New Insight into Bone Immunity in Marrow Cavity and Cancellous Bone Microenvironments and Their Regulation
Abstract
1. Introduction
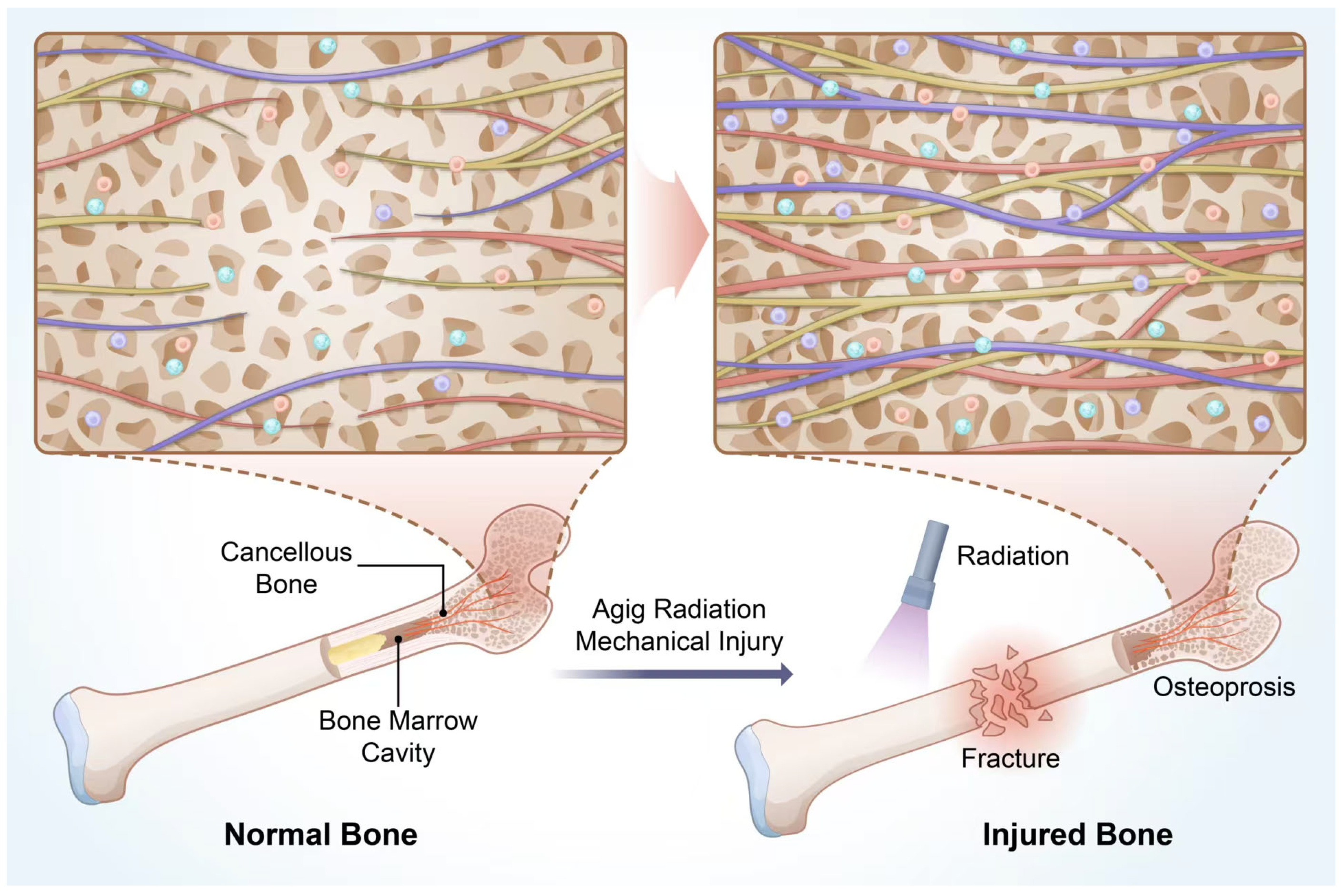
2. Bone Marrow Cavity and Cancellous Bone Immunity
2.1. Immunity of the Bone Marrow Cavity
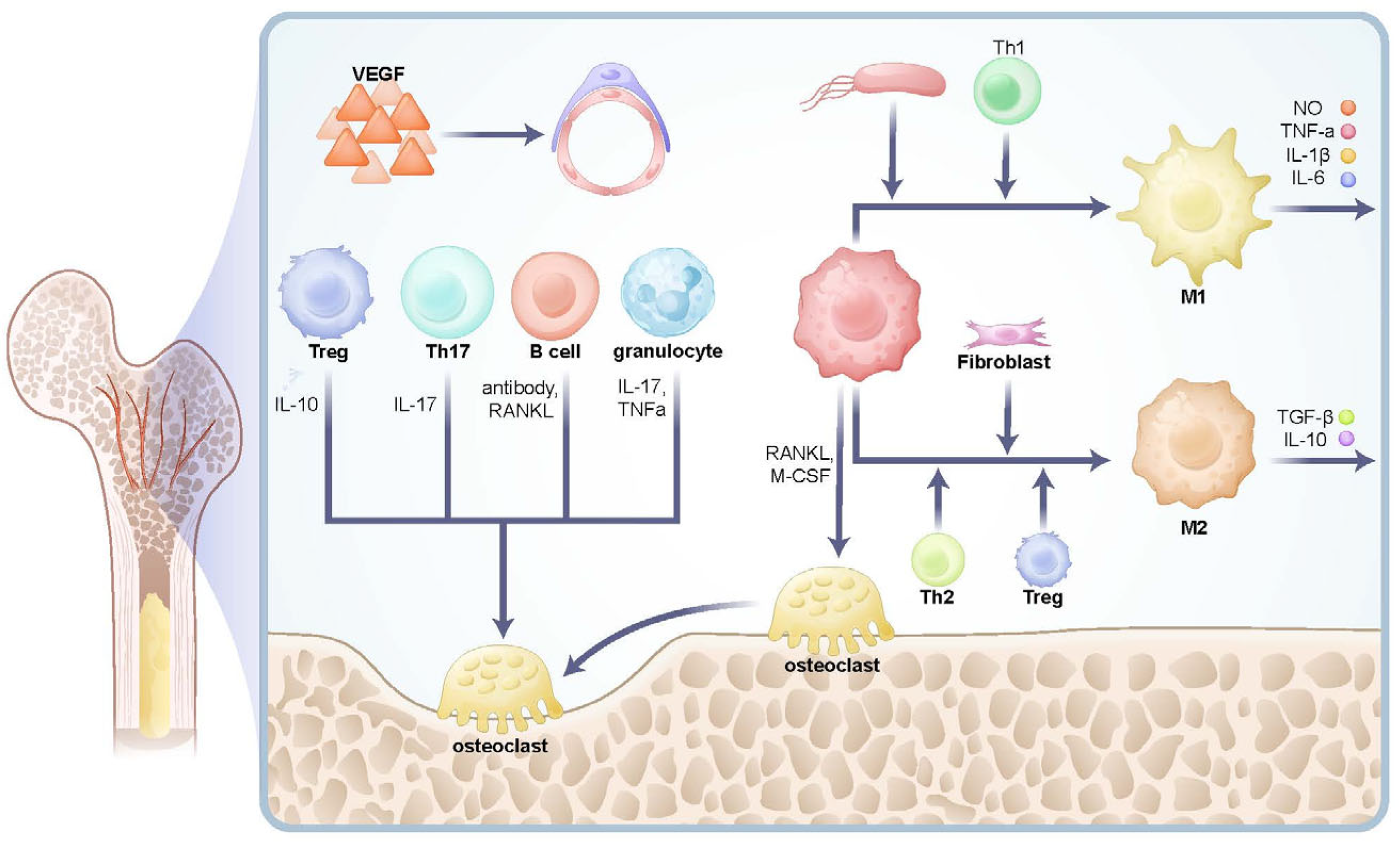
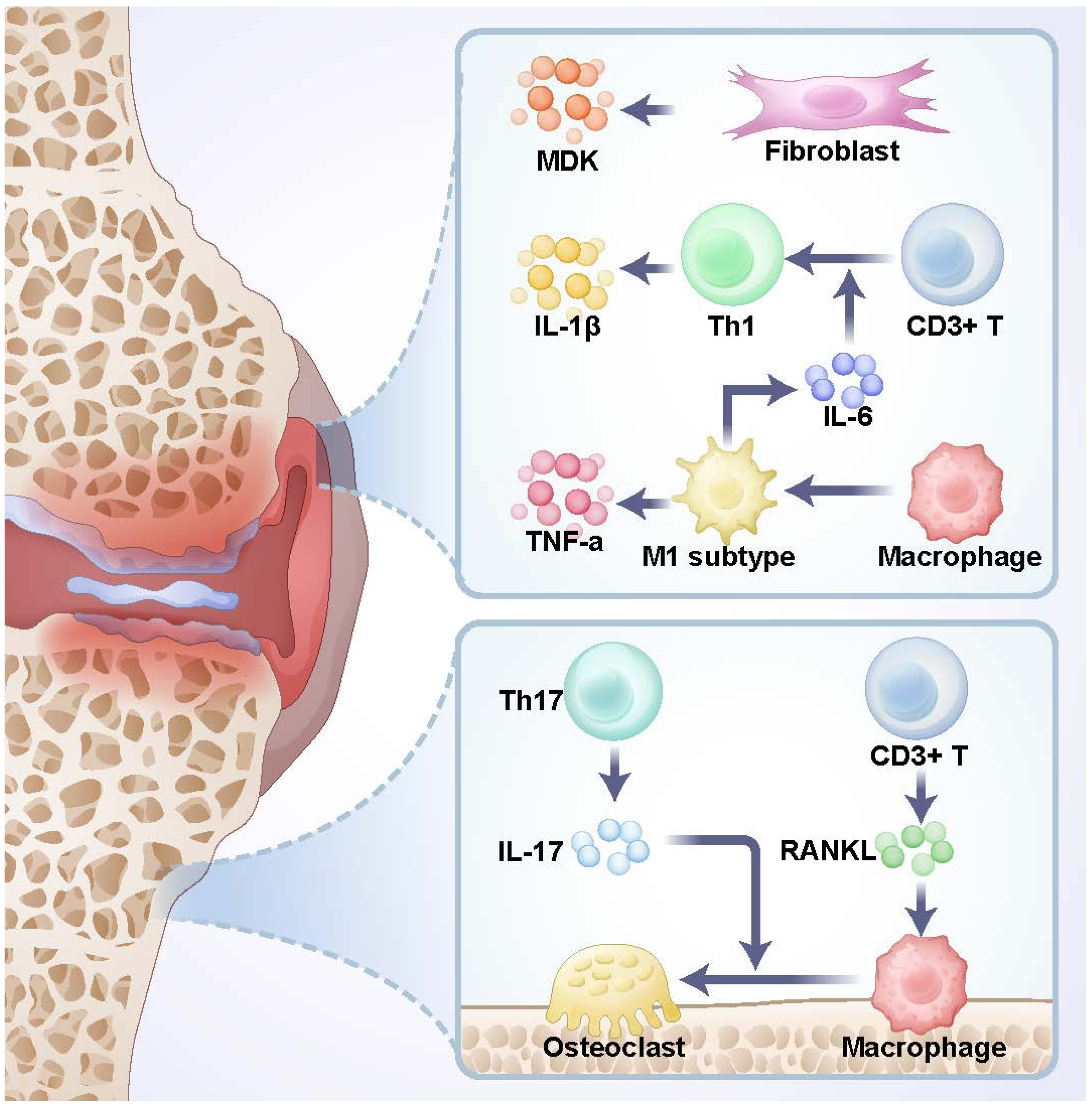
2.2. Immunity in Cancellous Bone Microenvironment
2.3. Differences in the Spatial Microenvironment Between Cancellous Bone and Bone Marrow Cavity
3. Regulation of Bone Immune Homeostasis
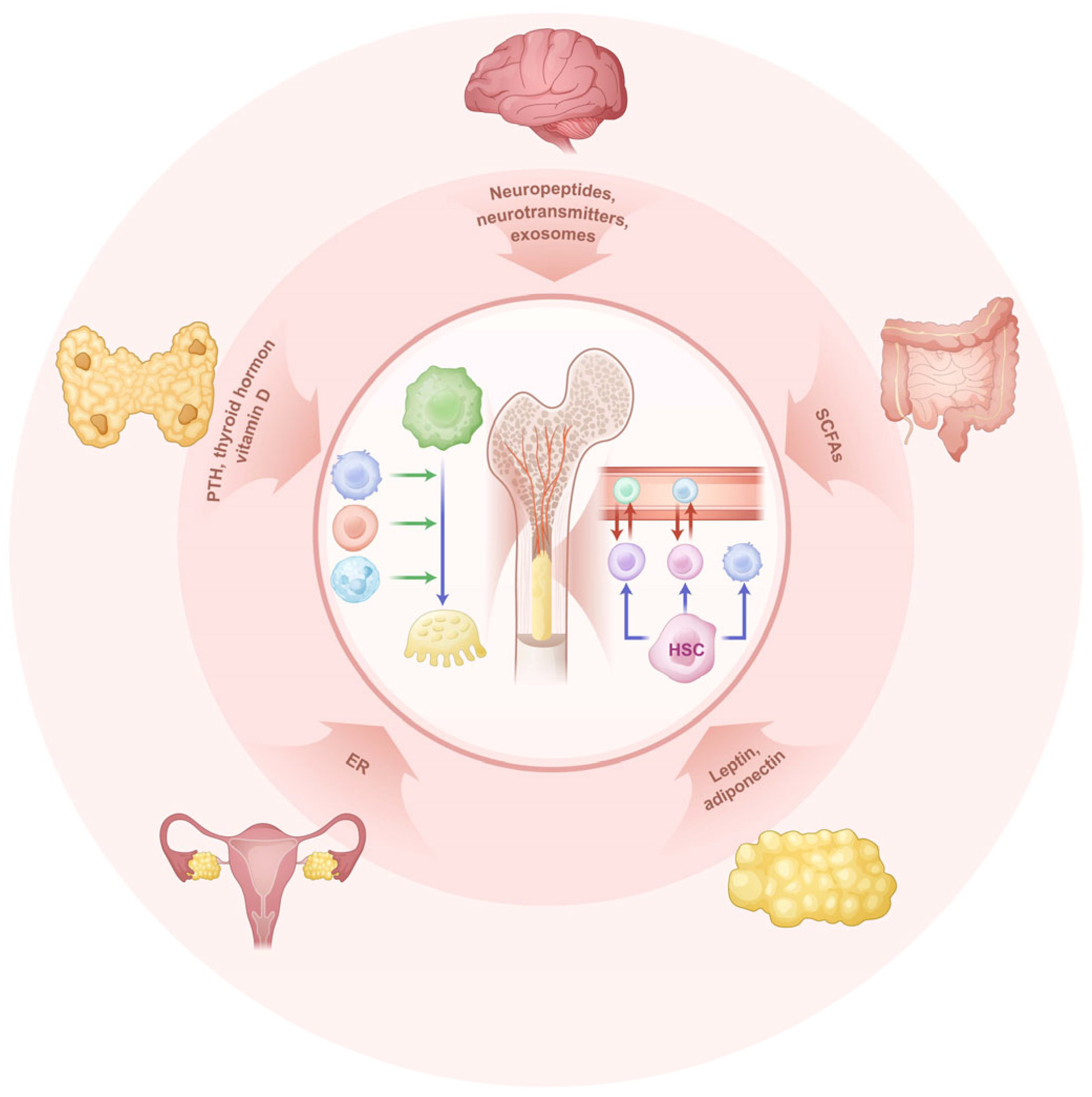
3.1. Brain-Gut-Bone Regulation and Mechanisms in Bone Immunity
3.2. The Tubular System and Bone Immunity
4. Discussion and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, A.F.; Wang, F.X.; Wang, S.C.; Zhang, Y.Z.; Bai, L.; Su, J.C. Bone-organ axes: Bidirectional crosstalk. Mil. Med. Res. 2024, 11, 37. [Google Scholar] [CrossRef]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, 261–262. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Montesi, M.; Mazzoni, E. Advances in Bone Biology. Int. J. Mol. Sci. 2024, 25, 6892. [Google Scholar] [CrossRef] [PubMed]
- Noble, D. Regulation of bone metabolism. Prog. Biophys. Mol. Biol. 2016, 122, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, W. Research progress in Alzheimer’s disease and bone-brain axis. Ageing Res. Rev. 2024, 98, 102341. [Google Scholar] [CrossRef]
- Mitroulis, I.; Hajishengallis, G.; Chavakis, T. Trained Immunity and Cardiometabolic Disease: The Role of Bone Marrow. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 48–54. [Google Scholar] [CrossRef]
- Berclaz, P.Y.; Shibata, Y.; Whitsett, J.A.; Trapnell, B.C. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood 2002, 100, 4193–4200. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2011, 12, 49–60. [Google Scholar] [CrossRef]
- Kalafati, L.; Hatzioannou, A.; Hajishengallis, G.; Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 2023, 314, 142–157. [Google Scholar] [CrossRef]
- Heras-Murillo, I.; Adán-Barrientos, I.; Galán, M.; Wculek, S.K.; Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, L.; Wang, T.; Li, Z. NK cell-based tumor immunotherapy. Bioact. Mater. 2024, 31, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Ng, J.C.; Wallis, G.; Tsioligka, V.; Fraternali, F.; Dunn-Walters, D.K. Single-Cell Transcriptomic Analyses Define Distinct Peripheral B Cell Subsets and Discrete Development Pathways. Front. Immunol. 2021, 12, 602539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Dirkx, L.; Loyens, M.; Van Acker, S.I.; Bulté, D.; Claes, M.; Radwanska, M.; Magez, S.; Caljon, G. Effect of Leishmania infantum infection on B cell lymphopoiesis and memory in the bone marrow and spleen. FASEB J. 2024, 38, e23893. [Google Scholar] [CrossRef]
- Collins, N.; Han, S.J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, E.A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 2019, 178, 1088–1101.e15. [Google Scholar] [CrossRef]
- Di Rosa, F. Two Niches in the Bone Marrow: A Hypothesis on Life-long T Cell Memory. Trends Immunol. 2016, 37, 503–512. [Google Scholar] [CrossRef]
- Day, R.B.; Link, D.C. Regulation of neutrophil trafficking from the bone marrow. Cell Mol. Life Sci. 2012, 69, 1415–1423. [Google Scholar] [CrossRef]
- de Jong, M.M.E.; Chen, L.; Raaijmakers, M.; Cupedo, T. Bone marrow inflammation in haematological malignancies. Nat. Rev. Immunol. 2024, 24, 543–558. [Google Scholar] [CrossRef]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Hu, Y.; Nie, W.; Lyu, L.; Zhang, X.; Wang, W.; Zhang, Y.; He, S.; Guo, A.; Liu, F.; Wang, B.; et al. Tumor-Microenvironment-Activatable Nanoparticle Mediating Immunogene Therapy and M2 Macrophage-Targeted Inhibitor for Synergistic Cancer Immunotherapy. ACS Nano 2024, 18, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Dobersalske, C.; Rauschenbach, L.; Hua, Y.; Berliner, C.; Steinbach, A.; Grüneboom, A.; Kokkaliaris, K.D.; Heiland, D.H.; Berger, P.; Langer, S.; et al. Cranioencephalic functional lymphoid units in glioblastoma. Nat. Med. 2024, 30, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Wear, K.A. Mechanisms of Interaction of Ultrasound With Cancellous Bone: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 454–482. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zhang, Z.; Zhang, J.; Xu, H. Microstructural Analysis of Cancellous Bone in Fluorosis Rats. Biol. Trace Elem. Res. 2023, 201, 4827–4833. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone-Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef]
