Sleep Disorders, Dysregulation of Circadian Rhythms, and Fatigue After Craniopharyngioma—A Narrative Review
Abstract
1. Introduction
2. Search Strategy
- The narrative review is based on a literature search of Web of Science, MEDLINE/PubMed, and Embase databases for initial identification of publications. The articles were identified using the following keywords: a) “craniopharyngioma” and b) “sleep,” “sleep disorders,” “sleep-related breathing disorders,” “sleep-disordered breathing,” “excessive daytime sleepiness,” “hypersomnolence,” “narcolepsy,” “fatigue,” “circadian rhythm,” “melatonin,” “stimulant.” Selected English language papers published between 1970 and February 2025 were included in our review. Only studies published in peer-reviewed journals were considered.
- The inclusion criteria, based on the PICOS approach, were as follows: participants (P): patients diagnosed with craniopharyngioma; intervention (I): no restrictions; comparison (C): no restrictions; outcomes (O): sleep-related parameters; study design (S): original studies. Exclusion criteria included: (1) non-English language publications; (2) animal studies or in vitro research; (3) studies involving participants with craniopharyngioma with additional central nervous system complications; (4) reviews, case report, thesis, and conference proceedings. In cases where multiple studies were published based on the same databases, only the study with the largest sample size was included in the analysis. Thirty-eight publications fulfilled the above-mentioned criteria (Figure 1).
- The data extracted from all studies in line with our research objectives included participants characteristics (i.e., age of the subjects, age of tumor onset, clinical characteristics), procedures used to evaluate sleep disorders (subjective and/or objective investigations), type of sleep disturbance, any additional examinations (melatonin dosage), treatment and efficacy, if available.
3. Results
3.1. Pathophysiology of Sleep Disorders in Craniopharyngioma
3.2. Assessment of Sleep Disturbances
3.3. Excessive Daytime Sleepiness
3.4. Secondary Narcolepsy
3.5. Sleep-Disordered Breathing/Obstructive Sleep Apnea
3.6. Disorders of Circadian Rhythms
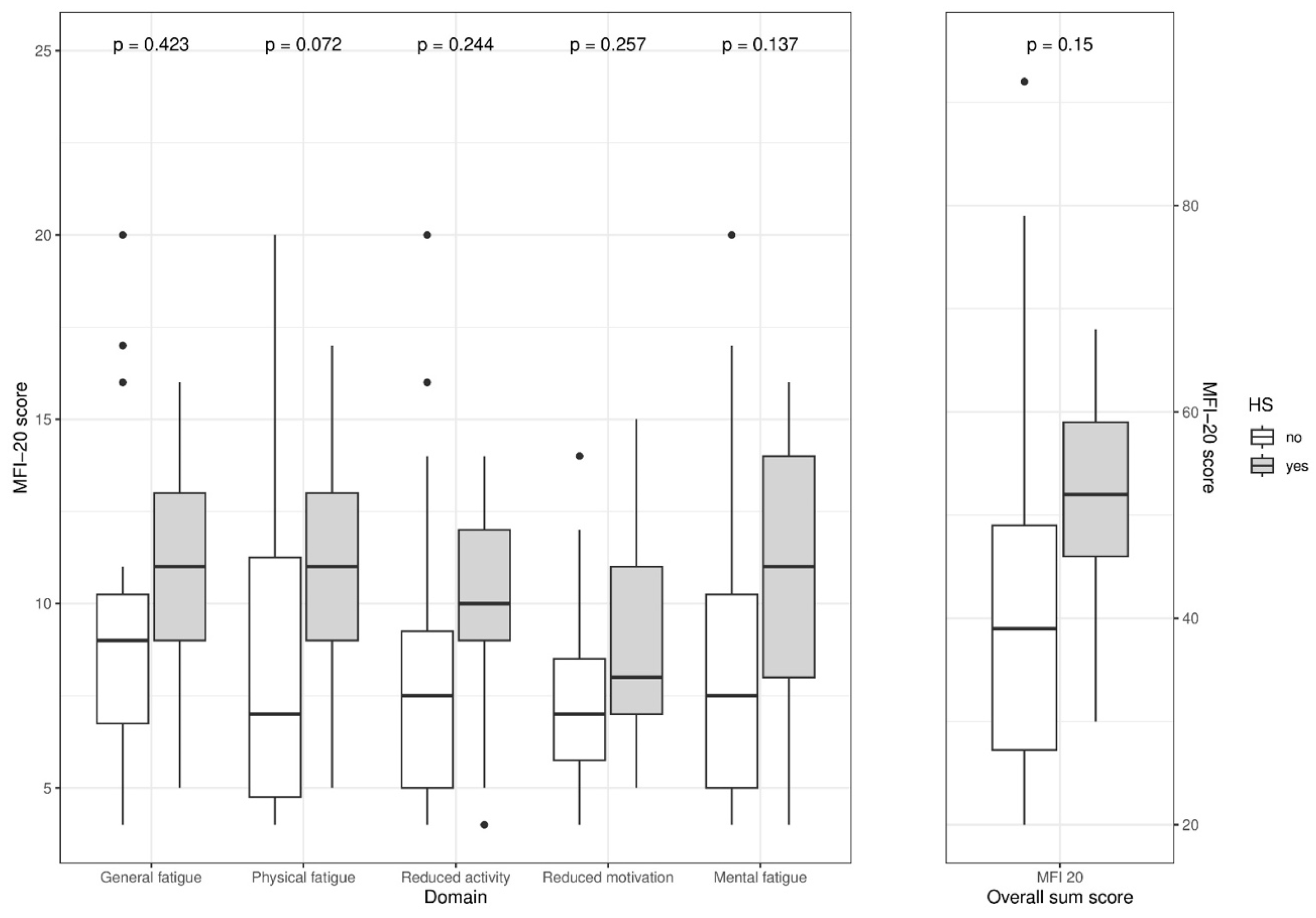
3.7. Fatigue
3.8. Treatment of Sleep Disturbances
4. Summary of Recommendations for Management of Sleep Disturbances
- Melatonin substitution should be initiated in patients with CP and sleep disturbances to reduce excessive daytime sleepiness (guideline: [114]).
- Central stimulants, for instance dextroamphetamine, modafinil, and methylphenidate, should be considered to reduce excessive daytime sleepiness in CP patients (guideline: [116]).
5. Future Perspectives in Management of Sleep Disturbances
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea hypopnea index |
| BMI | Body mass index |
| CI | Confidence interval |
| CNS | Central nervous system |
| CPAP | Continuous positive airway pressure |
| CP | Craniopharyngioma |
| CSF | Cerebrospinal fluid |
| EDS | Excessive daytime sleepiness |
| EEG | Electroencephalogram |
