Abstract
Objectives: miR-21-5p, miR-145-5p and miR-382-5p have been associated with angiogenesis, which plays a central role in tumor growth and metastasis formation. The aim of the study was to determine whether expression of these three potentially angiogenic miRNAs is related to the lymphatic spread capability of gastric adenocarcinoma and patient survival. Methods: Pathoclinical data of 123 patients who underwent elective gastric resection for adenocarcinoma between 1 August 2006 and 31 December 2013 were retrospectively retrieved. The major concerns were the total number of lymph nodes retrieved, the number of positive nodes, depth of the tumor invasion to the stomach wall, pTNM stage of the disease, Lauren histological tumor type, presence of a mucinous component in the cancer tissue, tumor location in the stomach and survival outcome. The cancer tissues of patients were examined for the expression levels of miR-21-5p, miR-145-5p and miR-382-5p. Results: Elevated hsa-miR-21-5p expression levels and downregulated hsa-miR-145-5p levels were observed in patients with a higher pT stage, lymph node metastasis and advanced pTNM stage. Additionally, hsa-miR-145-5p expression was lower in patients with cardia involvement and a Lauren intestinal-type carcinoma. hsa-miR-382-5p levels were higher in patients with non-mucinous gastric carcinoma. Both hsa-miR-145-5p and hsa-miR-21-5p were predictors of the presence of node metastasis, even when adjusted for pT status. hsa-miR-145-5p was significantly associated with improved survival. hsa-miR-145-5p was significantly associated with an increased probability of surviving 3 years, while increased hsa-miR-21 expression was significantly associated with reduced 3-year survival. All these associations were confirmed in multivariate models, which also included pT and M staging. Conclusions: The upregulation of miR-21-5p and downregulation of miR-145-5p are independent prognostic factors for lymph node metastasis and could serve as specific biomarkers of the lymphatic spread of gastric adenocarcinoma. miR-145-5p downregulation is an independent prognostic factor for overall survival.
1. Introduction
Despite the declining incidence and development of new treatment modalities, gastric cancer remains one of the deadliest oncological challenges [1]. Biological heterogeneity of the disease restricts prognostic predictions. One of the main causes of treatment failure is intense lymph node metastasis formation [2]. It is difficult to diagnose at an early stage, and the molecular pathways underlying lymphatic spread remain poorly understood [3]. Therefore, there is an increasing demand for novel biomarkers to predict the occurrence of lymph node metastases. Proper anticipation of lymphatic spread capability could increase the efficacy of comprehensive personalized patient management.
MicroRNAs are small non-coding nucleotides responsible for homeostatic regulation of gene expression and adaptation to transient changes in the cell microenvironment [4,5,6]. In pathological conditions, they play a remarkable role in tumorigenesis by altering the expression of oncogenes and tumor suppressors [5,6,7]. Specific miRNAs have been found to be related to tumor formation [4,8,9,10], angiogenesis [3,4,8,10,11,12], lymphatic spread [3], survival [4,13] and response to treatment [10,11,12,14]. Disorders of miRNA have been found in almost all types of cancer, and in every specific tumor, there is a distinct microRNA signature [4,8]. Two features make microRNAs unique: (i) stability in the tumor tissue and (ii) the possibility of miRNA modulation to reverse their oncogenic features [7]. Both are recognized as having substantial clinical potential as novel prognostic and predictive biomarkers in cancer diagnosis and treatment [4,8,9,10,15,16,17]. Their true diagnostic potential must be challenged in future clinical trials, and a combination of the most promising miRNAs as biomarkers with well-recognized pathoclinical parameters can enhance their value [6].
miR-21-5p, miR-145-5p and miR-382-5p have been associated with angiogenesis, which plays a central role in tumor growth and metastasis formation [7,10,11,12,18,19]. After reaching a diameter of about 1 mm, further expansion of the primary tumor is dependent on its own blood vessel formation [20]. For most carcinomas, including gastric cancer, lymphatic spread is preferential in comparison with blood vessel pathway dissemination, although lymphangiogenesis occurs after angiogenesis, and the formation of lymphatic spread is dependent on both angiogenetic and lymphangiogenetic factors [20].
The aim of the study was to determine whether expression of these three potentially angiogenic miRNAs is related to the lymphatic spread capability of gastric adenocarcinoma and patient survival in a population of Caucasian gastric cancer patients.
2. Materials and Methods
The analysis was designed to include all consecutive adult patients who underwent elective gastric resection for adenocarcinoma between 1 August 2006, and 31 December 2013, at the Oncological Surgery Department of the Medical University of Gdańsk. The exclusion criterion was the coexistence of any other malignancy, including lymphoma and stromal or neuroendocrine tumors.
