An Immuno-Fragile Profile Is Associated with Mortality Risk in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patient Population
2.2. Sample Collection and Measurements
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Participants
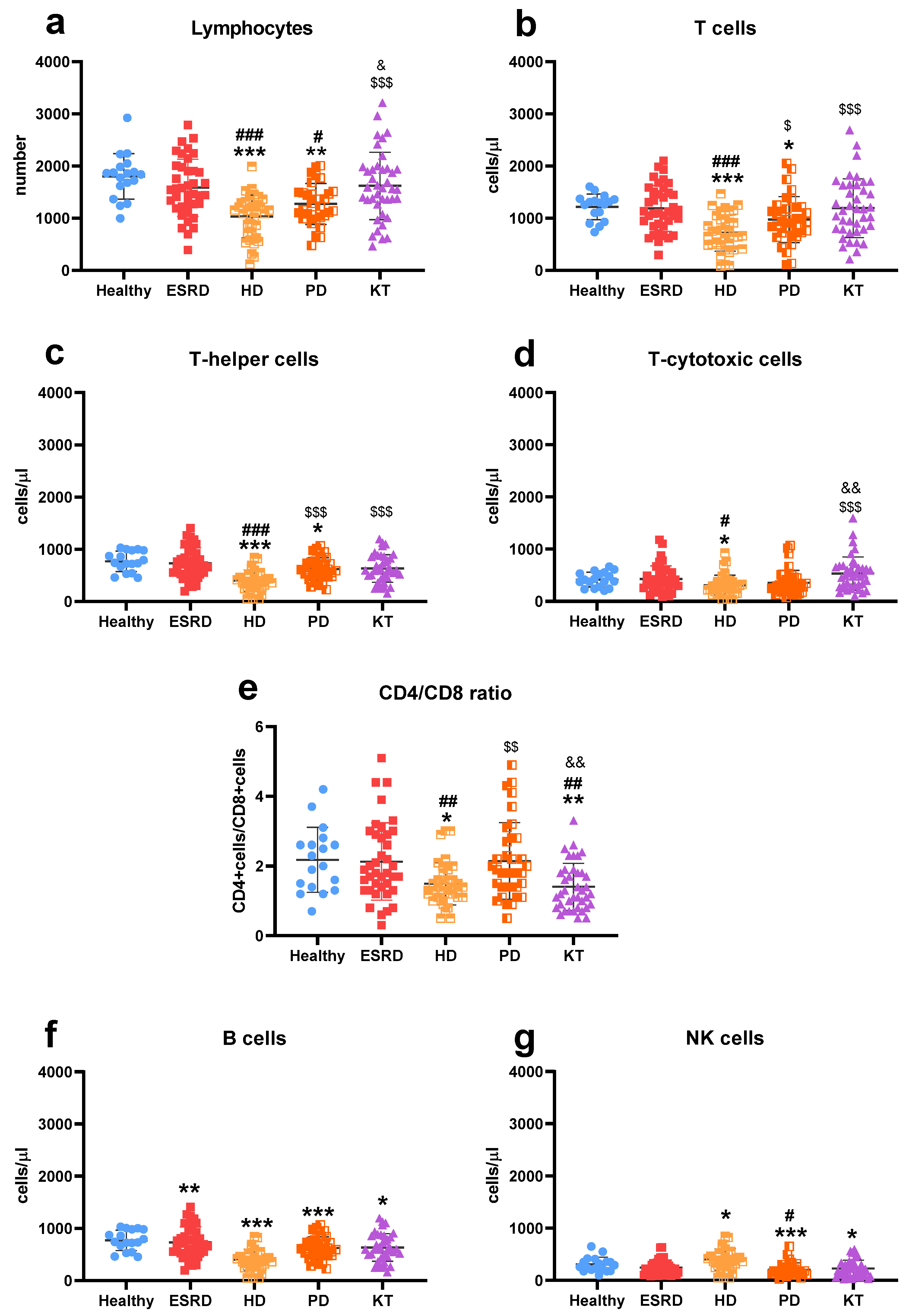
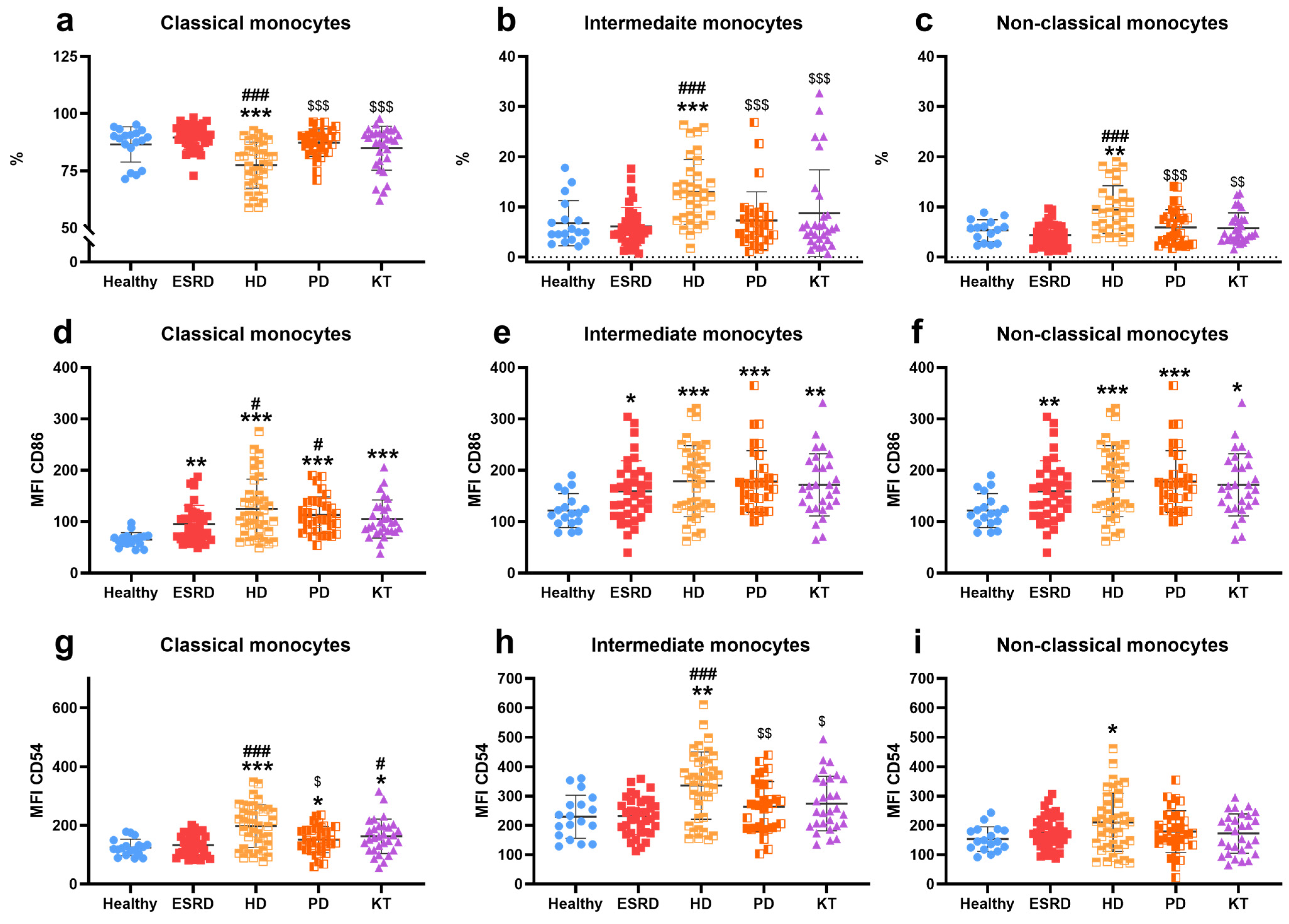
3.2. Immune Cells Characterization
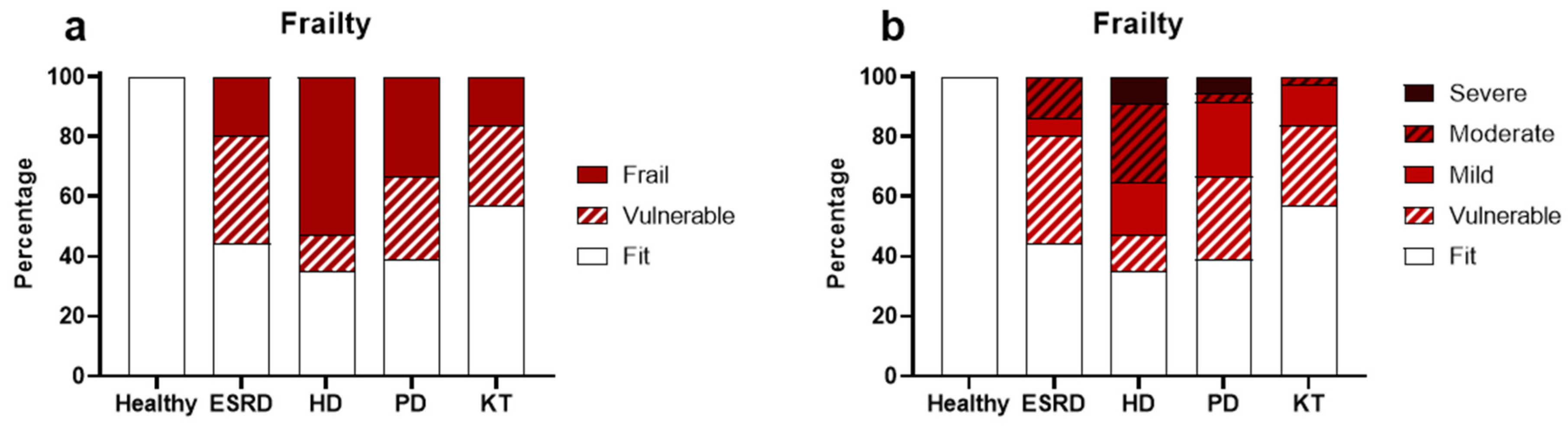
3.3. Frailty
3.4. Frailty and Immune Cells in CKD Patients
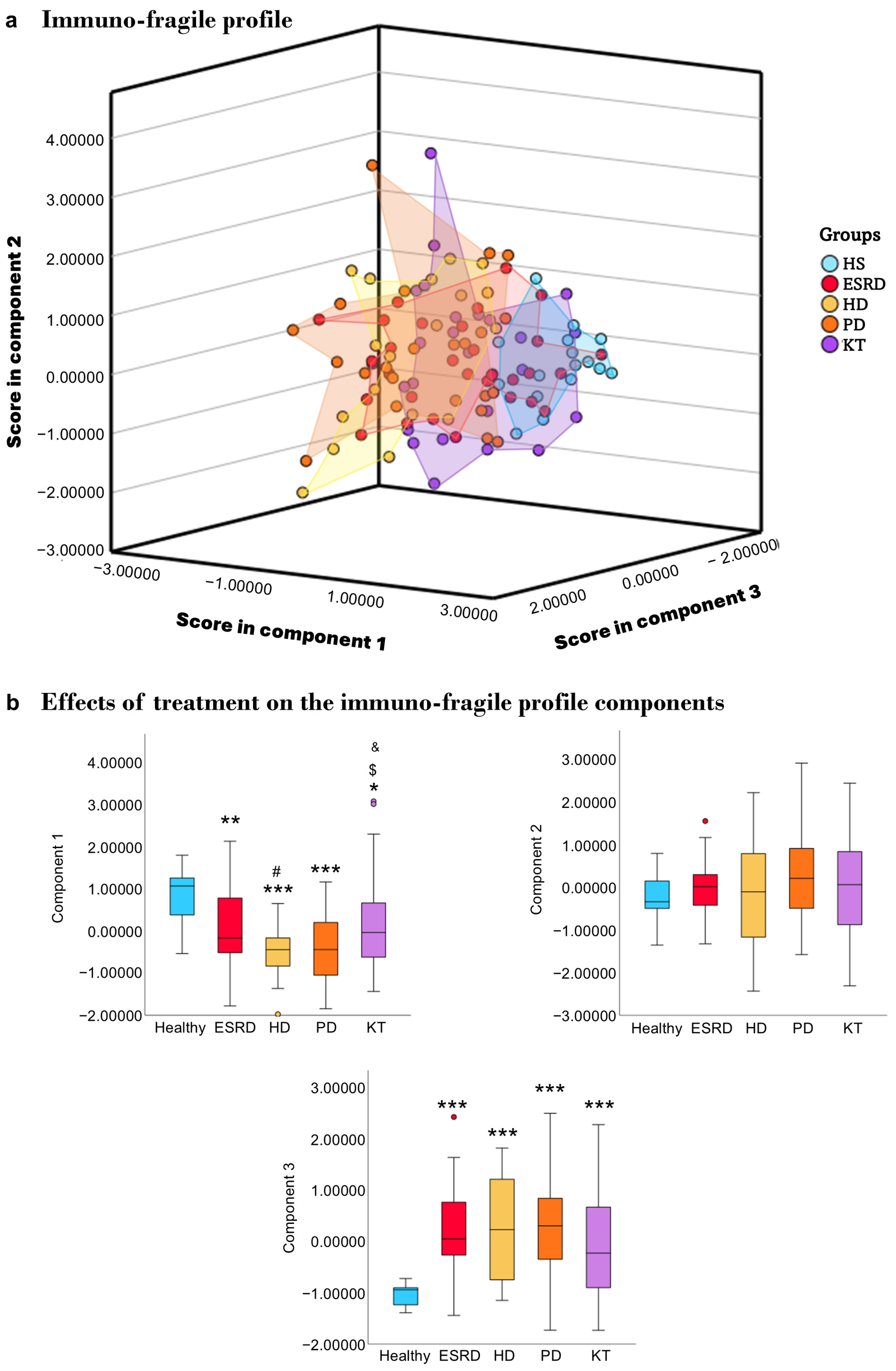
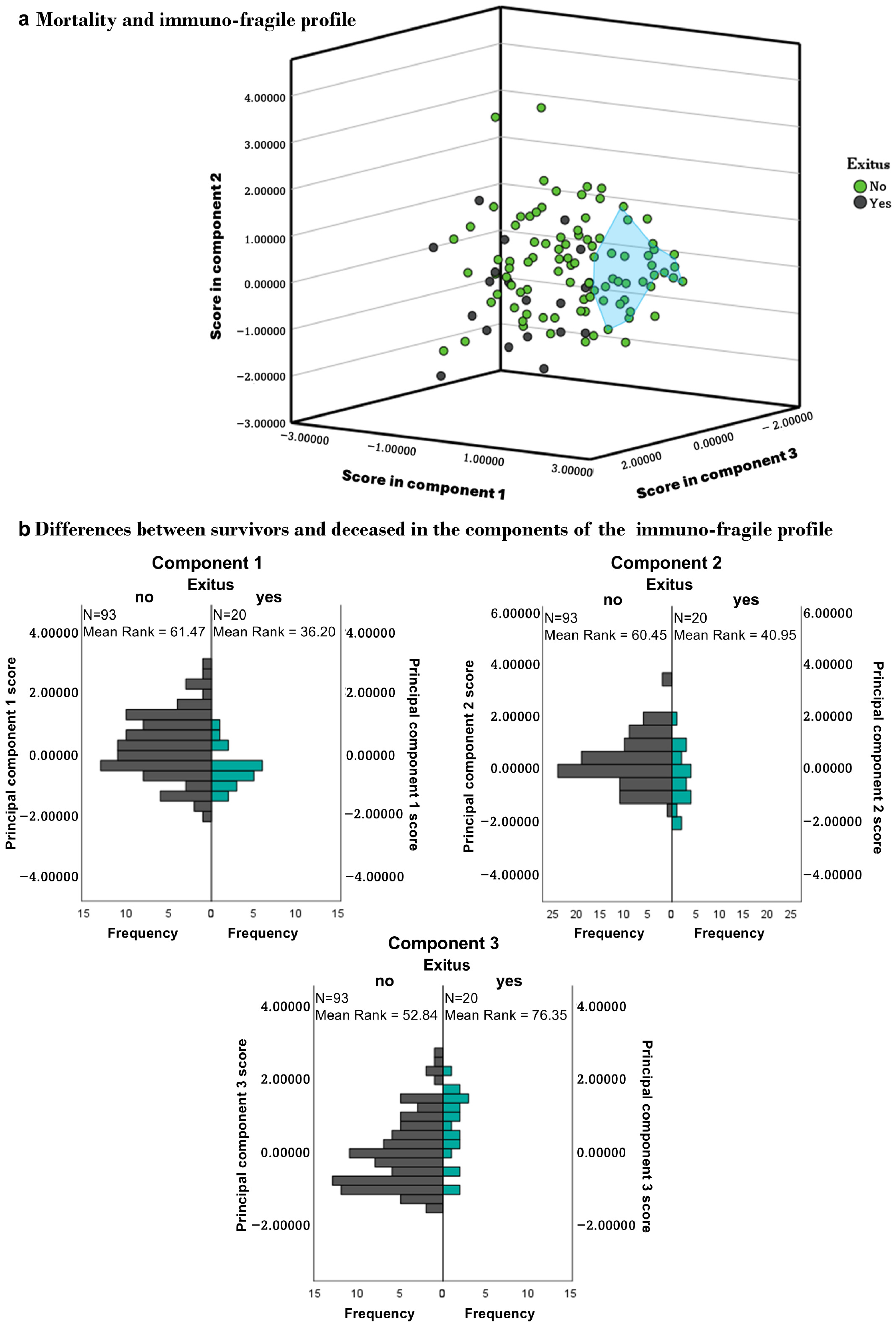
3.5. Immuno-Fragile Profile
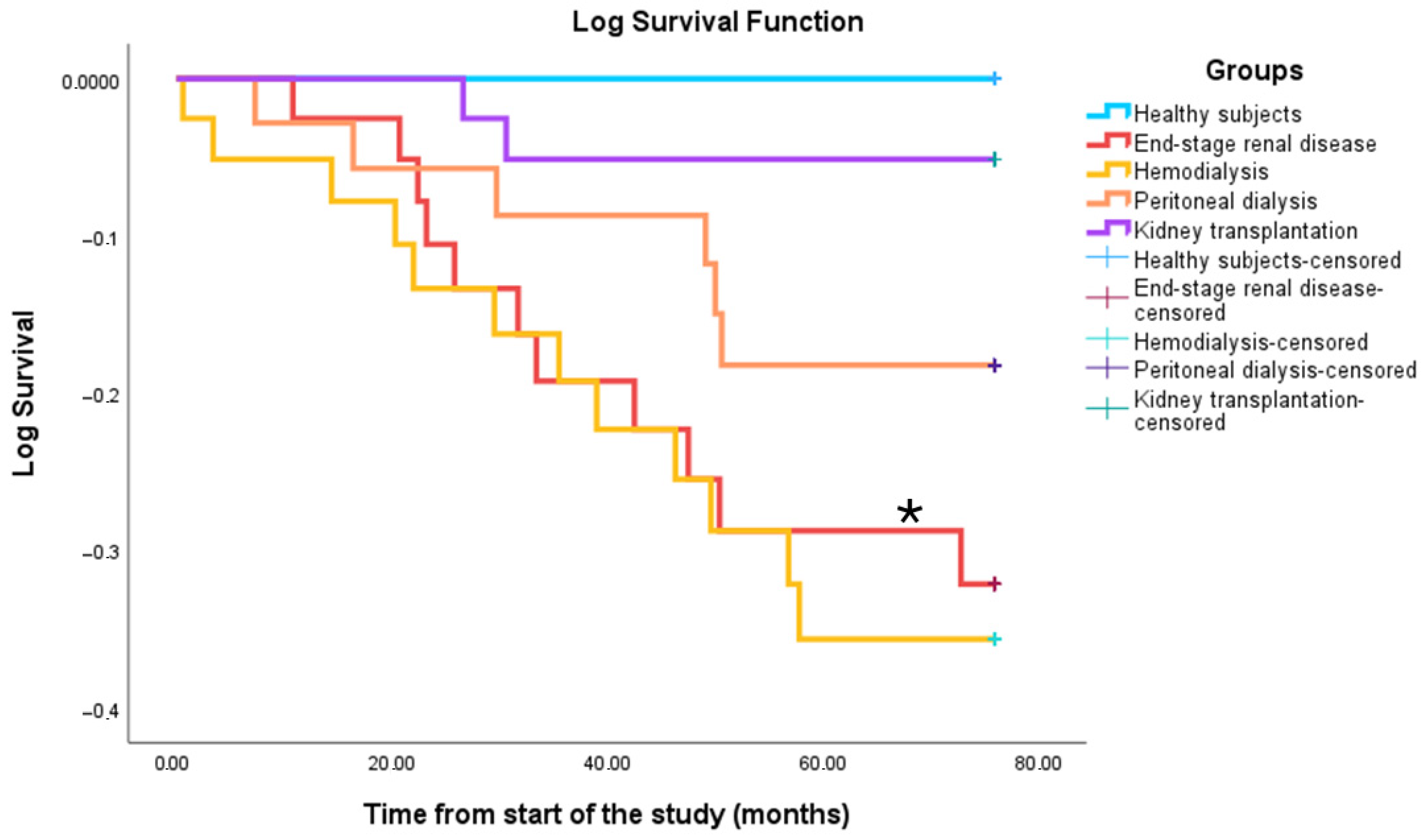
3.6. Survival
3.7. Validation of the Immunofragile Profile as a Prognostic Marker
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACVA | Acute cerebrovascular accident |
| BMI | Body mass index |
| CD | Cluster of differentiation |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| EFS | Edmonton Frail Scale |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End-stage renal disease |
| HD | Hemodialysis |
| HR | Hazard ratios |
| HS | Healthy subjects |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IS | Immune system |
| KT | Kidney transplant patients |
| PCA | Principal component analysis |
| PD | Peritoneal dialysis |
References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Ying, M.; Shao, X.; Qin, H.; Yin, P.; Lin, Y.; Wu, J.; Ren, J.; Zheng, Y. Disease Burden and Epidemiological Trends of Chronic Kidney Disease at the Global, Regional, National Levels from 1990 to 2019. Nephron 2024, 148, 113–123. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. (2011) 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, J.; Zhao, Y.; Du, D. Aging and Chronic Kidney Disease: Epidemiology, Therapy, Management and the Role of Immunity. Clin. Kidney J. 2024, 17, sfae235. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Ortiz, A.; Zoccali, C.; Stel, V.S.; Jager, K.J. The Impact of Population Ageing on the Burden of Chronic Kidney Disease. Nat. Rev. Nephrol. 2024, 20, 569–585. [Google Scholar] [CrossRef]
- He, Y.; Tang, W.; Chen, J.; Tang, J.; Zheng, Y.; Wang, X.; Xing, B.; Li, X.; Xu, Y.; Wang, X. Global Burden of Chronic Kidney Disease Due to Hypertension (1990–2021): A Systematic Analysis of Epidemiological Trends, Risk Factors, and Projections to 2036 from the Gbd 2021 Study. BMC Nephrol. 2025, 26, 448. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, C.; Li, X. Kidney Aging and Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 6585. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Back, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Yamamoto, T.; Isaka, Y. Pathological Mechanisms of Kidney Disease in Ageing. Nat. Rev. Nephrol. 2024, 20, 603–615. [Google Scholar] [CrossRef]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef]
- Kooman, J.P.; Dekker, M.J.; Usvyat, L.A.; Kotanko, P.; van der Sande, F.M.; Schalkwijk, C.G.; Shiels, P.G.; Stenvinkel, P. Inflammation and Premature Aging in Advanced Chronic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2017, 313, F938–F950. [Google Scholar] [CrossRef]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprian, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramirez, R. Mechanisms of Cardiovascular Disorders in Patients with Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef]
