Spectrum of Various Mosaicism Types According to Female Age: An Analysis of 36,506 Blastocysts Using Preimplantation Genetic Testing for Aneuploidy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. PGT-A
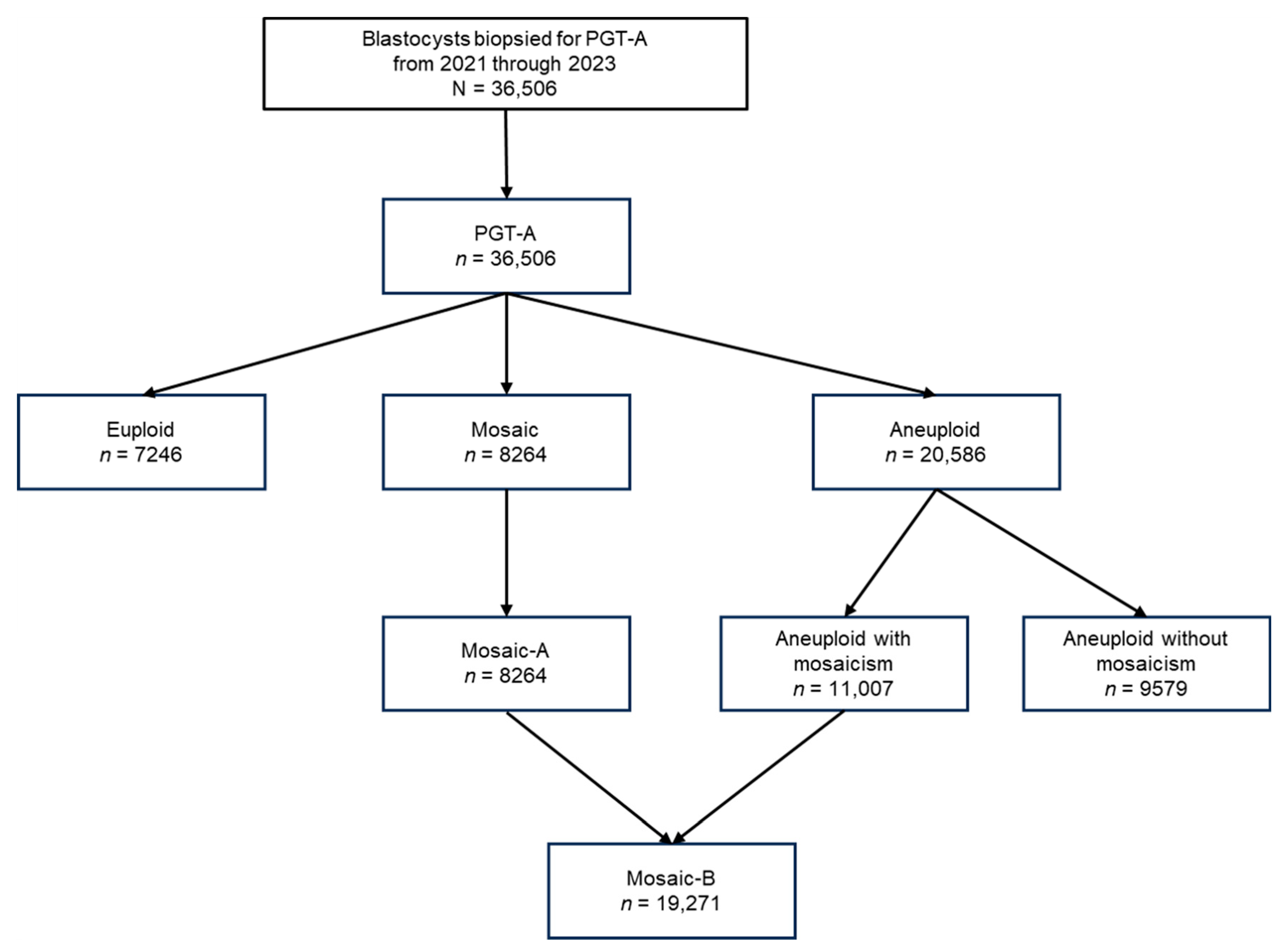
2.3. Definitions of Mosaic Traits
2.4. Statistical Analyses
3. Results
3.1. Frequencies of Mosaic-A and -B Across Female Age Groups
3.2. Analysis of the Frequencies of the Mosaicism Subtypes
3.3. Distribution of Single, Double, and Complex Mosaic Chromosomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASRM | American Society of Reproductive Medicine |
| ESHRE | European Society of Human Reproduction and Embryology |
| NGS | Next-Generation Sequencing |
| PGDIS | Preimplantation Genetic Diagnosis International Society |
| PGS | Preimplantation Genetic Screening |
| PGT-A | Preimplantation Genetic Testing for Aneuploidy |
| TE | Trophectoderm |
References
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. The Use of Preimplantation Genetic Testing for Aneuploidy (PGT-A): A Committee Opinion. Fertil. Steril. 2018, 109, 429–436. [Google Scholar] [CrossRef]
- ESHRE PGT Consortium Steering Committee; Carvalho, F.; Coonen, E.; Goossens, V.; Kokkali, G.; Rubio, C.; Meijer-Hoogeveen, M.; Moutou, C.; Vermeulen, N.; De Rycke, M. ESHRE PGT Consortium Good Practice Recommendations for the Organisation of PGT†. Hum. Reprod. Open 2020, 2020, hoaa021. [Google Scholar] [CrossRef]
- Cram, D.S.; Leigh, D.; Handyside, A.; Rechitsky, L.; Xu, K.; Harton, G.; Grifo, J.; Rubio, C.; Fragouli, E.; Kahraman, S.; et al. PGDIS Position Statement on the Transfer of Mosaic Embryos 2019. Reprod. BioMed. Online 2019, 39, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Hawke, D.C.; Watson, A.J.; Betts, D.H. Extracellular Vesicles, microRNA and the Preimplantation Embryo: Non-Invasive Clues of Embryo Well-Being. Reprod. Biomed. Online 2021, 42, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Viotti, M.; Victor, A.R.; Barnes, F.L.; Zouves, C.G.; Besser, A.G.; Grifo, J.A.; Cheng, E.-H.; Lee, M.-S.; Horcajadas, J.A.; Corti, L.; et al. Using Outcome Data from One Thousand Mosaic Embryo Transfers to Formulate an Embryo Ranking System for Clinical Use. Fertil. Steril. 2021, 115, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Minasi, M.G.; Fiorentino, F. Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts. N. Engl. J. Med. 2015, 373, 2089–2090. [Google Scholar] [CrossRef]