The pathoclinical data of the patients were retrospectively retrieved, and all samples were the subject of a thorough histopathological reexamination at the Department of Pathomorphology, Medical University of Gdańsk. The major concerns were the total number of lymph nodes retrieved, the number of positive nodes, depth of the tumor invasion to the stomach wall, pTNM stage of the disease according to the 8th edition of the AJCC Cancer Staging Manual [21], Lauren histological tumor type, presence of mucinous component in the cancer tissue, tumor location in the stomach and survival outcome.
MicroRNA assays were performed at the Molecular Oncology and Genetics Department, IFM, Łukaszczyk Oncology Centre in Bydgoszcz.
The study was approved by the Independent Ethics Committee at the Medical University of Gdańsk (NKBBN/90/2017).
Among 144 archival primary tumor specimens, 20 cases were excluded because of insufficient tumor material (19 cases) or concomitant gastric lymphoma (1 case). Because of the failure of a miRNA assessment in one of the specimens, the final studied material consisted of 123 cases.
For statistical reasons, we combined patients with pTis-2 stages into one group and pT3-4 into the second group. Similarly, we combined TNM stages I and II into one group and III and IV into the second group.
Mortality data were acquired from the Polish Ministry of Digitalization on 1 November 2023.
Isolation of microRNA derived from 32 gastric adenocarcinoma patients (three groups: pT1-T2N1, pT3-T4N0 and pT3-T4N3) was performed using the miRCURY RNA isolation kit dedicated for FFPE samples (Exiqon, Qiagen, Copenhagen, Denmark), and quality and quantity control were performed (Bioanalizator, Agilent, Santa Clara, CA, USA). Some of the FFPE samples derived between 2007 and 2013 yield degraded RNA, which could affect NGS and qPCR accuracy; therefore, not all 32 samples were taken for NGS analysis. Firstly, miRNA quality was evaluated, and we chose 20 of 32 FFPE samples based on spectrophotometry. The final decision on which samples should be selected for NGS was based on a miRNA quality analysis performed using an Agilent 2100 Bioanalyzer. Therefore, 10 of 32 of the samples with the highest microRNA abundance and the best miRNA quality, isolated from the FFPE samples derived between 2007 and 2013 and representing all three groups, were chosen for microRNA next-generation sequencing profiling. RIN analysis was performed in service.
A total of 100 ng of total RNA was converted into microRNA NGS libraries (Exiqon Services, Qiagen, Copenhagen, Denmark). Adapters were ligated to the RNA, and then the RNA was converted to cDNA. The cDNA was amplified using PCR (18 cycles), during which the PCR indices were added. After the PCR, the samples were purified. Library preparation QC was performed using the Bioanalyzer 2100 (Agilent). Based on the quality of the inserts and the concentration measurements, the libraries were pooled in equimolar ratios. The pool was then size-selected using the LabChip XT (PerkinElmer, Singapore), aiming to select the fraction with the size corresponding to the microRNA libraries (~145 nt). The library pool(s) were quantified using the qPCR KAPA Library Quantification Kit (KAPA Biosystems, Wilmington, MA, USA). The library pool was then sequenced on a NextSeq 500 (Illumina, San Diego, CA, USA) sequencing instrument according to the manufacturer’s instructions.
For each sample, an average of 10 microRNA reads was obtained using 51-nucleotide single-end sequencing. Following sequencing, intensity correction, base calling and assigning of Q-scores was performed. Subsequently, data quality was checked. All 10 samples chosen for NGS sequencing showed overall high quality, with the vast majority of the data obtained presenting a Q-score of more than Q30. The average reads Q-scores and base Q-scores of the NGS sequencing data for the samples are presented in Supplementary Figure S1 and Figure S2, respectively. After sequencing, the adapters were trimmed off as part of the base calling. Trimming of the adapters from the dataset revealed one distinct peak representing microRNA (~18–23 nt). On the other hand, as expected, sequencing of old archival material showed additional reads of other lengths, possibly reflecting degradation products from other RNA species. Comparison of experimental groups was performed.
Using NormFinder (MOMA, Aarhus, Denmark) analysis, the most stably expressed microRNA in the gastric adenocarcinoma FFPE samples based on microRNA NGS analysis was established. We chose hsa-miR-30c-5p, hsa-miR-125b-5p and has-miR125a-5p based on the NGS experimental approach and U6, UniSP3 and UniSp6 as control microRNAs for qPCR normalization. For the selected microRNA validation from the NGS data, we chose microRNAs based on more than 2-fold up or downregulation, together with supporting evidence in the literature. A total of 11 microRNAs were selected for validation using qPCR NGS on a miRCURY LNA miRNA custom PCR panel with exiLENT SYBR Green Master Mix kit (Exiqon): hsa-miR-375, hsa-miR-145-5p, hsa-miR-21-5p, hsa-miR-187-3p, hsa-miR-196a-5p, hsa-miR-708-3p, hsa-miR-142-3p, hsa-miR-27b-5p, hsa-miR-552-3p, hsa-miR-382-5p and hsa-miR-128-3p. MicroRNA expression was assessed in 124 samples due to assessment failure in one specimen; the final studied material consisted of 123 cases. For the purpose of this study, we chose to describe the role of three miRNAs only, which, according to the literature, have been found to be related to angiogenesis: hsa-miR-21-5p, hsa-miR-145-5p and hsa-miR-382-5p.