- Alioli, C.; Demesmay, L.; Peyruchaud, O.; Machuca-Gayet, I. Autotaxin/Lysophosphatidic Acid Axis: From Bone Biology to Bone Disorders. Int. J. Mol. Sci. 2022, 23, 3427. [Google Scholar] [CrossRef]
- Kim, T.S.; Silva, L.M.; Theofilou, V.I.; Greenwell-Wild, T.; Li, L.; Williams, D.W.; Ikeuchi, T.; Brenchley, L.; Bugge, T.H.; Diaz, P.I.; et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J. Exp. Med. 2023, 220, e20221751. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Ilchovska, D.D.; Barrow, D.M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun. Rev. 2021, 20, 102741. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Altomare, E.; Botta, C.; Gallo Cantafio, M.E.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F.; et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Donate, P.B.; Alves de Lima, K.; Peres, R.S.; Almeida, F.; Fukada, S.Y.; Silva, T.A.; Nascimento, D.C.; Cecilio, N.T.; Talbot, J.; Oliveira, R.D.; et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2017120118. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2021, 589, 442–447. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Ni, Q.; Wang, T.; Bao, C.; Geng, Y.; Lu, Y.; Cao, Y.; Li, Y.; Li, L.; et al. B-Cell-Derived TGF-β1 Inhibits Osteogenesis and Contributes to Bone Loss in Periodontitis. J. Dent. Res. 2023, 102, 767–776. [Google Scholar] [CrossRef]
- Jeljeli, M.M.; Adamopoulos, I.E. Innate immune memory in inflammatory arthritis. Nat. Rev. Rheumatol. 2023, 19, 627–639. [Google Scholar] [CrossRef]
- McDonald, M.M.; Khoo, W.H.; Ng, P.Y.; Xiao, Y.; Zamerli, J.; Thatcher, P.; Kyaw, W.; Pathmanandavel, K.; Grootveld, A.K.; Moran, I.; et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 2021, 184, 1330–1347.e13. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Weidner, D.; Kachler, K.; Faas, M.; Grüneboom, A.; Schlötzer-Schrehardt, U.; Muñoz, L.E.; Steffen, U.; Grötsch, B.; et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Investig. 2020, 130, 4811–4830. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, S.; Qiao, J.; Ji, Z.; Zhou, B.; Xu, W. CX3CL1 promotes M1 macrophage polarization and osteoclast differentiation through NF-κB signaling pathway in ankylosing spondylitis in vitro. J. Transl. Med. 2023, 21, 573. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Ma, X.; Yang, Y.; Deng, C.; Sun, M.; Lin, C.; Zhang, L. Periodontal ligament cells-derived exosomes promote osteoclast differentiation via modulating macrophage polarization. Sci. Rep. 2024, 14, 1465. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Li, M.O. Control of tumor-associated macrophage responses by nutrient acquisition and metabolism. Immunity 2023, 56, 14–31. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tong, D.; Song, H.; Ruan, R.; Sun, Y.; Lin, Y.; Wang, J.; Hou, L.; Dai, J.; Ding, J.; et al. Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv. Mater. 2022, 34, e2202044. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Duffy, M.P.; Ahn, K.J.; Sussman, J.H.; Pang, M.; Smith, D.; Duncan, G.; Zhang, I.; Huang, J.; Lin, Y.; et al. Mapping the cellular biogeography of human bone marrow niches using single-cell transcriptomics and proteomic imaging. Cell 2024, 187, 3120–3140.e29. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Juan, C.; Drennon, T.; Uytingco, C.R.; Vishlaghi, N.; Sokolowskei, D.; Xu, L.; Levi, B.; Sammarco, M.C.; Tower, R.J. Spatial transcriptomic interrogation of the murine bone marrow signaling landscape. Bone Res. 2023, 11, 59. [Google Scholar] [CrossRef]
- Iga, T.; Kobayashi, H.; Kusumoto, D.; Sanosaka, T.; Fujita, N.; Tai-Nagara, I.; Ando, T.; Takahashi, T.; Matsuo, K.; Hozumi, K.; et al. Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis. Nat. Cell Biol. 2023, 25, 1415–1425. [Google Scholar] [CrossRef]
- Tilburg, J.; Stone, A.P.; Billingsley, J.M.; Scoville, D.K.; Pavenko, A.; Liang, Y.; Italiano, J.E.; Machlus, K.R. Spatial transcriptomics of murine bone marrow megakaryocytes at single-cell resolution. Res. Pract. Thromb. Haemost. 2023, 7, 100158. [Google Scholar] [CrossRef]