- Capalbo, A.; Poli, M.; Rienzi, L.; Girardi, L.; Patassini, C.; Fabiani, M.; Cimadomo, D.; Benini, F.; Farcomeni, A.; Cuzzi, J.; et al. Mosaic Human Preimplantation Embryos and Their Developmental Potential in a Prospective, Non-Selection Clinical Trial. Am. J. Hum. Genet. 2021, 108, 2238–2247. [Google Scholar] [CrossRef]
- Abhari, S.; Kawwass, J.F. Pregnancy and Neonatal Outcomes after Transfer of Mosaic Embryos: A Review. J. Clin. Med. 2021, 10, 1369. [Google Scholar] [CrossRef]
- Yakovlev, P.; Vyatkina, S.; Polyakov, A.; Pavlova, M.; Volkomorov, V.; Yakovlev, M.; Filimonov, S.; Kazaryn, L.; Aizikovich, A.; Kornilov, N. Neonatal and Clinical Outcomes after Transfer of a Mosaic Embryo Identified by Preimplantation Genetic Testing for Aneuploidies. Reprod. BioMed. Online 2022, 45, 88–100. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, J.J.; Nabu, S.; Yeung, Q.S.Y.; Li, Y.; Tan, J.H.; Suksalak, W.; Chanchamroen, S.; Quangkananurug, W.; Wong, P.S.; et al. The Pregnancy Outcome of Mosaic Embryo Transfer: A Prospective Multicenter Study and Meta-Analysis. Genes 2020, 11, 973. [Google Scholar] [CrossRef]
- Munné, S.; Blazek, J.; Large, M.; Martinez-Ortiz, P.A.; Nisson, H.; Liu, E.; Tarozzi, N.; Borini, A.; Becker, A.; Zhang, J.; et al. Detailed Investigation into the Cytogenetic Constitution and Pregnancy Outcome of Replacing Mosaic Blastocysts Detected with the Use of High-Resolution next-Generation Sequencing. Fertil. Steril. 2017, 108, 62–71.e8. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Lee, C.-I.; Cheng, E.-H.; Huang, C.-C.; Lee, T.-H.; Shih, H.-H.; Pai, Y.-P.; Chen, Y.-C.; Lee, M.-S. Clinical Outcomes of Single Mosaic Embryo Transfer: High-Level or Low-Level Mosaic Embryo, Does It Matter? J. Clin. Med. 2020, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.J.; Kim, M.J.; Park, E.A.; Kang, I.S. Preimplantation Genetic Testing for Aneuploidy: The Management of Mosaic Embryos. Clin. Exp. Reprod. Med. 2022, 49, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Mercader, A.; Dominguez, F.; Quiñonero, A.; Perez, M.; Gonzalez-Martin, R.; Delgado, A.; Mifsud, A.; Pellicer, A.; De Los Santos, M.J. Mosaic Results after Preimplantation Genetic Testing for Aneuploidy May Be Accompanied by Changes in Global Gene Expression. Front. Mol. Biosci. 2023, 10, 1180689. [Google Scholar] [CrossRef]
- Mourad, A.; Antaki, R.; Bissonnette, F.; Al Baini, O.; Saadeh, B.; Jamal, W. Evidence-Based Clinical Prioritization of Embryos with Mosaic Results: A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2021, 38, 2849–2860. [Google Scholar] [CrossRef]