Statistical analysis was conducted in R (version 4.3.2). Fisher’s test was used to assess binary variables. The Freeman–Halton extension was used for categorical variables with multiple levels. For quantitative variables, the normality of distribution was evaluated using the Shapiro–Wilk test. Since the miRNA expression data did not follow a normal distribution, the Wilcoxon test was used for pairwise comparisons. For comparisons with multiple groups, the Kruskal–Wallis test was used. The Schoenfeld test was used to assess the proportionality of hazards; the proportional hazards assumption was met for all covariates in both univariate and multivariate Cox regression models (p > 0.05 for hsa-miR-21-5p [univariate: 0.2373; multivariate: 0.1871], hsa-miR-145-5p [univariate: 0.5245; multivariate: 0.5819] and hsa-miR-382-5p [univariate: 0.7804; multivariate: 0.8543]). Hazard ratios were evaluated using Cox regression models. Logistic regression models were built to assess odds ratios. Correlations were assessed using the Pearson method if the data followed a normal distribution, and the Spearman method if otherwise. No data imputation was used. p < 0.05 was considered significant.
3. Results
3.1. Characteristics of the Study Subjects
The mean (median) age of patients in the studied group was 62.7 (63) years. There were 87 (70.7%) males and 36 (29.3%) females. All 123 patients underwent major surgical resection for gastric adenocarcinoma; there were a total of 104 (84.6%) gastrectomies and 19 (15.4%) subtotal resections. The extent of lymphadenectomy reported by the operating surgeon was D2 in 21 (17.1%) and D1 or D1+ in 122 (82.9%) patients. The cardia was involved in 42 (34.1%) cases, and the remaining tumors were located distally in the stomach. Data from histopathological reports and survival are collected in Table 1.

Table 1.
Histopathological characteristics and survival in the studied group.
3.2. Univariate Analysis of the Correlation Between miRNA and Pathological Characteristics
Elevated hsa-miR-21-5p expression levels and downregulated hsa-miR-145-5p levels were observed in patients with a higher pT stage, lymph node metastasis and more advanced pTNM disease stage. Additionally, hsa-miR-145-5p expression was lower in patients with cardia involvement and Lauren intestinal-type carcinoma. A weak negative correlation was observed between has-miR-145-5p expression level and the number of nodes with metastasis. hsa-miR-382-5p levels were higher in patients with non-mucinous gastric carcinoma. Additionally, hsa-miR-382-5p expression was weakly positively correlated with the number of nodes with metastasis. No correlations were found between node ratio (ratio between the positive nodes and the total number of nodes resected) and any of the considered miRNAs. The statistics are presented in Table 2 and Table 3.

Table 2.
miRNA expression levels depending on the clinical and histopathological features.

Table 3.
Correlation between miRNA expression levels and number of positive nodes.
3.3. Multivariate Analysis of the Correlation Between miRNA and Pathological Characteristics
We further investigated the associations between miRNA expression levels and lymph node metastasis using logistic regression analyses. In univariate models, a doubling in hsa-miR-145-5p expression was associated with reduced odds of node metastasis presence (OR 0.572, 95% CI: 0.36–0.86, p = 0.0099), whereas a doubling in hsa-miR-21-5p expression was linked to increased odds (OR 1.646, 95% CI: 1.10–2.53, p = 0.0178). These findings persisted in multivariate models adjusted for pT status (hsa-miR-145-5p: adjusted OR 0.597, 95% CI: 0.38–0.89, p = 0.0174; hsa-miR-21-5p: adjusted OR 1.613, 95% CI: 1.07–2.49, p = 0.025). The relationship between miRNA expression and the number of node metastases was also evaluated. Neither hsa-miR-145-5p nor hsa-miR-21-5p significantly predicted the metastasis count. However, a doubling of hsa-miR-382-5p expression was significantly associated with a doubling of the number of node metastases in multivariate models adjusted for pT status (p = 0.0351; results not tabulated). Sex did not emerge as a significant covariate in any models, while age was a significant prognostic factor in several; however, inclusion of age and sex resulted in only minor changes to the miRNA effect estimates, with no impact on their statistical significance. Statistics for the presence of node metastasis (adjusted for tumor characteristics) are presented in Table 4. Detailed results from these additional analyses (further adjusted for age and sex) are provided in Table 5.