- Xia, W.; Xie, J.; Cai, Z.; Liu, X.; Wen, J.; Cui, Z.K.; Zhao, R.; Zhou, X.; Chen, J.; Mao, X.; et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021, 12, 6043. [Google Scholar] [CrossRef]
- Lin, Z.; Xiong, Y.; Sun, Y.; Zeng, R.; Xue, H.; Hu, Y.; Chen, L.; Liu, G.; Panayi, A.C.; Zhou, W.; et al. Circulating MiRNA-21-enriched extracellular vesicles promote bone remodeling in traumatic brain injury patients. Exp. Mol. Med. 2023, 55, 587–596. [Google Scholar] [CrossRef]
- Lv, X.; Gao, F.; Cao, X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 2022, 34, 1914–1931. [Google Scholar] [CrossRef]
- Oggero, S.; Cecconello, C.; Silva, R.; Zeboudj, L.; Sideris-Lampretsas, G.; Perretti, M.; Malcangio, M. Dorsal root ganglia CX3CR1 expressing monocytes/macrophages contribute to arthritis pain. Brain Behav. Immun. 2022, 106, 289–306. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Huang, J.; Wu, T.; Jiang, Y.R.; Zheng, X.Q.; Wang, H.; Liu, H.; Wang, H.; Leng, H.J.; Fan, D.W.; Yuan, W.Q.; et al. β-Receptor blocker enhances the anabolic effect of PTH after osteoporotic fracture. Bone Res. 2024, 12, 18. [Google Scholar] [CrossRef]
- Liu, W.; Chen, W.; Xie, M.; Chen, C.; Shao, Z.; Zhang, Y.; Zhao, H.; Song, Q.; Hu, H.; Xing, X.; et al. Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing. Signal Transduct. Target. Ther. 2023, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Knapstein, P.R.; Otto, E.; Köhli, P.; Sevecke, J.; Graef, F.; Graffmann, C.; Fuchs, M.; Jiang, S.; Rickert, M.; et al. Increased β(2)-adrenergic signaling promotes fracture healing through callus neovascularization in mice. Sci. Transl. Med. 2024, 16, eadk9129. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, R.; Cai, Z.; Yu, L.; Fei, Y.; Weng, L.; Wang, J.; Ge, X.; Zhu, T.; Wang, J.; et al. Salmeterol attenuates the inflammatory response in asthma and decreases the pro-inflammatory cytokine secretion of dendritic cells. Cell Mol. Immunol. 2012, 9, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Globig, A.M.; Zhao, S.; Roginsky, J.; Maltez, V.I.; Guiza, J.; Avina-Ochoa, N.; Heeg, M.; Araujo Hoffmann, F.; Chaudhary, O.; Wang, J.; et al. The β(1)-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 2023, 622, 383–392. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. Adrenergic Modulation of Hematopoiesis. J. Neuroimmune Pharmacol. 2020, 15, 82–92. [Google Scholar] [CrossRef]
- Akinyemi, D.E.; Chevre, R.; Soehnlein, O. Neuro-immune crosstalk in hematopoiesis, inflammation, and repair. Trends Immunol. 2024, 45, 597–608. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Kiss, M.G.; Rattik, S.; He, S.; Vassalli, A.; Valet, C.; Anzai, A.; Chan, C.T.; Mindur, J.E.; Kahles, F.; et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019, 566, 383–387. [Google Scholar] [CrossRef]
- Schloss, M.J.; Hulsmans, M.; Rohde, D.; Lee, I.H.; Severe, N.; Foy, B.H.; Pulous, F.E.; Zhang, S.; Kokkaliaris, K.D.; Frodermann, V.; et al. B lymphocyte-derived acetylcholine limits steady-state and emergency hematopoiesis. Nat. Immunol. 2022, 23, 605–618. [Google Scholar] [CrossRef]
- Wang, Y.; Anesi, J.C.; Panicker, I.S.; Cook, D.; Bista, P.; Fang, Y.; Oqueli, E. Neuroimmune Interactions and Their Role in Immune Cell Trafficking in Cardiovascular Diseases and Cancer. Int. J. Mol. Sci. 2025, 26, 2553. [Google Scholar] [CrossRef] [PubMed]
- Hedderich, J.; El Bagdadi, K.; Angele, P.; Grässel, S.; Meurer, A.; Straub, R.H.; Zaucke, F.; Jenei-Lanzl, Z. Norepinephrine Inhibits the Proliferation of Human Bone Marrow-Derived Mesenchymal Stem Cells via β2-Adrenoceptor-Mediated ERK1/2 and PKA Phosphorylation. Int. J. Mol. Sci. 2020, 21, 3924. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Báez-Pagán, C.A.; Delgado-Vélez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, F.; D’Souza, A.; Dhakal, B.; Pisano, M.; Chhabra, S.; Stolley, M.; Hari, P.; Janz, S. Autonomic nervous system control of multiple myeloma. Blood Rev. 2021, 46, 100741. [Google Scholar] [CrossRef]
- Gadomski, S.; Fielding, C.; García-García, A.; Korn, C.; Kapeni, C.; Ashraf, S.; Villadiego, J.; Toro, R.D.; Domingues, O.; Skepper, J.N.; et al. A cholinergic neuroskeletal interface promotes bone formation during postnatal growth and exercise. Cell Stem Cell 2022, 29, 528–544.e9. [Google Scholar] [CrossRef]