| ESS | Epworth sleepiness scale |
| HI | Hypothalamic injury |
| MFI | Multidimensional Fatigue Inventory |
| MRI | Magnetic resonance imaging |
| MSLT | Multiple sleep latency test |
| QoL | Quality of life |
| OSAS | Obstructive sleep apnea syndrome |
| PedsQL | Pediatric Quality of Life InventoryTM |
| PEDQOL | Padiatric Quality of Life Questionnaire |
| PSG | Polysomnography |
| PSQI | Pittsburgh Sleep Quality Index |
| REM | Rapid eye movement |
| SDB | Sleep disordered breathing |
| SOREMP | Sleep-onset rapid eye movement phase |
References
- Kultursay, N.; Gelal, F.; Mutluer, S.; Senrecper, S.; Oziz, E.; Oral, R. Antenatally diagnosed neonatal craniopharyngioma. J. Perinatol. Off. J. Calif. Perinat. Assoc. 1995, 15, 426–428. [Google Scholar]
- Muller, H.L. Craniopharyngioma. Endocr. Rev. 2014, 35, 513–543. [Google Scholar] [CrossRef]
- Muller, H.L.; Merchant, T.E.; Warmuth-Metz, M.; Martinez-Barbera, J.P.; Puget, S. Craniopharyngioma. Nat. Rev. Dis. Primers 2019, 5, 75. [Google Scholar] [CrossRef]
- Piloni, M.; Gagliardi, F.; Bailo, M.; Losa, M.; Boari, N.; Spina, A.; Mortini, P. Craniopharyngioma in Pediatrics and Adults. Adv. Exp. Med. Biol. 2023, 1405, 299–329. [Google Scholar] [CrossRef]
- Hamblin, R.; Tsermoulas, G.; Karavitaki, N. Craniopharyngiomas. Presse Med. 2021, 50, 104078. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.K.-D.B.; Komori, T.; Müller, H.L.; Pietsch, T. Adamantinomatous craniopharyngioma. In WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; Brat, D.J.G.A., Wesseling, P., Eds.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, pp. 393–396. [Google Scholar]
- Prieto, R.; Juratli, T.A.; Bander, E.D.; Santagata, S.; Barrios, L.; Brastianos, P.K.; Schwartz, T.H.; Pascual, J.M. Papillary Craniopharyngioma: An Integrative and Comprehensive Review. Endocr. Rev. 2025, 46, 151–213. [Google Scholar] [CrossRef]
- Beckhaus, J.; Boekhoff, S.; Scheinemann, K.; Schilling, F.H.; Fleischhack, G.; Binder, G.; Bison, B.; Pietsch, T.; Friedrich, C.; Müller, H.L. Perinatally diagnosed congenital craniopharyngiomas in the KRANIOPHARYNGEOM trials. Endocr. Connect. 2023, 12, e230294. [Google Scholar] [CrossRef] [PubMed]
- Witte, J.; Surmann, B.; Batram, M.; Weinert, M.; Flume, M.; Touchot, N.; Beckhaus, J.; Friedrich, C.; Muller, H.L. Hypothalamic obesity: Epidemiology in rare sellar/suprasellar tumors-A German claims database analysis. J. Neuroendocrinol. 2024, 36, e13439. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.L.; Witte, J.; Surmann, B.; Batram, M.; Braegelmann, K.; Flume, M.; Beckhaus, J.; Touchot, N.; Friedrich, C. Treatment of patients with tumor/treatment-related hypothalamic obesity in the first two years following surgical treatment or radiotherapy. Sci. Rep. 2025, 15, 2118. [Google Scholar] [CrossRef]
- Apps, J.R.; Muller, H.L.; Hankinson, T.C.; Yock, T.I.; Martinez-Barbera, J.P. Contemporary Biological Insights and Clinical Management of Craniopharyngioma. Endocr. Rev. 2023, 44, 518–538. [Google Scholar] [CrossRef]