Table 4.
Univariate and multivariate hazard/odds ratios for miRNA expression levels as predictors of survival and presence of node metastasis in gastric adenocarcinoma (multivariate models adjusted for tumor characteristics: pT status for node metastasis; pT and M staging for survival outcomes).

Table 5.
miRNA levels as predictors of survival and nodal involvement in gastric adenocarcinoma: univariate and multivariate hazard/odds ratios (adjusted for patient age, sex, pT status for node metastasis; pT and M staging for survival outcomes).
3.4. Analysis of the Correlation Between miRNA and Survival
We then analyzed the impact of selected miRNA expression levels on overall survival using univariate Cox proportional hazards regression models for each miRNA. Notably, higher hsa-miR-145-5p expression was significantly associated with improved survival, with a hazard ratio (HR) of 0.78 (95% CI: 0.64–0.95, p = 0.013) per doubling of expression levels. This association persisted in multivariate Cox models adjusted for pT and M staging (adjusted HR: 0.79, 95% CI: 0.65–0.97, p = 0.025).
We next evaluated the influence of miRNA expression on the probability of surviving specific time points (1, 2, 3, 4, and 5 years) using logistic regression. No miRNAs showed significant associations with 1-year (all p > 0.1) or 2-year survival (hsa-miR-145-5p: OR 1.441, 95% CI: 1.00–2.12, p = 0.0546 per doubling; results not tabulated). However, higher hsa-miR-145-5p expression was linked to an increased likelihood of 3-year survival (OR 1.863, 95% CI: 1.28–2.82, p = 0.0019), while elevated hsa-miR-21-5p expression was associated with reduced 3-year survival (OR 0.651, 95% CI: 0.45–0.93, p = 0.0219). These findings were robust in multivariate models adjusted for pT and M staging. For 4- and 5-year survival, hsa-miR-145-5p was significant in univariate analyses (4-year: OR 1.738, 95% CI: 1.20–2.61, p = 0.005; 5-year: OR 1.521, 95% CI: 1.06–2.25, p = 0.0273), but only the 4-year association was confirmed in multivariate models (adjusted OR 1.700, 95% CI: 1.16–2.58, p = 0.0087; 5-year adjusted OR 1.459, 95% CI: 1.01–2.18, p = 0.0539, approaching significance). hsa-miR-382-5p expression showed no significant associations with any survival outcomes. Detailed statistics are presented in Table 4 (adjusted for tumor characteristics) and Table 5 (additionally adjusted for age and sex). Kaplan–Meier survival curves are shown in Figure 1.
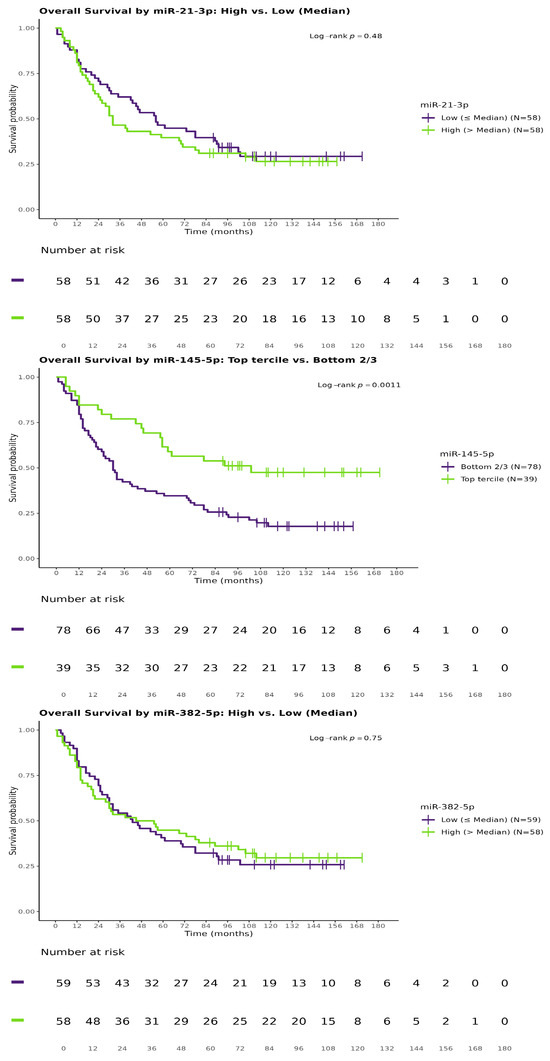
Figure 1.
Kaplan–Meier curves for survival probability.
4. Discussion
Recently, the Nobel Prize was awarded (jointly to Victor Ambros and Gary Ruvkun) for the discovery of microRNA and its role in post-transcriptional gene regulation, but a detailed understanding of the role of microRNA in carcinogenesis is still ongoing [22]. The influence of miR-21 on gastric cancer progression has been well documented [6,9]. Simultaneous suppression of miR-21-5p combined with miR-145-5p overexpression in vitro has been shown to decrease the proliferation of gastric cancer cells more efficiently than miR-21-5p suppression alone [23]. It was shown in vivo and in vitro that miR-145-5p is downregulated in gastric cancer and that it inhibits gastric cancer formation [10,17]. Our findings showed that the upregulation of miR-21-5p and downregulation of miR-145-5p in primary tumor cells have a statistically significant association with lymph nodes metastasis formation, independent of the depth of the primary tumor invasion into the stomach wall (pT stage). The impact of miR-382-5p expression on the presence of lymph node metastasis did not reach the level of statistical significance (p = 0.09); however, upregulation of miR-382-5p was statistically correlated with the number of positive nodes (Table 3). Multiple studies have demonstrated an association between numerous miRNAs and lymphatic status in univariate analyses [24,25,26,27]. It should be noted that higher pT status in gastric cancer increases the risk of lymph node involvement as a natural consequence of cancer progression, and up- or downregulation of these miRNAs might be associated with—or occur in the environment of—the more advanced tumor [28]. We also found an association between miR-21-5p upregulation and miR-145-5p downregulation and poor survival. For miR-21-5p, statistical calculations showed significant associations with reduced 3-year survival, both in the univariate and multivariate models, while for miR-145-5p, its downregulation had a significant impact on worse 3-year, 4-year, 5-year and overall survival. All associations were confirmed in the multivariate models, except for 5-year survival.
miR-21 belongs to the best-characterized cancer-associated microRNA, and the oncogenic pathways activated by miR-21 have already been quite widely described [4,5,6,8,9,14,23]. miR-21 is known to be associated with the regulation of multiple tumorigenic gene expressions, and it was found to be upregulated in nearly all solid tumors, including gastric cancer [3,14,16,23,29]. Its downregulation in saliva may indicate gastric cancer occurrence [15]. Estimated miR-21-5p levels in extracellular vesicles from the blood serum of gastric cancer patients may serve as a promising predictive and prognostic factor [16]. miR-21-5p is significantly elevated in the serum of cancer patients before surgery compared with its expression in healthy volunteers and significantly decreased after surgery [16]. In one study, it was overexpressed in 92% of gastric cancer cases [30]. The level of miR-21 expression is significantly related to the advanced stage of the disease [3]; however, it is also frequently overexpressed in Helicobacter-pylori-infected mucosa [14], suggesting the occurrence of miRNA dysregulation at an early stage of the disease. An in vitro study showed that miR-21 had been induced by Helicobacter pylori infection, and knockdown of miR-21 had remarkably decreased gastric cell invasion and migration and increased apoptosis [14]. Therefore, miR-21 could have multiple functions in both cancer genesis and progression [5].
To our knowledge, only one other study has, to date, reported that high expression of miR-21 is an independent prognostic factor for lymphatic spread capability [31]. In the study conducted on 86 gastric adenocarcinoma specimens, it was found in the multivariate analysis that high expression of miR-21 was related to lymph node metastasis and a higher pTNM score [31].
A meta-analysis of eight studies concerning the prognostic value of miR-21 found an association between high miR-21 expression and poor tumor differentiation, lymph node metastasis, higher TNM stage and poor survival [25]. The authors, however, did not carry out a multivariate analysis to conclude whether miR-21 could work as an independent prognostic factor for lymphatic spread [25]. An original study on 50 gastric cancer specimens found an association between high expression of miR-21 and metastases, as well as overall survival, but also between high miR-21 expression and tumor size [26]. In another meta-analysis concerning, among others, miR-21 and miR-145, a high expression of miR-21 and low expression of miR-145 were associated with shorter overall survival [13].
In contrast to miR-21, miR-145 is usually downregulated in gastric cancer tissue, and its overexpression suppresses tumorigenesis [10,12,17]. In a study involving 145 patients, low expression of miR-145-5p was significantly associated with both lymph node and distant metastasis, as well as overall survival. In their multivariate analysis, miR-145-5p was an independent prognostic factor for overall survival only [27].
The results of another study suggest that miR-145-5p targets SERPINE1 and inhibits gastric cancer development [17]. It was also shown that miR-145-5p affects gastric cancer progression via the ANGPT2/NLR axis and participates in angiogenesis, proliferation and migration of cancer cells [12]. In another study, miR-145 was found to increase the sensitivity of gastric adenocarcinoma cell line MKN-1 to 5-FU, which remains one of the main chemotherapeutic agents applied in gastric cancer [32].
What seems to be very interesting about this particular tumor-suppressing miRNA is that it was found to be overexpressed in stromal fibroblasts with a scirrhous-type histology of gastric adenocarcinoma, which is well recognized to be associated with extremely poor patient prognosis [33]. The explanation given by the authors is that it participates in the transforming growth factor-β pathway by enhancing the expression of α-smooth muscle actin, which eventually results in the activation of peri-tumoral fibroblasts and cancer progression [33]. In our study, apart from the impact on lymphatic spread, we found an association between miR-145-5p downregulation and cardia involvement and intestinal type according to the Lauren classification.
In contrast to miR-21 and miR-145, miR-382 is not well described in the literature. It belongs to non-coding RNAs that induce ferroptosis, a type of non-apoptotic cell death [34]. It was chosen for this study because it has been known to promote angiogenesis [19]. In an experimental study on MKN-1 human gastric cancer cell lines, the authors found that miR-382 upregulation had been induced by hypoxia, and it had acted like an angiogenic oncogene by repressing the well-recognized tumor suppressor, the phosphatase and tensin homolog (PTEN) gene [19]. Hypoxia is known to increase angiogenesis, limit the efficacy of oncologic treatment and the adaptation of malignant cells to hypoxic conditions, all of which result in an increase in tumor aggressiveness [35]. The same authors working on the clinical material of gastric adenocarcinoma found a correlation between the expression of miR-382 and higher T-stage, N-stage, pTNM stage, lymphovascular, venous and perineural invasion, as well as overall survival [35]. However, the results of their study were not subjected to multivariate analysis, and the real impact of miR-382 upregulation on gastric cancer progression and prognosis in their material remained unknown [35]. In our study, upregulation of miR-382 was statistically related only to the number of metastatic nodes, and we failed to demonstrate the impact of miR-382 on the presence of lymph node metastasis in both univariate and multivariate models. However, downregulation of miR-382 was associated with the mucinous component of gastric tumors.
The results of another study concerning the impact of different microRNAs on lymph node involvement in gastric cancer also lacked a multivariate analysis [24]. The authors examined the impact of six miRNA expressions on predicting lymph node metastasis in 102 gastric tumor samples [24]. They identified four miRNAs (miR-27b, miR-128, miR-100 and miR-214) that were associated with the presence of positive nodes [24]. Some of the studied miRNAs were also associated with pT stage [24]. The authors did not conclude whether these four miRNAs could be recognized as an independent prognostic factor for the occurrence of lymph node metastases [24].
In conclusion, it is noting that the vast majority of studies concerning the value of miRNA expression in gastric cancer cells as a prognostic and predictive biomarker, including all the studies citied in this paper, come from Asia [8,11,12,13,14,15,16,17,23,24,25,26,27,29,30,31,32,33,34,35]. Gastric cancer is a highly heterogeneous disease, and many disparities exist concerning gastric adenocarcinoma incidence, risk factors, histology and molecular characteristics between different ethnic groups and races [36]. Our study, to our knowledge, is the first performed on a Caucasian cohort to reveal the impact of miR-21-5p and miR-145-5p expression on lymphatic spread capability.
Limitations of the Study
There are some limitations to our study. First, because of its retrospective nature, we were not able to gather fair data concerning neoadjuvant and adjuvant therapy. Second, we did not assess miRNA expression in the adjacent non-tumor tissue mucosa for a reference to the normal expression level. We also did not collect tumor types according to the Borrmann classification. Last, another limitation of our study was the lack of a separate patient population for NGS analysis and qPCR validation and the use of archival FFPE material of gastric resections performed between 2007 and 2013, which could have an impact on miRNA degradation and miRNA selection. In addition, at the time when the miRNA NGS experiment was performed (March 2017), a typical microRNA sequencing experiment yielded approximately 10–60% microRNAs mapping to the reference genome, which, together with archival FFPE miRNAs, limited the selection of miRNAs.
5. Conclusions
The upregulation of miR-21-5p and downregulation of miR-145-5p are independent prognostic factors for lymph node metastasis and could serve as specific biomarkers of lymphatic spread of gastric adenocarcinoma. miR-145-5p downregulation is an independent prognostic factor for overall survival.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13102393/s1; Figure S1: The average reads Q-scores of the NGS sequencing data for the samples; Figure S2: The average base Q-scores of the NGS sequencing data for the samples.
Author Contributions
Conceptualization, M.C. and M.A.L.; methodology, M.C., M.A.L., M.S., R.P. and K.P.; validation, M.C., M.A.L. and W.J.K.; formal analysis, M.C.; investigation, M.C., M.A.L., M.S. and J.W.; resources, M.C., M.A.L., J.Z., R.P. and J.W.; data curation, M.C., M.A.L., J.Z., R.P., P.K., J.W. and K.P.; writing—original draft preparation, M.C., M.A.L. and P.K.; writing—review and editing, P.K., M.A.L., J.Z. and W.J.K.; visualization, P.K.; supervision, W.J.K.; project administration, M.S. and M.C.; funding acquisition, M.C. and W.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Medical University of Gdańsk (ST 02/130/07/308).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Independent Ethic Committee at the Medical University of Gdańsk (NKBBN/90/2017; 20 March 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used OpenAI for the purposes of English editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FFPE | Formalin-fixed paraffin-embedded |
| NGS | Next-generation sequencing |
| qPCR | Quantitative polymerase chain reaction |
| OR | Odds ratio |
| HR | Hazard ratio |
| AJCC | American Joint Committee on Cancer |
| pTNM | Pathological tumor-node-metastasis |
| MOMA | Aarhus University Molecular Medicine |
| 5-FU | 5-Fluorouracil |
| PTEN | Phosphatase and tensin homolog |
| ANGPT2 | Angiopoietin-2 |
| NLR | Nod-like receptor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Deng, J.Y.; Liang, H. Clinical significance of lymph node metastasis in gastric cancer. World J. Gastroenterol. 2014, 20, 3967–3975. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lai, M. The microRNA network and tumor metastasis. Oncogene 2010, 29, 937–948. [Google Scholar]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Vakilzadehian, N.; Moradi, Y.; Allela, O.Q.B.; Al-Hussainy, A.F.; Al-Nuaimi, A.M.A.; Al-Hussein, R.K.; Jawad, M.J.; Gandomkar, H.; Moradi, S. Non-coding RNA in the regulation of gastric cancer tumorigenesis: Focus on microRNAs and exosomal microRNAs. Int. J. Mol. Cell. Med. 2025, 13, 417–435. [Google Scholar]
- Bakinowska, E.; Kiełbowski, K.; Skórka, P.; Dach, A.; Olejnik-Wojciechowska, J.; Szwedkowicz, A.; Pawlik, A. Non-coding RNA as biomarkers and their role in the pathogenesis of gastric cancer—A narrative review. Int. J. Mol. Sci. 2024, 25, 5144. [Google Scholar] [CrossRef]
- Tsai, M.M.; Wang, C.S.; Tsai, C.Y.; Huang, H.W.; Chi, H.C.; Lin, Y.H.; Lu, P.H.; Lin, K.H. Potential diagnostic, prognostic and therapeutic targets of microRNAs in human gastric cancer. Int. J. Mol. Sci. 2016, 17, 945. [Google Scholar] [CrossRef]
- Sun, Q.H.; Kuang, Z.Y.; Zhu, G.H.; Ni, B.Y.; Li, J. Multifaceted role of microRNAs in gastric cancer stem cells: Mechanisms and potential biomarkers. World J. Gastrointest. Oncol. 2024, 16, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Kalajahi, H.G.; Yari, A.H.; Amini, M.; Catal, T.; Youshanlui, M.A.; Pourbagherian, O.; Zhmurov, C.S.; Mokhtarzadeh, A. Therapeutic effect of microRNA-21 on differentially expressed hub genes in gastric cancer based on systems biology. Sci. Rep. 2023, 13, 21906. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y. Role of miRNA-145-5p in cancer (Review). Oncol. Rep. 2025, 53, 39. [Google Scholar]
- Shin, V.Y.; Chu, K.M. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J. Gastroenterol. 2014, 20, 10432–10439. [Google Scholar] [CrossRef]
- Zhou, K.; Song, B.; Wei, M.; Fang, J.; Xu, Y. miR-145-5p suppresses the proliferation, migration and invasion of gastric cancer epithelial cells via the ANGPT2/NOD_LIKE_RECEPTOR axis. Cancer Cell Int. 2020, 20, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, D.H.; Bi, R.X.; Xie, J.; Yang, C.H.; Jiang, Y.H. Prognostic value of microRNAs in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 55489–55510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Gao, C.; Chen, P.; Chen, J.; Liu, W.; Lu, H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab. Investig. 2008, 88, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Koopaie, M.; Arian-Kia, S.; Manifar, S.; Fatahzadeh, M.; Kolahdooz, S.; Davoudi, M. Expression of salivary miRNAs, clinical, and demographic features in the early detection of gastric cancer: A statistical and machine learning analysis. J. Gastrointest. Cancer 2025, 56, 15. [Google Scholar] [CrossRef]