- Li, F.X.; Xu, F.; Lin, X.; Wu, F.; Zhong, J.Y.; Wang, Y.; Guo, B.; Zheng, M.H.; Shan, S.K.; Yuan, L.Q. The Role of Substance P in the Regulation of Bone and Cartilage Metabolic Activity. Front. Endocrinol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, K.; Chen, X.; Deng, L.; Zhang, L.; Li, Y.; Gao, A.; Gao, J.; Wu, C.; Yang, X.; et al. Neuron-derived neuropeptide Y fine-tunes the splenic immune responses. Neuron 2022, 110, 1327–1339.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Liu, Y.B.; Liu, W.F.; Zhou, Y.Y.; He, H.F.; Lin, S. Neuropeptide Y Is an Immunomodulatory Factor: Direct and Indirect. Front. Immunol. 2020, 11, 580378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.Y.; Liu, Y.W.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Xia, K.; Huang, J.; Liu, X.X.; Hong, C.G.; et al. Neuronal Induction of Bone-Fat Imbalance through Osteocyte Neuropeptide Y. Adv. Sci. 2021, 8, e2100808. [Google Scholar] [CrossRef]
- Pannell, M.; Labuz, D.; Celik, M.; Keye, J.; Batra, A.; Siegmund, B.; Machelska, H. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflamm. 2016, 13, 262. [Google Scholar] [CrossRef]
- Zhou, Y.; Sheng, Y.J.; Li, C.Y.; Zou, L.; Tong, C.Y.; Zhang, Y.; Cao, G.; Shou, D. Beneficial effect and mechanism of natural resourced polysaccharides on regulating bone metabolism through intestinal flora: A review. Int. J. Biol. Macromol. 2023, 253, 127428. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The enteric nervous system. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Our Mental Health Is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nerves, and Gut Microbiota. Int. J. Mol. Sci. 2023, 25, 38. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, L.; Du, M.; Müller, D.; Gu, Y.; Gao, Z.; Xin, X.; Gu, Y.; He, M.; Marquardt, T.; et al. Sacral Neural Crest-Independent Origin of the Enteric Nervous System in Mouse. Gastroenterology 2024, 166, 1085–1099. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Song, P.R.; Wang, S.C.; Liu, H.; Shi, Z.M.; Su, J.C. Diets intervene osteoporosis via gut-bone axis. Gut Microbes 2024, 16, 2295432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Lu, P.P.; Dai, G.C.; Zhang, M.; Wang, H.; Rui, Y.F. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J. Orthop. Translat. 2022, 37, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, C.; Huang, Y.; Yang, J.; Zhou, F.; Zeng, J.; Lin, Y. Jiangu granule ameliorated OVX rats bone loss by modulating gut microbiota-SCFAs-Treg/Th17 axis. Biomed. Pharmacother. 2022, 150, 112975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Zhao, J.; Wang, D.; Liu, H.; Gao, P.; Shen, Y.; Wu, T.; Wu, X.; Zhao, Y.; et al. Ento-A alleviates DSS-induced experimental colitis in mice by remolding intestinal microbiota to regulate SCFAs metabolism and the Th17 signaling pathway. Biomed. Pharmacother. 2024, 170, 115985. [Google Scholar] [CrossRef]
- Wu, Y.L.; Zhang, C.H.; Teng, Y.; Pan, Y.; Liu, N.C.; Liu, P.X.; Zhu, X.; Su, X.L.; Lin, J. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil. Med. Res. 2022, 9, 46. [Google Scholar] [CrossRef]
- Larsen, M.C.; Rondelli, C.M.; Almeldin, A.; Song, Y.S.; N’Jai, A.; Alexander, D.L.; Forsberg, E.C.; Sheibani, N.; Jefcoate, C.R. AhR and CYP1B1 Control Oxygen Effects on Bone Marrow Progenitor Cells: The Enrichment of Multiple Olfactory Receptors as Potential Microbiome Sensors. Int. J. Mol. Sci. 2023, 24, 16884. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Lei, H.; Zhang, C.; Wu, M.; Huang, S.; Li, X.; Xie, D.; Liu, M.; Zhang, L.; et al. Microbial Tryptophan Metabolites Ameliorate Ovariectomy-Induced Bone Loss by Repairing Intestinal AhR-Mediated Gut-Bone Signaling Pathway. Adv. Sci. 2024, 11, e2404545. [Google Scholar] [CrossRef]
- Kong, S.H.; Kim, J.H.; Shin, C.S. Serum Spermidine as a Novel Potential Predictor for Fragility Fractures. J. Clin. Endocrinol. Metab. 2021, 106, e582–e591. [Google Scholar] [CrossRef]
- Ou, Q.; Tang, S.; Zhu, J.; Xue, S.; Huang, H.; Zhao, Y.; Cai, Y.; Wu, C.; Chen, J.; Ruan, G.; et al. Spermidine ameliorates osteoarthritis via altering macrophage polarization. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167083. [Google Scholar] [CrossRef]
- Guan, B.; Li, C.; Yang, Y.; Lu, Y.; Sun, Y.; Su, L.; Shi, G.; Bai, L.; Liu, J.; Meng, A. Effect of spermidine on radiation-induced long-term bone marrow cell injury. Int. Immunopharmacol. 2023, 114, 109557. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Turan, S. Endocrine disrupting chemicals and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef] [PubMed]