- van Schaik, J.; Hoving, E.W.; Muller, H.L.; van Santen, H.M. Hypothalamic-Pituitary Outcome after Treatment for Childhood Craniopharyngioma. Front. Horm. Res. 2021, 54, 47–57. [Google Scholar] [CrossRef]
- van Roessel, I.; Hulsmann, S.C.; Schouten-van Meeteren, A.Y.N.; Hoving, E.W.; Janssens, G.O.; Raphael, M.F.; Zijtregtop-Blom, E.A.M.; de Vos-Kerkhof, E.; Bakker, B.; Tissing, W.J.E.; et al. The many different clinical faces of acquired hypothalamic dysfunction: A retrospective cohort study in the Netherlands. EClinicalMedicine 2025, 85, 103313. [Google Scholar] [CrossRef]
- Dimitri, P. The management of hypothalamic obesity in craniopharyngioma. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 102018. [Google Scholar] [CrossRef]
- Blacha, A.K.; Kropp, P.; Rahvar, A.H.; Flitsch, J.; van de Loo, I.; Harbeck, B. Poor quality of life and sleep in patients with adrenal insufficiency-another cause of increased mortality? Ir. J. Med. Sci. 2022, 191, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.W.; Cerbone, M.; Dattani, M.T. Appetite- and Weight-Regulating Neuroendocrine Circuitry in Hypothalamic Obesity. Endocr. Rev. 2024, 45, 309–342. [Google Scholar] [CrossRef] [PubMed]
- Argente, J.; Farooqi, I.S.; Chowen, J.A.; Kuhnen, P.; Lopez, M.; Morselli, E.; Gan, H.W.; Spoudeas, H.A.; Wabitsch, M.; Tena-Sempere, M. Hypothalamic obesity: From basic mechanisms to clinical perspectives. Lancet Diabetes Endocrinol. 2025, 13, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; McCormack, S.E. Acquired hypothalamic obesity: A clinical overview and update. Diabetes Obes. Metab. 2024, 26 (Suppl. 2), 34–45. [Google Scholar] [CrossRef]
- Beckhaus, J.; Friedrich, C.; Boekhoff, S.; Calaminus, G.; Bison, B.; Eveslage, M.; Timmermann, B.; Flitsch, J.; Müller, H.L. Outcome after pediatric craniopharyngioma—The role of age at diagnosis and hypothalamic damage. Eur. J. Endocrinol. 2023, 188, 300–309. [Google Scholar] [CrossRef]
- Sterkenburg, A.S.; Hoffmann, A.; Gebhardt, U.; Warmuth-Metz, M.; Daubenbuchel, A.M.; Muller, H.L. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: Newly reported long-term outcomes. Neuro Oncol. 2015, 17, 1029–1038. [Google Scholar] [CrossRef]
- Eveslage, M.; Calaminus, G.; Warmuth-Metz, M.; Kortmann, R.D.; Pohl, F.; Timmermann, B.; Schuhmann, M.U.; Flitsch, J.; Faldum, A.; Muller, H.L. The Postoperative Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch. Arztebl. Int. 2019, 116, 321–328. [Google Scholar] [CrossRef]
- Muller, H.L. Long-term quality of life and hypothalamic dysfunction after craniopharyngioma. J. Neuro-Oncol. 2025, 173, 233–244. [Google Scholar] [CrossRef]
- Perez-Torres Lobato, M.R.; Morell, M.; Solano-Paez, P.; Ortiz-Palacios, M.; Menargez, A.; Panesso, M.; Vazquez-Gomez, F.; Moreno-Carrasco, J.; Lassaletta, A.; Santa-Maria, V.; et al. Long-term sequelae and quality of life after childhood-onset craniopharyngioma: Results of a Spanish multicenter study. Pediatr. Blood Cancer 2024, 71, e31343. [Google Scholar] [CrossRef]