- Crepin, T.; Legendre, M.; Carron, C.; Vachey, C.; Courivaud, C.; Rebibou, J.M.; Ferrand, C.; Laheurte, C.; Vauchy, C.; Gaiffe, E.; et al. Uraemia-Induced Immune Senescence and Clinical Outcomes in Chronic Kidney Disease Patients. Nephrol. Dial. Transplant. 2020, 35, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic Kidney Disease: A Clinical Model of Premature Aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Claro, L.M.; Moreno-Amaral, A.N.; Gadotti, A.C.; Dolenga, C.J.; Nakao, L.S.; Azevedo, M.L.V.; de Noronha, L.; Olandoski, M.; de Moraes, T.P.; Stinghen, A.E.M.; et al. The Impact of Uremic Toxicity Induced Inflammatory Response on the Cardiovascular Burden in Chronic Kidney Disease. Toxins 2018, 10, 384. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, J.J.; Wu, C.Y.; Lin, T.Y.; Hung, S.C.; Yang, H.Y. Immunosenescence, Gut Dysbiosis, and Chronic Kidney Disease: Interplay and Implications for Clinical Management. Biomed. J. 2024, 47, 100638. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Magagnoli, L.; Ciceri, P. From Physicochemical Classification to Multidimensional Insights: A Comprehensive Review of Uremic Toxin Research. Toxins 2025, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Allende, L.M.; Ramos, L.E.; Gutierrez, E.; Pleguezuelo, D.E.; Hernandez, E.R.; Rios, F.; Fernandez, C.; Praga, M.; Morales, E. CD19(+) B-Cells, a New Biomarker of Mortality in Hemodialysis Patients. Front. Immunol. 2018, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.; Litjens, N.H. Chronic Kidney Disease and Premature Ageing of the Adaptive Immune Response. Curr. Urol. Rep. 2015, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Ducloux, D.; Legendre, M.; Bamoulid, J.; Saas, P.; Courivaud, C.; Crepin, T. End-Stage Renal Disease-Related Accelerated Immune Senescence: Is Rejuvenation of the Immune System a Therapeutic Goal? Front. Med. 2021, 8, 720402. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of Uremia on Structure and Function of Immune System. J. Ren. Nutr. 2012, 22, 149–156. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Menezes-Silva, L.; Leite, J.A.; Andrade-Silva, M.; Camara, N.O.S. Immunology of Kidney Disease. Annu. Rev. Immunol. 2024, 42, 207–233. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Q.; Liu, B.; Ma, Q.; Zhang, T.; Huang, T.; Lv, Z.; Wang, R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022, 10, 868715. [Google Scholar] [CrossRef]
- Alvarez-Lara, M.A.; Carracedo, J.; Ramirez, R.; Martin-Malo, A.; Rodriguez, M.; Madueno, J.A.; Aljama, P. The Imbalance in the Ratio of Th1 and Th2 Helper Lymphocytes in Uraemia Is Mediated by an Increased Apoptosis of Th1 Subset. Nephrol. Dial. Transplant. 2004, 19, 3084–3090. [Google Scholar] [CrossRef]
- Dounousi, E.; Duni, A.; Naka, K.K.; Vartholomatos, G.; Zoccali, C. The Innate Immune System and Cardiovascular Disease in Eskd: Monocytes and Natural Killer Cells. Curr. Vasc. Pharmacol. 2021, 19, 63–76. [Google Scholar] [CrossRef]