- Banoriya, G.K.; Singh, V.K.; Maurya, R.; Kharwar, R.K. Neuro-Immuno-Endocrine Regulation of Bone Homeostasis. Discov. Med. 2025, 37, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Sandor, L.F.; Ragacs, R.; Gyori, D.S. Local Effects of Steroid Hormones within the Bone Microenvironment. Int. J. Mol. Sci. 2023, 24, 17482. [Google Scholar] [CrossRef]
- Lademann, F.; Tsourdi, E.; Rijntjes, E.; Köhrle, J.; Hofbauer, L.C.; Heuer, H.; Rauner, M. Lack of the Thyroid Hormone Transporter Mct8 in Osteoblast and Osteoclast Progenitors Increases Trabecular Bone in Male Mice. Thyroid 2020, 30, 329–342. [Google Scholar] [CrossRef]
- Starosz, A.; Stożek, K.; Opęchowska, A.; Bossowski, F.; Moniuszko, M.; Grubczak, K.; Bossowski, A. Effect of methimazole treatment on Th1, Th17, and Th22 lymphocytes in pediatric Graves’ disease patients. Front. Immunol. 2024, 15, 1431686. [Google Scholar] [CrossRef]
- Yu, M.; Malik Tyagi, A.; Li, J.Y.; Adams, J.; Denning, T.L.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat. Commun. 2020, 11, 468. [Google Scholar] [CrossRef]
- Martin, T.J. PTH1R Actions on Bone Using the cAMP/Protein Kinase A Pathway. Front. Endocrinol. 2021, 12, 833221. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef]
- Marino, S.; Bellido, T. PTH receptor signalling, osteocytes and bone disease induced by diabetes mellitus. Nat. Rev. Endocrinol. 2024, 20, 661–672. [Google Scholar] [CrossRef]
- Ding, P.; Gao, C.; Zhou, J.; Mei, J.; Li, G.; Liu, D.; Li, H.; Liao, P.; Yao, M.; Wang, B.; et al. Mitochondria from osteolineage cells regulate myeloid cell-mediated bone resorption. Nat. Commun. 2024, 15, 5094. [Google Scholar] [CrossRef]
- Wang, G.; Ma, C.; Mo, L.; Chen, J.; Yuan, J.; Xu, J.; He, W. Cycloastragenol prevents bone loss via inhibiting osteoclast activity in glucocorticoid-induced osteonecrosis of the femoral head: An in vivo study. J. Orthop. Translat. 2024, 45, 178–187. [Google Scholar] [CrossRef]
- Chen, M.; Fu, W.; Xu, H.; Liu, C.J. Pathogenic mechanisms of glucocorticoid-induced osteoporosis. Cytokine Growth Factor. Rev. 2023, 70, 54–66. [Google Scholar] [CrossRef]
- Chavakis, T.; Alexaki, V.I.; Ferrante, A.W., Jr. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat. Immunol. 2023, 24, 757–766. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, K.; Upadhyay, J.; Ramirez-Cisneros, A.; Patel, N.; Sahai, A.; Mantzoros, C.S. Leptin physiology and pathophysiology in energy homeostasis, immune function, neuroendocrine regulation and bone health. Metabolism 2024, 161, 156056. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P. Leptin, Adiponectin, and Sam68 in Bone Metastasis from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1051. [Google Scholar] [CrossRef]
- Gao, X.; Murphy, M.M.; Peyer, J.G.; Ni, Y.; Yang, M.; Zhang, Y.; Guo, J.; Kara, N.; Embree, C.; Tasdogan, A.; et al. Leptin receptor(+) cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat. Cell Biol. 2023, 25, 1746–1757. [Google Scholar] [CrossRef]
- Hirakawa, H.; Gao, L.; Tavakol, D.N.; Vunjak-Novakovic, G.; Ding, L. Cellular plasticity of the bone marrow niche promotes hematopoietic stem cell regeneration. Nat. Genet. 2023, 55, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Klibanski, A. Neuroendocrine consequences of anorexia nervosa in adolescents. Endocr. Dev. 2010, 17, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Feng, X.; Wang, L.W.; Zhou, R.; Sun, H.; Chen, X.; Lu, R.B.; Huang, Y.; Guo, Q.; Luo, X.H. Bone marrow immune cells respond to fluctuating nutritional stress to constrain weight regain. Cell Metab. 2023, 35, 1915–1930.e8. [Google Scholar] [CrossRef]
- Clahsen, T.; Hadrian, K.; Notara, M.; Schlereth, S.L.; Howaldt, A.; Prokosch, V.; Volatier, T.; Hos, D.; Schroedl, F.; Kaser-Eichberger, A.; et al. The novel role of lymphatic vessels in the pathogenesis of ocular diseases. Prog. Retin. Eye Res. 2023, 96, 101157. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, B.; Wang, Y.; Jiang, R.; Liu, J.; Wei, Y.; Gao, X.; Zhu, Y.; Wang, X.; Sun, M.; et al. An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature 2023, 622, 834–841. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.; Zhang, C.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef]
- Qin, Q.; Lee, S.; Patel, N.; Walden, K.; Gomez-Salazar, M.; Levi, B.; James, A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022, 54, 1844–1849. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Zhai, D.; Qin, C.; Wang, Y.; Ma, J.; Zhuang, H.; Shi, Z.; Wang, L.; Wu, C. Polyhedron-Like Biomaterials for Innervated and Vascularized Bone Regeneration. Adv. Mater. 2023, 35, e2302716. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L. Gorham-Stout Syndrome: A Case of a 7-Year-Old Girl with Massive Osteolysis and Chylothorax. Am. J. Respir. Crit. Care Med. 2024, 209, 101–103. [Google Scholar] [CrossRef]
- Ozeki, M.; Fujino, A.; Matsuoka, K.; Nosaka, S.; Kuroda, T.; Fukao, T. Clinical Features and Prognosis of Generalized Lymphatic Anomaly, Kaposiform Lymphangiomatosis, and Gorham-Stout Disease. Pediatr. Blood Cancer 2016, 63, 832–838. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, S.; Luo, W.; Gao, X.; Men, Y.; Ma, C.; Liu, X.; Yi, Y.; Bugde, A.; Zhou, B.O.; et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 2018, 28, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Martin, B.L.; Matus, D.Q.; Gao, L. Imaging multicellular specimens with real-time optimized tiling light-sheet selective plane illumination microscopy. Nat. Commun. 2016, 7, 11088. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.; Chen, J.; De Angelis, J.; Singh, A.; Owen-Woods, C.; Ding, Z.; Pujol, J.M.; Kumar, N.; Zeng, F.; Ramasamy, S.K.; et al. Lymphatic vessels in bone support regeneration after injury. Cell 2023, 186, 382–397.e24. [Google Scholar] [CrossRef] [PubMed]
- Jannaway, M.; Iyer, D.; Mastrogiacomo, D.M.; Li, K.; Sung, D.C.; Yang, Y.; Kahn, M.L.; Scallan, J.P. VEGFR3 is required for button junction formation in lymphatic vessels. Cell Rep. 2023, 42, 112777. [Google Scholar] [CrossRef]
- Korhonen, E.A.; Murtomäki, A.; Jha, S.K.; Anisimov, A.; Pink, A.; Zhang, Y.; Stritt, S.; Liaqat, I.; Stanczuk, L.; Alderfer, L.; et al. Lymphangiogenesis requires Ang2/Tie/PI3K signaling for VEGFR3 cell-surface expression. J. Clin. Investig. 2022, 132, e155478. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Zhao, L.; Xing, L.; Xu, H.; Li, N.; Zhao, Y.; Shi, Q.; Liang, Q.; Wang, Y. A novel therapy for fracture healing by increasing lymphatic drainage. J. Orthop. Translat. 2024, 45, 66–74. [Google Scholar] [CrossRef]
- Burgan, J.; Rahmati, M.; Lee, M.; Saiz, A.M. Innate immune response to bone fracture healing. Bone 2025, 190, 117327. [Google Scholar] [CrossRef]
- Hachemi, Y.; Perrin, S.; Ethel, M.; Julien, A.; Vettese, J.; Geisler, B.; Göritz, C.; Colnot, C. Multimodal analyses of immune cells during bone repair identify macrophages as a therapeutic target in musculoskeletal trauma. Bone Res. 2024, 12, 56. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, H.; Yan, X.; Jiang, Z.; Feng, L.; Hu, W.; Fan, Y.; Lin, S.; Li, G. T cell related osteoimmunology in fracture healing: Potential targets for augmenting bone regeneration. J. Orthop. Translat. 2025, 51, 82–93. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Liu, D.; Bai, J.; Haugen, H.J.; Li, B.; Yan, H. Early osteoimmunomodulation by mucin hydrogels augments the healing and revascularization of rat critical-size calvarial bone defects. Bioact. Mater. 2023, 25, 176–188. [Google Scholar] [CrossRef]
- Ben Amara, H.; Martinez, D.C.; Iskhakova, K.; Emanuelsson, L.; Norlindh, B.; Johansson Loo, A.; Wieland, D.C.F.; Zeller-Plumhoff, B.; Willumeit-Römer, R.; Plocinski, T.; et al. Multifaceted bone response to immunomodulatory magnesium implants: Osteopromotion at the interface and adipogenesis in the bone marrow. Biomaterials 2025, 314, 122779. [Google Scholar] [CrossRef]
- Li, J.; Yao, Z.; Liu, X.; Duan, R.; Yi, X.; Ayoub, A.; Sanders, J.O.; Mesfin, A.; Xing, L.; Boyce, B.F. TGFβ1(+)CCR5(+) neutrophil subset increases in bone marrow and causes age-related osteoporosis in male mice. Nat. Commun. 2023, 14, 159. [Google Scholar] [CrossRef]
- Lu, K.; Shi, T.S.; Shen, S.Y.; Shi, Y.; Gao, H.L.; Wu, J.; Lu, X.; Gao, X.; Ju, H.X.; Wang, W.; et al. Defects in a liver-bone axis contribute to hepatic osteodystrophy disease progression. Cell Metab. 2022, 34, 441–457.e7. [Google Scholar] [CrossRef]
- Peng, H.; Hu, B.; Xie, L.Q.; Su, T.; Li, C.J.; Liu, Y.; Yang, M.; Xiao, Y.; Feng, X.; Zhou, R.; et al. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis. Cell Metab. 2022, 34, 1168–1182.e6. [Google Scholar] [CrossRef]
- Ma, C.; Gao, J.; Liang, J.; Wang, F.; Xu, L.; Bu, J.; He, B.; Liu, G.; Niu, R.; Liu, G. CCL12 induces trabecular bone loss by stimulating RANKL production in BMSCs during acute lung injury. Exp. Mol. Med. 2023, 55, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Chalitsios, C.V.; Shaw, D.E.; McKeever, T.M. Risk of osteoporosis and fragility fractures in asthma due to oral and inhaled corticosteroids: Two population-based nested case-control studies. Thorax 2021, 76, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chalitsios, C.V.; McKeever, T.M.; Shaw, D.E. Incidence of osteoporosis and fragility fractures in asthma: A UK population-based matched cohort study. Eur. Respir. J. 2021, 57, 2001251. [Google Scholar] [CrossRef] [PubMed]
- Cowman, S.J.; Koh, M.Y. Revisiting the HIF switch in the tumor and its immune microenvironment. Trends Cancer 2022, 8, 28–42. [Google Scholar] [CrossRef]
- Dong, Y.; Kang, H.; Peng, R.; Liu, Z.; Liao, F.; Hu, S.A.; Ding, W.; Wang, P.; Yang, P.; Zhu, M.; et al. A clinical-stage Nrf2 activator suppresses osteoclast differentiation via the iron-ornithine axis. Cell Metab. 2024, 36, 1679–1695. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, G.; Liu, J.; Su, W.; Li, C.; Liu, Y.; Meng, F.; Zhao, J.; Gao, N.; Chang, Y.; et al. Brain-wide mapping of c-Fos expression in nitroglycerin-induced models of migraine. J. Headache Pain 2024, 25, 136. [Google Scholar] [CrossRef]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef]
- Fan, Y.; Bian, X.; Meng, X.; Li, L.; Fu, L.; Zhang, Y.; Wang, L.; Zhang, Y.; Gao, D.; Guo, X.; et al. Unveiling inflammatory and prehypertrophic cell populations as key contributors to knee cartilage degeneration in osteoarthritis using multi-omics data integration. Ann. Rheum. Dis. 2024, 83, 926–944. [Google Scholar] [CrossRef]
- Pu, H.; Gao, C.; Zou, Y.; Zhao, L.; Li, G.; Liu, C.; Zhao, L.; Zheng, M.; Sheng, G.; Sun, X.; et al. Single cell transcriptome profiling of infrapatellar fat pad highlights the role of interstitial inflammatory fibroblasts in osteoarthritis. Int. Immunopharmacol. 2024, 131, 111888. [Google Scholar] [CrossRef]
- Tang, S.; Yao, L.; Ruan, J.; Kang, J.; Cao, Y.; Nie, X.; Lan, W.; Zhu, Z.; Han, W.; Liu, Y.; et al. Single-cell atlas of human infrapatellar fat pad and synovium implicates APOE signaling in osteoarthritis pathology. Sci. Transl. Med. 2024, 16, eadf4590. [Google Scholar] [CrossRef] [PubMed]
- Sieper, J.; Poddubnyy, D.; Miossec, P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat. Rev. Rheumatol. 2019, 15, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Forte, D.; García-Fernández, M.; Sánchez-Aguilera, A.; Stavropoulou, V.; Fielding, C.; Martín-Pérez, D.; López, J.A.; Costa, A.S.H.; Tronci, L.; Nikitopoulou, E.; et al. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020, 32, 829–843.e9. [Google Scholar] [CrossRef]
- Moore, J.A.; Mistry, J.J.; Hellmich, C.; Horton, R.H.; Wojtowicz, E.E.; Jibril, A.; Jefferson, M.; Wileman, T.; Beraza, N.; Bowles, K.M.; et al. LC3-associated phagocytosis in bone marrow macrophages suppresses acute myeloid leukemia progression through STING activation. J. Clin. Investig. 2022, 132, e153157. [Google Scholar] [CrossRef]
- Lasry, A.; Nadorp, B.; Fornerod, M.; Nicolet, D.; Wu, H.; Walker, C.J.; Sun, Z.; Witkowski, M.T.; Tikhonova, A.N.; Guillamot-Ruano, M.; et al. An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat. Cancer 2023, 4, 27–42. [Google Scholar] [CrossRef]
- Kulasekararaj, A.; Cavenagh, J.; Dokal, I.; Foukaneli, T.; Gandhi, S.; Garg, M.; Griffin, M.; Hillmen, P.; Ireland, R.; Killick, S.; et al. Guidelines for the diagnosis and management of adult aplastic anaemia: A British Society for Haematology Guideline. Br. J. Haematol. 2024, 204, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Rojas-Hernandez, C.; Yee, C. Hematologic complications of immune checkpoint inhibitors. Blood 2022, 139, 3594–3604. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, A.; Pawelec, K.; Szmigielska-Kapłon, A.; Ussowicz, M. The state of the art in the treatment of severe aplastic anemia: Immunotherapy and hematopoietic cell transplantation in children and adults. Front. Immunol. 2024, 15, 1378432. [Google Scholar] [CrossRef] [PubMed]