- Illumina. VeriSeq PGS Library Prep Reference Guide. Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/veriseq-pgs/veriseq-pgs-library-prep-reference-guide-15052877-05.pdf (accessed on 26 September 2024).
- Viotti, M.; McCoy, R.C.; Griffin, D.K.; Spinella, F.; Greco, E.; Madjunkov, M.; Madjunkova, S.; Librach, C.L.; Victor, A.R.; Barnes, F.L.; et al. Let the Data Do the Talking: The Need to Consider Mosaicism during Embryo Selection. Fertil. Steril. 2021, 116, 1212–1219. [Google Scholar] [CrossRef]
- Daniluk, J.C.; Koert, E. Fertility Awareness Online: The Efficacy of a Fertility Education Website in Increasing Knowledge and Changing Fertility Beliefs. Hum. Reprod. 2015, 30, 353–363. [Google Scholar] [CrossRef]
- Popa, T.; Davis, C.; Xanthopoulou, L.; Bakosi, E.; He, C.; O’Neill, H.; Ottolini, C.S. Current Quantitative Methodologies for Preimplantation Genetic Testing Frequently Misclassify Meiotic Aneuploidies as Mosaic. Fertil. Steril. 2025, 124, 307–318. [Google Scholar] [CrossRef]
- Sachdev, N.M.; Maxwell, S.M.; Besser, A.G.; Grifo, J.A. Diagnosis and Clinical Management of Embryonic Mosaicism. Fertil. Steril. 2017, 107, 6–11. [Google Scholar] [CrossRef]
- Ottolini, C.S.; Newnham, L.J.; Capalbo, A.; Natesan, S.A.; Joshi, H.A.; Cimadomo, D.; Griffin, D.K.; Sage, K.; Summers, M.C.; Thornhill, A.R.; et al. Genome-Wide Maps of Recombination and Chromosome Segregation in Human Oocytes and Embryos Show Selection for Maternal Recombination Rates. Nat. Genet. 2015, 47, 727–735. [Google Scholar] [CrossRef]
- Swain, J.E. Controversies in ART: Can the IVF Laboratory Influence Preimplantation Embryo Aneuploidy? Reprod. BioMed. Online 2019, 39, 599–607. [Google Scholar] [CrossRef]


| Age group | Total | <35 Years | 35-37 Years | 38–40 Years | 41–42 Years | >42 Years | |
|---|---|---|---|---|---|---|---|
| No. of Embryos | N = 36,506 | n = 6049 | n = 7505 | n = 10,634 | n = 7434 | n = 4884 | p-Value |
| Result Type | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |
| Euploid | 19.8% (7246) | 34.9% (2110) (33.7~36.1%) | 29.1% (2181) (28.0~30.1%) | 18.8% (1996) (18.0~19.5%) | 10.2% (761) (9.5~10.9%) | 4.1% (198) (3.5~4.6%) | <0.001 |
| Mosaic (Mosaic-A, B) | 22.6% (8264) | 31.2% (1890) (30.1~32.4%) | 29.8% (2234) (28.7~30.8%) | 23.3% (2474) (22.5~24.1%) | 16.1% (1200) (15.3~17.0%) | 9.5% (466) (8.7~10.4%) | |
| Aneuploid with mosaicism (Mosaic-B) | 30.2% (11,007) | 14.5% (877) (13.6~15.4%) | 19.3% (1449) (18.4~20.2%) | 29.6% (3143) (28.7~30.4%) | 40.2% (2989) (39.1~41.3%) | 52.2% (2549) (50.8~53.6%) | |
| Aneuploid without mosaicism | 26.2% (9579) | 18.2% (1099) (17.2~19.1%) | 20.8% (1561) (19.9~21.7%) | 27.3% (2905) (18.9~20.5%) | 32.3% (2401) (31.2~33.4%) | 33.0% (1613) (31.7~34.3%) | |
| No Call | 1.1% (410) | 1.2% (73) (0.9~1.5%) | 1.1% (80) (0.8~1.3%) | 1.1% (116) (0.9~1.3%) | 1.1% (83) (0.9~1.4%) | 1.2% (58) (0.9~1.5%) |
| Mosaic-A | |||||||
| Age Group | Total | <35 Years | 35–37 Years | 38–40 Years | 41–42 Years | >42 Years | |
| No. of Embryos | n = 8264 | n = 1890 | n = 2234 | n = 2474 | n = 1200 | n = 466 | p-Value |
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | ||
| Low-segmental mosaic | 10.7% (881) (10.0~11.3%) | 14.1% (266) (12.5~15.6%) | 11.5% (257) (10.2~12.8%) | 10.3% (254) (9.1~11.5%) | 7.2% (86) (5.7~8.6%) | 3.9% (18) (2.1~5.6%) | <0.001 |
| High-segmental mosaic | 4.2% (346) (3.8~4.6%) | 5.7% (107) (4.6~6.7%) | 4.1% (92) (3.3~4.9%) | 3.6% (90) (2.9~4.4%) | 3.7% (44) (2.6~4.7%) | 2.8% (13) (1.3~4.3%) | |
| One low mosaic | 6.8% (564) (6.3~7.4%) | 7.6% (143) (6.4~8.8%) | 8.2% (183) (7.1~9.3%) | 6.1% (152) (5.2~7.1%) | 5.3% (64) (4.1~6.6%) | 4.7% (22) (2.8~6.6%) | |
| Two low mosaic | 12% (990) (11.3~12.7%) | 12.9% (244) (11.4~14.4%) | 13% (291) (11.6~14.4%) | 12.3% (305) (11.0~13.6%) | 9.9% (119) (8.2~11.6%) | 6.7% (31) (4.4~8.9%) | |
| One high mosaic | 10.1% (834) (9.4~10.7%) | 5.8% (110) (4.8~6.9%) | 9.0% (201) (7.8~10.2%) | 11.4% (281) (10.1~12.6%) | 14.9% (179) (12.9~16.9%) | 13.5% (63) (10.4~16.6%) | |
| Two high mosaic | 10.2% (842) (9.5~10.8%) | 7.7% (145) (6.5~8.9%) | 10.2% (227) (8.9~11.4%) | 10.6% (262) (9.4~11.8%) | 11.9% (143) (10.1~13.7%) | 13.9% (65) (10.8~17.1%) | |
| Low-complex mosaic | 19.9% (1647) (19.1~20.8%) | 23.9% (452) (22.0~25.8%) | 21.8% (488) (20.1~23.6%) | 19.5% (482) (17.9~21.0%) | 14.7% (176) (12.7~16.7%) | 10.5% (49) (7.7~13.3%) | |
| High-complex mosaic | 26.1% (2160) (25.2~27.1%) | 22.4% (423) (20.5~24.3%) | 22.2% (495) (20.4~23.9%) | 26.2% (648) (24.5~27.9%) | 32.4% (389) (29.8~35.1%) | 44.0% (205) (39.5~48.5%) | |
| Mosaic-B | |||||||
| Age Group | Total | <35 Years | 35–37 Years | 38–40 Years | 41–42 Years | >42 Years | |
| No. of Embryos | n = 19,271 | n = 2767 | n = 3683 | n = 5617 | n = 4189 | n = 3015 | p-Value |
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | ||
| Low-segmental mosaic | 10% (1922) (9.6~10.4%) | 13.4% (372) (12.2~14.7%) | 11.5% (424) (10.5~12.5%) | 10.3% (581) (9.5~11.1%) | 8.1% (340) (7.3~8.9%) | 6.8% (205) (5.9~7.7%) | <0.001 |
| High-segmental mosaic | 4.1% (784) (3.8~4.3%) | 5.6% (156) (4.8~6.5%) | 4.6% (170) (3.9~5.3%) | 3.9% (218) (3.4~4.4%) | 3.7% (154) (3.1~4.2%) | 2.9% (86) (2.3~3.4%) | |
| One low mosaic | 6.8% (1320) (6.5~7.2%) | 7.4% (206) (6.5~8.4%) | 7.6% (280) (6.7~8.5%) | 7% (392) (6.3~7.6%) | 6.5% (272) (5.7~7.2%) | 5.6% (170) (4.8~6.5%) | |
| Two low mosaic | 9.4% (1807) (9.0~9.8%) | 11.2% (310) (10.0~12.4%) | 11.8% (435) (10.8~12.9%) | 10% (560) (9.2~10.8%) | 7.6% (320) (6.8~8.4%) | 6% (182) (5.2~6.9%) | |
| One high mosaic | 10.6% (2048) (10.2~11.1%) | 5.5% (153) (4.7~6.4%) | 8.2% (301) (7.3~9.1%) | 11.2% (630) (10.4~12.0%) | 13.2% (553) (12.2~14.2%) | 13.6% (411) (12.4~14.9%) | |
| Two high mosaic | 10.1% (1953) (9.7~10.6%) | 7.4% (204) (6.4~8.3%) | 9.4% (345) (8.4~10.3%) | 9.8% (553) (9.1~10.6%) | 10.9% (458) (10.0~11.9%) | 13.0% (393) (11.8~14.2%) | |
| Low-complex mosaic | 16.5% (3186) (16.0~17.1%) | 21.3% (590) (19.8~22.8%) | 20.4% (753) (19.1~21.7%) | 17.0% (953) (16.0~17.9%) | 13.7% (575) (12.7~14.8%) | 10.4% (315) (9.4~11.5%) | |
| High-complex mosaic | 32.4% (6251) (31.8~33.1%) | 28.0% (776) (26.4~29.7%) | 26.5% (975) (25.0~27.9%) | 30.8% (1730) (29.6~32.0%) | 36.2% (1517) (34.8~37.7%) | 41.6% (1253) (39.8~43.3%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, M.S.; Kim, M.J.; Choi, N.; Hong, J.; Choi, R.; Jeong, Y.; Lee, H.-S.; Lee, K.A.; Yu, E.J.; Kang, I.S. Spectrum of Various Mosaicism Types According to Female Age: An Analysis of 36,506 Blastocysts Using Preimplantation Genetic Testing for Aneuploidy. Biomedicines 2025, 13, 2380. https://doi.org/10.3390/biomedicines13102380
Jeon MS, Kim MJ, Choi N, Hong J, Choi R, Jeong Y, Lee H-S, Lee KA, Yu EJ, Kang IS. Spectrum of Various Mosaicism Types According to Female Age: An Analysis of 36,506 Blastocysts Using Preimplantation Genetic Testing for Aneuploidy. Biomedicines. 2025; 13(10):2380. https://doi.org/10.3390/biomedicines13102380
Chicago/Turabian StyleJeon, Min Seo, Min Jee Kim, Nayeon Choi, Jiseon Hong, Rosa Choi, Yebin Jeong, Hyoung-Song Lee, Kyung Ah Lee, Eun Jeong Yu, and Inn Soo Kang. 2025. "Spectrum of Various Mosaicism Types According to Female Age: An Analysis of 36,506 Blastocysts Using Preimplantation Genetic Testing for Aneuploidy" Biomedicines 13, no. 10: 2380. https://doi.org/10.3390/biomedicines13102380
APA StyleJeon, M. S., Kim, M. J., Choi, N., Hong, J., Choi, R., Jeong, Y., Lee, H.-S., Lee, K. A., Yu, E. J., & Kang, I. S. (2025). Spectrum of Various Mosaicism Types According to Female Age: An Analysis of 36,506 Blastocysts Using Preimplantation Genetic Testing for Aneuploidy. Biomedicines, 13(10), 2380. https://doi.org/10.3390/biomedicines13102380