- Baqai, M.W.S.; Shah, Z.; Malik, M.J.A.; Zia, N.; Shafqat, S.; Zahid, N.; Shamim, M.S. Quality of life of pediatric patients with craniopharyngioma: A retrospective series from a low-middle-income country with more than 4 years follow-up. Surg. Neurol. Int. 2024, 15, 199. [Google Scholar] [CrossRef]
- Sowithayasakul, P.; Beckhaus, J.; Boekhoff, S.; Friedrich, C.; Calaminus, G.; Müller, H.L. Vision-related quality of life in patients with childhood-onset craniopharyngioma. Sci. Rep. 2023, 13, 19599. [Google Scholar] [CrossRef] [PubMed]
- Boekhoff, S.; Bison, B.; Genzel, D.; Eveslage, M.; Otte, A.; Friedrich, C.; Flitsch, J.; Müller, H.L. Cerebral Infarction in Childhood-Onset Craniopharyngioma Patients: Results of KRANIOPHARYNGEOM 2007. Front. Oncol. 2021, 11, 698150. [Google Scholar] [CrossRef]
- Sowithayasakul, P.; Buschmann, L.K.; Boekhoff, S.; Muller, H.L. Cardiac remodeling in patients with childhood-onset craniopharyngioma—Results of HIT-Endo and KRANIOPHARYNGEOM 2000/2007. Eur. J. Pediatr. 2021, 180, 1593–1602. [Google Scholar] [CrossRef]
- van Santen, H.M. The Central Control of Energy Metabolism: Hypothalamic Obesity Is Not One Disease. Horm. Res. Paediatr. 2025, 1–10. [Google Scholar] [CrossRef]
- Siklar, Z.; Ozsu, E.; Kizilcan Cetin, S.; Ozen, S.; Cizmecioglu-Jones, F.; Balki, H.G.; Aycan, Z.; Goksen, D.; Kilci, F.; Abseyi, S.N.; et al. Comprehensive Insights Into Pediatric Craniopharyngioma: Endocrine and Metabolic Profiles, Treatment Challenges, and Long-term Outcomes from a Multicenter Study. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 288–296. [Google Scholar] [CrossRef]
- Kayadjanian, N.; Hsu, E.A.; Wood, A.M.; Carson, D.S. Caregiver Burden and Its Relationship to Health-Related Quality of Life in Craniopharyngioma Survivors. J. Clin. Endocrinol. Metab. 2023, 109, e76–e87. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, I.; de Graaf, J.P.; Biermasz, N.R.; Charmandari, E.; van Santen, H.M. Acquired hypothalamic dysfunction in childhood: ‘what do patients need?’—An Endo-ERN survey. Endocr. Connect. 2023, 12, e230147. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Barrea, L.; Solari, D.; Riccio, E.; Arianna, R.; Cavallo, L.M.; Romano, F.; Di Benedetto, E.; Rodriguez, A.; et al. Craniopharyngioma, Chronotypes and Metabolic Risk Profile. Nutrients 2021, 13, 3444. [Google Scholar] [CrossRef]
- Witte, J.; Touchot, N.; Surmann, B.; Braegelmann, K.; Flume, M.; Beckhaus, J.; Friedrich, C.; Muller, H.L. Economics of hypothalamic obesity in patients with craniopharyngioma and other rare sellar/suprasellar tumors. Eur. J. Health Econ. 2025, 1–11. [Google Scholar] [CrossRef]
- Muller, H.L.; Muller-Stover, S.; Gebhardt, U.; Kolb, R.; Sorensen, N.; Handwerker, G. Secondary narcolepsy may be a causative factor of increased daytime sleepiness in obese childhood craniopharyngioma patients. J. Pediatr. Endocrinol. Metab. 2006, 19 (Suppl. 1), 423–429. [Google Scholar] [CrossRef]
- Müller, H.L.; Handwerker, G.; Gebhardt, U.; Faldum, A.; Emser, A.; Kolb, R.; Sorensen, N. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 2006, 17, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.L.; Handwerker, G.; Wollny, B.; Faldum, A.; Sorensen, N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J. Clin. Endocrinol. Metab. 2002, 87, 3993–3996. [Google Scholar] [CrossRef]
- Cordani, R.; Veneruso, M.; Napoli, F.; Di Iorgi, N.; Milanaccio, C.; Consales, A.; Disma, N.; De Grandis, E.; Maghnie, M.; Nobili, L. Sleep Disturbances in Pediatric Craniopharyngioma: A Systematic Review. Front. Neurol. 2022, 13, 876011. [Google Scholar] [CrossRef] [PubMed]
- Romigi, A.; Mercuri, N.B.; Caccamo, M.; Testa, F.; Vitrani, G.; Tripaldi, M.C.; Centonze, D.; Jacoangeli, F. Subjective sleep disorders and daytime sleepiness in patients with restrictive type anorexia nervosa and effects on quality of life: A case-control study. Sleep Biol. Rhythm. 2022, 20, 181–189. [Google Scholar] [CrossRef]
- Romigi, A.; Feola, T.; Cappellano, S.; De Angelis, M.; Pio, G.; Caccamo, M.; Testa, F.; Vitrani, G.; Centonze, D.; Colonnese, C.; et al. Sleep Disorders in Patients With Craniopharyngioma: A Physiopathological and Practical Update. Front. Neurol. 2021, 12, 817257. [Google Scholar] [CrossRef] [PubMed]
- Dodet, P.; Noiray, C.; Leu-Semenescu, S.; Lefevre, E.; Nigam, M.; Faucher, P.; Maranci, J.B.; Jublanc, C.; Poitou, C.; Arnulf, I. Hypersomnia and narcolepsy in 42 adult patients with craniopharyngioma. Sleep 2023, 46, zsad032. [Google Scholar] [CrossRef]
- Müller, H.L.; Tauber, M.; Lawson, E.A.; Özyurt, J.; Bison, B.; Martinez-Barbera, J.-P.; Puget, S.; Merchant, T.E.; van Santen, H.M. Hypothalamic syndrome. Nat. Rev. Dis. Primers 2022, 8, 24. [Google Scholar] [CrossRef]
- Beckhaus, J.; Friedrich, C.; Müller, H.L. Childhood-onset craniopharyngioma—A life-long family burden? J. Clin. Endocrinol. Metab. 2023, 109, e1404–e1405. [Google Scholar] [CrossRef]
- Semko, J.; Al Ghriwati, N.; Winter, M.; Merchant, T.E.; Crabtree, V.M. Sleep-related challenges and family functioning in children and adolescents previously treated for craniopharyngioma. J. Psychosoc. Oncol. 2024, 42, 32–47. [Google Scholar] [CrossRef]
- Witcraft, S.M.; Wickenhauser, M.E.; Russell, K.M.; Mandrell, B.N.; Conklin, H.M.; Merchant, T.E.; Crabtree, V.M. A Latent Profile Analysis of Sleep, Anxiety, and Mood in Youth with Craniopharyngioma. Behav. Sleep Med. 2022, 20, 762–773. [Google Scholar] [CrossRef]
- van Santen, H.M.; Muller, H.L. Management of Acquired Hypothalamic Dysfunction and the Hypothalamic Syndrome; it is more than obesity. Endocr. Rev. 2025. [Google Scholar] [CrossRef]
- Erfurth, E.M.; Muller, H.L. Metabolic complications and their mechanisms in patients with craniopharyngioma. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 101999. [Google Scholar] [CrossRef]
- Niel, K.A.; Klages, K.L.; Merchant, T.E.; Wise, M.S.; Hancock, D.; Caples, M.; Mandrell, B.N.; Conklin, H.M.; Crabtree, V.M. Impact of sleep, neuroendocrine, and executive function on health-related quality of life in young people with craniopharyngioma. Dev. Med. Child Neurol. 2021, 63, 984–990. [Google Scholar] [CrossRef]
- Cleare, A.J. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol. Metab. 2004, 15, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Landmark-Høyvik, H.; Reinertsen, K.V.; