath. Med. 2024, 124, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Bermudez, E.A.; Ridker, P.M.; Hurwitz, S.; Serhan, C.N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 2004, 101, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Kasikara, C.; Dora, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Invest. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Salic, K.; Morrison, M.C.; Verschuren, L.; Wielinga, P.Y.; Wu, L.; Kleemann, R.; Gjorstrup, P.; Kooistra, T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 2016, 250, 158–165. [Google Scholar] [CrossRef]
- Fredman, G.; Hellmann, J.; Proto, J.D.; Kuriakose, G.; Colas, R.A.; Dorweiler, B.; Connolly, E.S.; Solomon, R.; Jones, D.M.; Heyer, E.J.; et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 2016, 7, 12869. [Google Scholar] [CrossRef]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016, 119, 1030–11038. [Google Scholar] [CrossRef]
- Colas, R.A.; Souza, P.R.; Walker, M.E.; Burton, M.; Zasłona, Z.; Curtis, A.M.; Marques, R.M.; Dalli, J. Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease. Circ. Res. 2018, 122, 855–863. [Google Scholar] [CrossRef]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, C.; Yang, L.; Zhang, Z.; Zhang, Q.; Wang, B.; Wang, X. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019, 217, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Aspects Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Busnelli, M.; Ganzetti, G.S.; Dellera, F.; Manzini, S.; Scanziani, E.; Johnson, J.L.; Sirtori, C.R.; Chiesa, G. Magnetic resonance imaging visualization of vulnerable atherosclerotic plaques at the brachiocephalic artery of apolipoprotein E knockout mice by the blood-pool contrast agent B22956/1. Mol. Imaging 2014, 13, 7290.2014. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Fernandez, A.; Checa, A.; Carracedo, M.; Artiach, G.; Petri, M.H.; Baumgartner, R.; Forteza, M.J.; Jiang, X.; Andonova, T.; Walker, M.E.; et al. ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages. Circulation 2018, 138, 1693–1705. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef]
- Spite, M.; Fredman, G. Insights into the role of the resolvin D2-GPR18 signaling axis in cardiovascular physiology and disease. Adv. Pharmacol. 2023, 97, 257–281. [Google Scholar] [CrossRef]
- Honkisz-Orzechowska, E.; Łażewska, D.; Baran, G.; Kieć-Kononowicz, K. Uncovering the Power of GPR18 Signalling: How RvD2 and Other Ligands Could Have the Potential to Modulate and Resolve Inflammation in Various Health Disorders. Molecules 2024, 29, 1258. [Google Scholar] [CrossRef]
- Ji, R.-R. Specialized Pro-Resolving Mediators as Resolution Pharmacology for the Control of Pain and Itch. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 273–293. [Google Scholar] [CrossRef]
- Peh, H.Y.; Chen, J. Pro-resolving lipid mediators and therapeutic innovations in resolution of inflammation. Pharmacol. Ther. 2025, 265, 108753. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Spite, M.; Yang, R.; Flower, R.J.; Perretti, M.; Serhan, C.N. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011, 186, 5543–5547. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Kleinbölting, J.; Börner, F.; Jordan, P.M.; Werz, O.; Pelzer, S.; Dieck, H.T.; Wagner, T.; Schön, C. Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients 2022, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- Elajami, T.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Welty, F.K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016, 30, 2792–2801. [Google Scholar] [CrossRef]
- Balfegó, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martínez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N.; et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 78. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Husson, M.-O.; Ley, D.; Portal, C.; Gottrand, M.; Hueso, T.; Desseyn, J.-L.; Gottrand, F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J. Infect. 2016, 73, 523–535. [Google Scholar] [CrossRef]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Deficiency of essential dietary n-3 PUFA disrupts the caecal microbiome and metabolome in mice. Br. J. Nutr. 2017, 118, 959–970. [Google Scholar] [CrossRef]
- Vijay, A.; Astbury, S.; Le Roy, C.; Spector, T.D.; Valdes, A.M. The prebiotic effects of omega-3 fatty acid supplementation: A six-week randomised intervention trial. Gut Microbes 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergstrom, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Backhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Fu, J.; Bonder Mj Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; Franke, L.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Awoyemi, A.; Troseid, M.; Arnesen, H.; Solheim, S.; Sljeflot, I. Effects of dietary intervention and n-3 PUFA supplementation on markers of gut-related inflammation and their association with cardiovascular events in a high-risk population. Atherosclerosis 2019, 286, 53–59. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal Disorders and Metabolic Syndrome: Dysbiosis as a Key Link and Common Bioactive Dietary Components Useful for their Treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Loukil, I.; Mutch, D.M.; Plourde, M. Genetic association between FADS and ELOVL polymorphisms and the circulating levels of EPA/DHA in humans: A scoping review. Genes. Nutr. 2024, 19, 11. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J.; et al. Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Mol. Nutr. Food Res. 2020, 64, e1900616. [Google Scholar] [CrossRef]
- de Oliveira, Y.; Cavalcante, R.G.S.; Cavalcanti Neto, M.P.; Magnani, M.; de Andrade Braga, V.; de Souza, E.L.; de Brito Alves, J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020, 11, 5581–5594. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Chan, J.Y.H.; Lee, C.-T.; Tain, Y.-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; Rukavina Mikusic, N.L.; Lee, H.J.; García Menéndez, S.; Choi, M.R.; Manucha, W. Physiopathological mechanisms involved in the development of hypertension associated with gut dysbiosis and the effect of nutritional/pharmacological interventions. Biochem. Pharmacol. 2022, 204, 115213. [Google Scholar] [CrossRef] [PubMed]
- Szklany, K.; Engen, P.A.; Naqib, A.; Green, S.J.; Keshavarzian, A.; Lopez Rincon, A.; Siebrand, C.J.; Diks, M.A.P.; van de Kaa, M.; Garssen, J.; et al. Dietary Supplementation throughout Life with Non-Digestible Oligosaccharides and/or n-3 Poly-Unsaturated Fatty Acids in Healthy Mice Modulates the Gut-Immune System-Brain Axis. Nutrients 2021, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Wagner, T.; Jordan, P.M.; Werz, O.; Wilhelm, M.; Dieck, H.T.; Schön, C. Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study. Nutrients 2024, 16, 1354. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Kadooka, Y.; Kato, K.; Shirouchi, B.; Sato, M. Lactobacillus gasseri SBT2055 reduces postprandial and fasting serum non-esterified fatty acid levels in Japanese hypertriacylglycerolemic subjects. Lipids Health Dis. 2014, 13, 36. [Google Scholar] [CrossRef]
- Hoppu, U.; Isolauri, E.; Laakso, P.; Matomäki, J.; Laitinen, K. Probiotics and dietary counselling targeting maternal dietary fat intake modifies breast milk fatty acids and cytokines. Eur. J. Nutr. 2012, 51, 211–219. [Google Scholar] [CrossRef]
- Houttu, N.; Vahlberg, T.; Miles, E.A.; Calder, P.C.; Laitinen, K. The impact of fish oil and/or probiotics on serum fatty acids and the interaction with low-grade inflammation in pregnant women with overweight and obesity: Secondary analysis of a randomised controlled trial. Br. J. Nutr. 2024, 131, 296–311. [Google Scholar] [CrossRef]
- Marcus, M.D.; Link, M.S. Omega-3 Fatty Acids and Arrhythmias. Circulation 2024, 150, 488–503. [Google Scholar] [CrossRef]
| Study Type | Intervention | Main Effects | Refs. |
|---|---|---|---|
| Clinical trial | Probiotics plus | ||
| EPA + DHA | Increase in SPMs | [167] | |
| Clinical trial | Probiotics | Reduction in FFA concentration | [168] |
| Clinical trial | Probiotics | Improvement of FFA and cytokine profile | [169] |
| Clinical trial | Probiotics plus | Reduction in CRP | [170] |
| EPA + DHA | Increase in EPA and DHA levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amedei, A.; Lamminpää, I.; Parolini, C. Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation. Biomedicines 2025, 13, 2428. https://doi.org/10.3390/biomedicines13102428
Amedei A, Lamminpää I, Parolini C. Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation. Biomedicines. 2025; 13(10):2428. https://doi.org/10.3390/biomedicines13102428
Chicago/Turabian StyleAmedei, Amedeo, Ingrid Lamminpää, and Cinzia Parolini. 2025. "Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation" Biomedicines 13, no. 10: 2428. https://doi.org/10.3390/biomedicines13102428
APA StyleAmedei, A., Lamminpää, I., & Parolini, C. (2025). Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation. Biomedicines, 13(10), 2428. https://doi.org/10.3390/biomedicines13102428