- Valera, G.; Figuer, A.; Caro, J.; Yuste, C.; Morales, E.; Ceprian, N.; Bodega, G.; Ramirez, R.; Alique, M.; Carracedo, J. Plasma Glycocalyx Pattern: A Mirror of Endothelial Damage in Chronic Kidney Disease. Clin. Kidney J. 2023, 16, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Bernelot Moens, S.J.; Verweij, S.L.; van der Valk, F.M.; van Capelleveen, J.C.; Kroon, J.; Versloot, M.; Verberne, H.J.; Marquering, H.A.; Duivenvoorden, R.; Vogt, L.; et al. Arterial and Cellular Inflammation in Patients with Ckd. J. Am. Soc. Nephrol. 2017, 28, 1278–1285. [Google Scholar] [CrossRef]
- Heine, G.H.; Ortiz, A.; Massy, Z.A.; Lindholm, B.; Wiecek, A.; Martinez-Castelao, A.; Covic, A.; Goldsmith, D.; Suleymanlar, G.; London, G.M.; et al. Monocyte Subpopulations and Cardiovascular Risk in Chronic Kidney Disease. Nat. Rev. Nephrol. 2012, 8, 362–369. [Google Scholar] [CrossRef]
- Roshanravan, B.; Khatri, M.; Robinson-Cohen, C.; Levin, G.; Patel, K.V.; de Boer, I.H.; Seliger, S.; Ruzinski, J.; Himmelfarb, J.; Kestenbaum, B. A Prospective Study of Frailty in Nephrology-Referred Patients with Ckd. Am. J. Kidney Dis. 2012, 60, 912–921. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Marshall, L.M.; Michael, Y.; Dam, T.T.; Ensrud, K.E.; Barrett-Connor, E.; Orwoll, E.S.; Osteoporotic Fractures in Men Research, G. Frailty in Older Men: Prevalence, Progression, and Relationship with Mortality. J. Am. Geriatr. Soc. 2007, 55, 1216–1223. [Google Scholar] [CrossRef]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty Syndrome: Implications and Challenges for Health Care Policy. Risk Manag. Healthc. Policy 2019, 12, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Midao, L.; Almada, M.; Costa, E. Pre-Frailty and Frailty in Dialysis and Pre-Dialysis Patients: A Systematic Review of Clinical and Biochemical Markers. Int. J. Environ. Res. Public Health 2021, 18, 9579. [Google Scholar] [CrossRef]
- Ballew, S.H.; Chen, Y.; Daya, N.R.; Godino, J.G.; Windham, B.G.; McAdams-DeMarco, M.; Coresh, J.; Selvin, E.; Grams, M.E. Frailty, Kidney Function, and Polypharmacy: The Atherosclerosis Risk in Communities (Aric) Study. Am. J. Kidney Dis. 2017, 69, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Brar, R.; Eng, F.; Komenda, P.; Rigatto, C.; Prasad, B.; Bohm, C.J.; Storsley, L.J.; Tangri, N. Frailty and Physical Function in Chronic Kidney Disease: The Canfit Study. Can. J. Kidney Health Dis. 2015, 2, 32. [Google Scholar] [CrossRef]
- Ceprian, N.; Valera, G.; Caro, J.; Yuste, C.; Serroukh, N.; Gonzalez de Pablos, I.; Oliva, C.; Figuer, A.; Praga, M.; Alique, M.; et al. Effect of Kidney Transplantation on Accelerated Immunosenescence and Vascular Changes Induced by Chronic Kidney Disease. Front. Med. 2021, 8, 705159. [Google Scholar] [CrossRef]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and Reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ospina, D.; Mas-Fontao, S.; Gracia-Iguacel, C.; Avello, A.; Gonzalez de Rivera, M.; Mujika-Marticorena, M.; Gonzalez-Parra, E. Displacing the Burden: A Review of Protein-Bound Uremic Toxin Clearance Strategies in Chronic Kidney Disease. J. Clin. Med. 2024, 13, 1428. [Google Scholar] [CrossRef]
- Ramírez, R.; Ceprian, N.; Figuer, A.; Valera, G.; Bodega, G.; Alique, M.; Carracedo, J. Endothelial Senescence and the Chronic Vascular Diseases: Challenges and Therapeutic Opportunities in Atherosclerosis. J. Pers. Med. 2022, 12, 215. [Google Scholar] [CrossRef]
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Gonzalez-Cuadrado, C.; Caro-Espada, P.J.; Chivite-Lacaba, M.; Utrero-Rico, A.; Lozano-Yuste, C.; Gutierrez-Solis, E.; Morales, E.; Sandino-Perez, J.; Gil-Etayo, F.J.; Allende-Martinez, L.; et al. Hemodialysis-Associated Immune Dysregulation in SARS-CoV-2-Infected End-Stage Renal Disease Patients. Int. J. Mol. Sci. 2023, 24, 1712. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Borges, M.; Fernandes, J.; Nascimento, H.; Sameiro-Faria, M.; Miranda, V.; Reis, F.; Belo, L.; Costa, E.; Santos-Silva, A. Apoptosis of Peripheral Cd4(+) T-Lymphocytes in End-Stage Renal Disease Patients under Hemodialysis and Rhepo Therapies. Ren. Fail. 2011, 33, 138–143. [Google Scholar] [CrossRef]
- Xiang, F.F.; Zhu, J.M.; Cao, X.S.; Shen, B.; Zou, J.Z.; Liu, Z.H.; Zhang, H.; Teng, J.; Liu, H.; Ding, X.Q. Lymphocyte Depletion and Subset Alteration Correlate to Renal Function in Chronic Kidney Disease Patients. Ren. Fail. 2016, 38, 7–14. [Google Scholar] [CrossRef]
- Moser, B.; Roth, G.; Brunner, M.; Lilaj, T.; Deicher, R.; Wolner, E.; Kovarik, J.; Boltz-Nitulescu, G.; Vychytil, A.; Ankersmit, H.J. Aberrant T Cell Activation and Heightened Apoptotic Turnover in End-Stage Renal Failure Patients: A Comparative Evaluation between Non-Dialysis, Haemodialysis, and Peritoneal Dialysis. Biochem. Biophys. Res. Commun. 2003, 308, 581–585. [Google Scholar] [CrossRef]
- Fernandez-Fresnedo, G.; Ramos, M.A.; Gonzalez-Pardo, M.C.; de Francisco, A.L.; Lopez-Hoyos, M.; Arias, M. B Lymphopenia in Uremia Is Related to an Accelerated in Vitro Apoptosis and Dysregulation of Bcl-2. Nephrol. Dial. Transplant. 2000, 15, 502–510. [Google Scholar] [CrossRef]
- Litjens, N.H.; van Druningen, C.J.; Betjes, M.G. Progressive Loss of Renal Function Is Associated with Activation and Depletion of Naive T Lymphocytes. Clin. Immunol. 2006, 118, 83–91. [Google Scholar] [CrossRef]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent Cd14+Cd16+ Monocytes Exhibit Proinflammatory and Proatherosclerotic Activity. J. Immunol. 2011, 186, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.H.; Ulrich, C.; Seibert, E.; Seiler, S.; Marell, J.; Reichart, B.; Krause, M.; Schlitt, A.; Kohler, H.; Girndt, M. Cd14(++)Cd16+ Monocytes but Not Total Monocyte Numbers Predict Cardiovascular Events in Dialysis Patients. Kidney Int. 2008, 73, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.; Carracedo, J.; Berdud, I.; Carretero, D.; Merino, A.; Rodriguez, M.; Tetta, C.; Martin-Malo, A.; Aljama, P. Microinflammation in Hemodialysis Is Related to a Preactivated Subset of Monocytes. Hemodial. Int. 2006, 10 (Suppl. S1), S24–S27. [Google Scholar] [CrossRef]