- Wang, J.H.; Bai, Z.Z.; Niu, X.D.; Zhu, C.I.; Liang, T.; Hu, Y.L.; Gao, Z.H.; Da, M.X. Serum extracellular vesicle-derived miR-21-5p and miR-26a-5p as non-invasive diagnostic potential biomarkers for gastric cancer: A preliminary study. Int. J. Biol. Markers 2024, 39, 217–225. [Google Scholar] [CrossRef]
- Bai, H.X.; Qiu, X.M.; Xu, C.H.; Guo, J.Q. miRNA-145-5p inhibits gastric cancer progression via the serpin family E member 1–extracellular signal-regulated kinase-1/2 axis. World J. Gastrointest. Oncol. 2024, 16, 2123–2140. [Google Scholar] [CrossRef]
- Ciesielski, M.; Szajewski, M.; Peksa, R.; Lewandowska, M.A.; Zielinski, J.; Walczak, J.; Szefel, J.; Kruszewski, W.J. The relationship between HER2 overexpression and angiogenesis in gastric cancer. Medicine 2018, 97, e12851. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, S.H.; Kim, M.J.; Lee, Y.M. MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014, 42, 8062–8072. [Google Scholar] [CrossRef]
- Paduch, R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell. Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Mueller, C.; Gao, G.; Flotte, T.R. The 2024 Nobel Prize: Impact of the discovery of miRNA on the field to gene therapy. Hum. Gene Ther. 2025, 36, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Bilan, F.; Amini, M.; Doustvandi, M.A.; Tohidast, M.; Baghbanzadeh, A.; Hosseini, S.S.; Mokhtarzadeh, A.; Baradaran, B. Simultaneous suppression of miR-21 and restoration of miR-145 in gastric cancer cells; a promising strategy for inhibition of cell proliferation and migration. BioImpacts 2024, 14, 27764. [Google Scholar] [CrossRef]
- Liu, H.T.; Wang, Y.W.; Xing, A.Y.; Shi, D.B.; Zhang, H.; Guo, X.Y.; Xu, J.; Gao, P. Prognostic value of microRNA signature in patients with gastric cancers. Sci. Rep. 2017, 7, 42806. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Q.; Jiang, Z.; Liu, B.; Zhu, Z.; Li, C. Prognostic role of microRNA-21 in gastric cancer: A meta-analysis. Med. Sci. Monit. 2014, 20, 1668–1674. [Google Scholar]
- Wang, D.; Fan, Z.; Liu, F.; Zuo, J. Hsa-miR-21 and Hsa-miR-29 in tissue as potential diagnostic and prognostic biomarkers for gastric cancer. Cell. Physiol. Biochem. 2015, 37, 1454–1462. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, X.; Hu, X.L.; Cheng, L.Z.; Yu, J.Y.; Wei, Z.B. Downregulation of miR-145-5p correlates with poor prognosis in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3026–3030. [Google Scholar]
- de Jong, M.H.S.; Gisbertz, S.S.; van Berge Henegouwen, M.I.; Draaisma, W.A. Prevalence of nodal metastases in the individual lymph node stations for different T-stages in gastric cancer: A systematic review. Updates Surg. 2023, 75, 281–290. [Google Scholar]
- Zhang, B.G.; Li, J.F.; Yu, B.Q.; Zhu, Z.G.; Liu, B.Y.; Yan, M.I.N. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol. Rep. 2012, 27, 1019–1026. [Google Scholar] [CrossRef]
- Chan, S.H.; Wu, C.W.; Li, A.F.; Chi, C.W.; Lin, W.C. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008, 28, 907–911. [Google Scholar] [PubMed]
- Xu, Y.; Sun, J.; Xu, J.; Li, Q.; Guo, Y.; Zhang, Q. miR-21 is a promising novel biomarker for lymph node metastasis in patients with gastric cancer. Gastroenterol. Res. Pract. 2012, 2012, 640168. [Google Scholar] [PubMed]
- Takagi, T.; Iio, A.; Nakagawa, Y.; Naoe, T.; Tanigawa, N.; Akao, Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 2009, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yasuno, K.; Tagawa, H.; Sakamoto, N.; Oue, N.; Yashiro, M.; Sentani, K.; Goto, K.; Shinmei, S.; Oo, H.Z.; et al. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol. Rep. 2014, 32, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Wu, S.; Xiao, J.; Zhang, Z. Ferroptosis: Opening up potential targets for gastric cancer treatment. Mol. Cell. Biochem. 2024, 479, 2863–2874. [Google Scholar] [CrossRef]
- Seo, A.N.; Jung, Y.; Jang, H.; Lee, E.; Bae, H.I.; Son, T.; Kwon, O.; Chung, H.Y.; Yu, W.; Lee, Y.M. Clinical significance and prognostic role of hypoxia-induced microRNA 382 in gastric adenocarcinoma. PLoS ONE 2019, 14, e0223608. [Google Scholar] [CrossRef]
- López, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, D.; Araujo, J.M.; Quispe, L.; Zevallos, A.; Buleje, J.L.; Cho, C.E.; et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol. Hematol. 2023, 181, 103841. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).