- Specht, A.J.; Hoover, C.; Grier, T. Portable x-ray fluorescence for bone lead measurement: Current approaches and future directions. Curr. Environ. Health Rep. 2024, 11, 443–451. [Google Scholar] [CrossRef]
- Pulous, F.E.; Cruz-Hernández, J.C.; Yang, C.; Kaya, Ζ.; Paccalet, A.; Wojtkiewicz, G.; Capen, D.; Brown, D.; Wu, J.W.; Schloss, M.J.; et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat. Neurosci. 2022, 25, 567–576. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, C.; Dai, Y.; Tang, T.; Hou, C.; Yang, H.; Wang, Y.; Xu, J.; Lu, Y.; Wang, Y.; et al. Single-cell, single-nucleus and xenium-based spatial transcriptomics analyses reveal inflammatory activation and altered cell interactions in the hippocampus in mice with temporal lobe epilepsy. Biomark. Res. 2024, 12, 103. [Google Scholar] [CrossRef]

| Source | Molecular Classification | Molecular | Target | Function | References |
|---|---|---|---|---|---|
| The traumatized brain | Exosomes | miR-21-5p, miR-328a-3p, miR-150-5p | SMAD7, FOXO4, CBL | accelerating bone regeneration | [49,50] |
| Sympathetic nervous system | Neurotransmitters | Norepinephrine | β2-AR, β1-AR, β3-AR | Enhances RANKL-induced osteoclastogenesis and bone resorption; | [54,55,56,57,58] |
| Parasympathetic nervous system | Neurotransmitters | Acetylcholine | α7nAChR | Suppresses M1 macrophage polarization; reduces IL-17 secretion by Th17 | [65,66,67] |
| Sensory nerves | Neuropeptide | Substance P | Bone and cartilage cells | Regulates bone metabolism and fracture healing via autocrine/paracrine | [70,71] |
| Sympathetic nerves | Neuropeptide | NPY | Y1, Y2, Y5 receptors | Inhibits immune cell activation; reduces bone resorption | [73,74] |
| Central nervous system | Endogenous opioids | Enkephalins | μ-opioid receptors | Modulates sympathetic, parasympathetic activity | [75] |
| Gut microbiota | Metabolites (SCFAs) | Butyrate, Propionate | HDACs | Inhibit Th17-derived IL-17; promote Treg proliferation and activation. | [82,83,84,85] |
| Gut microbiota | Tryptophan metabolites | Tryptophan metabolites | aryl hydrocarbon receptor | Promote Treg differentiation; Th17 cell activity; drive M2 and suppress macrophage polarization | [86,87] |
| Gut microbiota | Polyamines | Spermidine | Foxp3 expression | promote Treg proliferation and differentiation; reduce the secretion of IL-17 | [88,89] |
| Gut MALT | Mucosal immune system | sIgA | Pro-inflammatory factors | Downregulates RANKL and suppresses osteoclast activation | [90] |
| Ovaries | Hormone | Estrogen | ERα, ERβ receptors | Inhibits osteoclast activity; promotes Treg proliferation | [93] |
| Thyroid gland | Hormone | T3/T4 | Thyroid hormone receptors | Promotes osteoclast formation; regulates Th1 activity | [96,97] |
| Parathyroid gland | Hormone | Parathyroid hormone | PTH1R | Enhances Th1/Th17 proliferation; increases RANKL expression | [98,99] |
| Adipocytes | Adipokine | Leptin | Ob-R receptor | Stimulates Th1/Th17 differentiation; enhances osteoclast activity | [110,111,112] |
| Adrenal glands | Hormone | Cortisol | Glucocorticoid receptor | Suppresses osteoblast function; enhances osteoclast activity and bone loss | [102,103,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, H.; Ding, L.; Jiang, P.; Li, G.; Wang, K.; Jiang, J.; Gan, X. New Insight into Bone Immunity in Marrow Cavity and Cancellous Bone Microenvironments and Their Regulation. Biomedicines 2025, 13, 2426. https://doi.org/10.3390/biomedicines13102426
Pu H, Ding L, Jiang P, Li G, Wang K, Jiang J, Gan X. New Insight into Bone Immunity in Marrow Cavity and Cancellous Bone Microenvironments and Their Regulation. Biomedicines. 2025; 13(10):2426. https://doi.org/10.3390/biomedicines13102426
Chicago/Turabian StylePu, Hongxu, Lanping Ding, Pinhui Jiang, Guanghao Li, Kai Wang, Jiawei Jiang, and Xin Gan. 2025. "New Insight into Bone Immunity in Marrow Cavity and Cancellous Bone Microenvironments and Their Regulation" Biomedicines 13, no. 10: 2426. https://doi.org/10.3390/biomedicines13102426
APA StylePu, H., Ding, L., Jiang, P., Li, G., Wang, K., Jiang, J., & Gan, X. (2025). New Insight into Bone Immunity in Marrow Cavity and Cancellous Bone Microenvironments and Their Regulation. Biomedicines, 13(10), 2426. https://doi.org/10.3390/biomedicines13102426