- Girndt, M.; Sester, M.; Sester, U.; Kaul, H.; Kohler, H. Defective Expression of B7-2 (Cd86) on Monocytes of Dialysis Patients Correlates to the Uremia-Associated Immune Defect. Kidney Int. 2001, 59, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Verkade, M.A.; van Druningen, C.J.; Vaessen, L.M.; Hesselink, D.A.; Weimar, W.; Betjes, M.G. Functional Impairment of Monocyte-Derived Dendritic Cells in Patients with Severe Chronic Kidney Disease. Nephrol. Dial. Transplant. 2007, 22, 128–138. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Tai, J.J.; Ong, S.M.; Dang, T.M.; Wong, S.C. The Three Human Monocyte Subsets: Implications for Health and Disease. Immunol. Res. 2012, 53, 41–57. [Google Scholar] [CrossRef]
- Goldstein, J.S.; Chen, T.; Gubina, E.; Pastor, R.W.; Kozlowski, S. Icam-1 Enhances Mhc-Peptide Activation of Cd8(+) T Cells without an Organized Immunological Synapse. Eur. J. Immunol. 2000, 30, 3266–3270. [Google Scholar] [CrossRef]
- Petho, A.G.; Fulop, T.; Orosz, P.; Szenasi, G.; Tapolyai, M.; Dezsi, L. Increased Cardiovascular Mortality in Hemodialysis: The Role of Chronic Inflammation, Complement Activation, and Non-Biocompatibility. Toxins 2025, 17, 345. [Google Scholar] [CrossRef]
- Song, Z.; Tsou, S.; Martin, F.; Kayumov, M.; Xiao, Y.; Zhou, H.; Abdi, R.; Tullius, S.G. Kidney Disease as a Driver of Immunosenescence: Mechanisms and Potential Interventions. J. Am. Soc. Nephrol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Francis, M.D.; Bologna, C.; Moncaglieri, F.; Riva, A.; Morazzoni, P.; Allegrini, P.; Isu, A.; Vigo, B.; Guerriero, F.; et al. Performance of Edmonton Frail Scale on Frailty Assessment: Its Association with Multi-Dimensional Geriatric Conditions Assessed with Specific Screening Tools. BMC Geriatr. 2017, 17, 2. [Google Scholar] [CrossRef]
- Chowdhury, R.; Peel, N.M.; Krosch, M.; Hubbard, R.E. Frailty and Chronic Kidney Disease: A Systematic Review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef]
- Kennard, A.; Glasgow, N.; Rainsford, S.; Talaulikar, G. Frailty in Chronic Kidney Disease: Challenges in Nephrology Practice. A Review of Current Literature. Intern. Med. J. 2023, 53, 465–472. [Google Scholar] [CrossRef]
- Wesson, D.E.; Mathur, V.; Tangri, N. Metabolic Basis and Pathogenesis of Skeletal Muscle Dysfunction as Cause of Frailty in Chronic Kidney Disease. Am. J. Nephrol. 2022, 53, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Gill, J.S.; Bello, A.K.; Hemmelgarn, B.R.; Chan, C.T.; Lloyd, A.; Thadhani, R.I.; Thompson, S. Frailty and Clinical Outcomes in Patients Treated with Hemodialysis: A Prospective Cohort Study. Kidney Med. 2023, 5, 100684. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, A.; Ma, L.; Jia, L.; Chhetri, J.K.; Chan, P. Severity of Frailty as a Significant Predictor of Mortality for Hemodialysis Patients: A Prospective Study in China. Int. J. Med. Sci. 2021, 18, 3309–3317. [Google Scholar] [CrossRef]
- Hubbard, R.E.; O’Mahony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and Frailty Measures in Older People. J. Cell. Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hamilton, R.G.; Weng, N.P.; Xue, Q.L.; Bream, J.H.; Li, H.; Tian, J.; Yeh, S.H.; Resnick, B.; Xu, X.; et al. Frailty Is Associated with Impairment of Vaccine-Induced Antibody Response and Increase in Post-Vaccination Influenza Infection in Community-Dwelling Older Adults. Vaccine 2011, 29, 5015–5021. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Cattaneo, F.; D’Amelio, P. Association between Immunosenescence, Mitochondrial Dysfunction and Frailty Syndrome in Older Adults. Cells 2022, 12, 44. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Akbar, A.N. Aging and frailty immune landscape. Nat. Aging 2022, 2, 280–281. [Google Scholar] [CrossRef]
- Navarro-Martinez, R.; Cauli, O. Lymphocytes as a Biomarker of Frailty Syndrome: A Scoping Review. Diseases 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Cybularz, M.; Wydra, S.; Berndt, K.; Poitz, D.M.; Barthel, P.; Alkouri, A.; Heidrich, F.M.; Ibrahim, K.; Jellinghaus, S.; Speiser, U.; et al. Frailty Is Associated with Chronic Inflammation and Pro-Inflammatory Monocyte Subpopulations. Exp. Gerontol. 2021, 149, 111317. [Google Scholar] [CrossRef]
- Cafiero, C.; Gigante, M.; Brunetti, G.; Simone, S.; Chaoul, N.; Oranger, A.; Ranieri, E.; Colucci, S.; Pertosa, G.B.; Grano, M.; et al. Inflammation Induces Osteoclast Differentiation from Peripheral Mononuclear Cells in Chronic Kidney Disease Patients: Crosstalk between the Immune and Bone Systems. Nephrol. Dial. Transplant. 2018, 33, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.R.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of Sarcopenia in Patients with Chronic Kidney Disease: A Global Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Martinez de Toda, I.; Vida, C.; Diaz-Del Cerro, E.; De la Fuente, M. The Immunity Clock. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1939–1945. [Google Scholar] [CrossRef]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A Clinically Meaningful Metric of Immune Age Derived from High-Dimensional Longitudinal Monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Crepin, T.; Gaiffe, E.; Courivaud, C.; Roubiou, C.; Laheurte, C.; Moulin, B.; Frimat, L.; Rieu, P.; Mousson, C.; Durrbach, A.; et al. Pre-Transplant End-Stage Renal Disease-Related Immune Risk Profile in Kidney Transplant Recipients Predicts Post-Transplant Infections. Transpl. Infect. Dis. 2016, 18, 415–422. [Google Scholar] [CrossRef]
- Zaza, G.; Granata, S.; Rascio, F.; Pontrelli, P.; Dell’Oglio, M.P.; Cox, S.N.; Pertosa, G.; Grandaliano, G.; Lupo, A. A Specific Immune Transcriptomic Profile Discriminates Chronic Kidney Disease Patients in Predialysis from Hemodialyzed Patients. BMC Med. Genom. 2013, 6, 17. [Google Scholar] [CrossRef]
- Kim, K.W.; Chung, B.H.; Jeon, E.J.; Kim, B.M.; Choi, B.S.; Park, C.W.; Kim, Y.S.; Cho, S.G.; Cho, M.L.; Yang, C.W. B Cell-Associated Immune Profiles in Patients with End-Stage Renal Disease (Esrd). Exp. Mol. Med. 2012, 44, 465–472. [Google Scholar] [CrossRef]
- Sasaki, K.; Shoji, T.; Kabata, D.; Shintani, A.; Okute, Y.; Tsuchikura, S.; Shimomura, N.; Tsujimoto, Y.; Nakatani, S.; Mori, K.; et al. Oxidative Stress and Inflammation as Predictors of Mortality and Cardiovascular Events in Hemodialysis Patients: The DREAM Cohort. J. Atheroscler. Thromb. 2021, 28, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation Enhances Cardiovascular Risk and Mortality in Hemodialysis Patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef]
- Greeviroj, P.; Lertussavavivat, T.; Thongsricome, T.; Takkavatakarn, K.; Phannajit, J.; Avihingsanon, Y.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. The World Prevalence, Associated Risk Factors and Mortality of Hepatitis C Virus Infection in Hemodialysis Patients: A Meta-Analysis. J. Nephrol. 2022, 35, 2269–2282. [Google Scholar] [CrossRef]
- Sinnakirouchenan, R.; Holley, J.L. Peritoneal Dialysis Versus Hemodialysis: Risks, Benefits, and Access Issues. Adv. Chronic Kidney Dis. 2011, 18, 428–432. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Huang, C.; Wang, Y.; Lou, L.; Zhao, L.; Xu, S.; Zheng, M.; Li, S. Development of a Prediction Model for in-Hospital Mortality in Immunocompromised Chronic Kidney Diseases Patients with Severe Infection. BMC Nephrol. 2025, 26, 78. [Google Scholar] [CrossRef]
- Kennard, A.; Richardson, A.; Rainsford, S.; Hamilton, K.; Glasgow, N.; Pumpa, K.; Douglas, A.; Talaulikar, G.S. Longitudinal Frailty Assessment in the Prediction of Survival Among Patients with Advanced Chronic Kidney Disease: A Prospective Observational Single-Centre Cohort Study. BMJ Open 2024, 14, e087189. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.; Chen, J.; Hsu, J.; Zhang, X.; Saunders, M.R.; Brown, J.; McAdams-DeMarco, M.; Mohanty, M.J.; Vyas, R.; Hajjiri, Z.; et al. Frailty and Cardiovascular Outcomes in Adults with Ckd: Findings from the Chronic Renal Insufficiency Cohort (Cric) Study. Am. J. Kidney Dis. 2024, 83, 208–215. [Google Scholar] [CrossRef] [PubMed]
| HS | ESRD | HD | PD | KT | |
|---|---|---|---|---|---|
| Number of patients | 18 | 40 | 40 | 36 | 40 |
| Age (years), mean (range) | 51 (21–79) | 61 a (22–82) | 57 (21–84) | 56 (24–82) | 56 (25–80) |
| Female, nº (%) | 9 (50%) | 14 (35%) | 13 (29%) | 17 (47%) | 15 (33%) |
| Etiology of CKD nº (%) | |||||
| Diabetic nephropathy | - | 13 (33%) | 4 (10%) | 5 (14%) | 8 (21%) |
| Nephroangiosclerosis | - | 7 (17%) | 6 (15%) | 9 (26%) | 6 (16%) |
| Glomerular nephropathy | - | 6 (15%) | 11 (28%) | 11 (31%) | 4 (11%) |
| Interstitial nephritis | - | 6 (15%) | 8 (20%) | 4 (11%) | 7 (18%) |
| Polycystic kidney disease | - | 4 (10%) | 1 (2%) | 2 (6%) | 8 (21%) |
| Others | - | 4 (10%) | 10 (25%) | 4 (11%) | 5 (13%) |
| Body composition, mean (range) | |||||
| BMI | 24.6 (20.6–33.7) | 27.4 a (15.7–41.1) | 24.3 bb (17.3–36.4) | 24.8 b (19.0–36.4) | 27.0 c (17.0–39.5) |
| Cardiovascular risk factors,nº (%) | |||||
| Hypertension | 1 (6%) | 36 (90%) aaa | 33 (83%) aaa | 33 (92%) aaa | 39 (98%) aaac |
| Diabetes mellitus | 2 (11%) | 18 (45%) a | 7 (18%) bb | 10 (28%) | 15 (40%) ac |
| Dyslipidaemia | 0 (0%) | 31 (78%) aaa | 23 (58%) aaa | 22 (61%) aaa | 21 (53%) aaab |
| Obesity (BMI ≥ 30 kg/m2) | 2 (11%) | 10 (25%) | 3 (8%) b | 4 (11%) | 8 (24%) |
| Smoking history | 4 (22%) | 11 (28%) | 15 (38%) | 4 (11%) cc | 10 (25%) |
| Treatment,nº (%) | |||||
| Statins | 0 (0) | 30 (75%) aaa | 16 (40%) aabb | 20 (56%) aaa | 23 (58%) aaa |
| Allopurinol | 0 (0) | 24 (60%) aaa | 8 (20%) abbb | 17 (47%) aaac | 11 (28%) abb |
| Antiplatelet | 0 (0) | 8 (20%) a | 16 (40%) aa | 11 (31%) aa | 11 (28%) a |
| Erythropoietin | 0 (0) | 19 (48%) aaa | 40 (100%) aaabbb | 20 (56%) aaaccc | 8 (21%) abbcccddd |
| HS | ESRD | HD | PD | KT | |
|---|---|---|---|---|---|
| eGFR (mL/min/1.73 m2), mean (range) | >90 | 15 aaa (0–86) | 16 aaa (0–66) | 17 aaa (3–80) | 47 bbbcccddd (8–81) |
| Creatinine (mg/dL), mean (range) | 0.8 (0.6–1.1) | 4.3 (2.4–7.8) aaa | 7.8 (2.8–2.5) aaabbb | 7.3 (4.0–5.3) aaabbb | 1.6 (0.9–3.9) abbbcccddd |
| Albumin (g/dL), mean (range) | 4.7 (4.1–5.0) | 4.3 (3.6–5.0) aaa | 4.1 (3.3–4.8) aaa | 3.8 (2.6–4.5) aaabbbc | 4.5 (3.4–5.2) bbbcccddd |
| Proteins (mg/dL), mean (range) | 7.1 (6.5–8.0) | 6.9 (5.5–7.9) | 6.7 (5.2–8.6) aab | 6.5 (4.1–13.6) aaabbb | 7.0 (5.0–7.9) bbccddd |
| Uric acid (mg/dL), mean (range) | 5.0 (2.9–7.1) | 6.5 aa (2.8–11.4) | 5.8 (3.6–8.3) | 5.7 (2.8–8.8) | 6.9 aaacccddd (3.5–9.2) |
| CRP (mg/dL), mean (range) | 0.3 (0.0–2.0) | 0.5 aa (0.0–1.7) | 0.9 aaa (0.0–12.8) | 0.9 a (0.0–10.4) | 0.5 a (0.0–5.5) |
| Component 1 | Component 2 | Component 3 | |
|---|---|---|---|
| Frailty (score) | −0.242 | 0.003 | 0.927 |
| Total lymphocytes (nº) | 0.924 | 0.196 | 0.123 |
| T lymphocytes (nº) | 0.858 | 0.210 | 0.197 |
| B lymphocytes (nº) | 0.554 | 0.165 | −0.137 |
| Intermediate monocytes (%) | −0.201 | 0.619 | 0.004 |
| Amount of B7.2/CD86 expressed by non-classical monocytes | −0.326 | 0.733 | −0.276 |
| Amount of ICAM-1/CD54 expressed by non-classical monocytes | 0.362 | 0.694 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceprián, N.; Martínez de Toda, I.; Caro, P.J.; Yuste, C.; Valera-Arévalo, G.; González de Pablos, I.; Figuer, A.; Alique, M.; Ramírez, R.; Morales, E.; et al. An Immuno-Fragile Profile Is Associated with Mortality Risk in Patients with Chronic Kidney Disease. Biomedicines 2025, 13, 2370. https://doi.org/10.3390/biomedicines13102370
Ceprián N, Martínez de Toda I, Caro PJ, Yuste C, Valera-Arévalo G, González de Pablos I, Figuer A, Alique M, Ramírez R, Morales E, et al. An Immuno-Fragile Profile Is Associated with Mortality Risk in Patients with Chronic Kidney Disease. Biomedicines. 2025; 13(10):2370. https://doi.org/10.3390/biomedicines13102370
Chicago/Turabian StyleCeprián, Noemí, Irene Martínez de Toda, Paula Jara Caro, Claudia Yuste, Gemma Valera-Arévalo, Ignacio González de Pablos, Andrea Figuer, Matilde Alique, Rafael Ramírez, Enrique Morales, and et al. 2025. "An Immuno-Fragile Profile Is Associated with Mortality Risk in Patients with Chronic Kidney Disease" Biomedicines 13, no. 10: 2370. https://doi.org/10.3390/biomedicines13102370
APA StyleCeprián, N., Martínez de Toda, I., Caro, P. J., Yuste, C., Valera-Arévalo, G., González de Pablos, I., Figuer, A., Alique, M., Ramírez, R., Morales, E., & Carracedo, J. (2025). An Immuno-Fragile Profile Is Associated with Mortality Risk in Patients with Chronic Kidney Disease. Biomedicines, 13(10), 2370. https://doi.org/10.3390/biomedicines13102370








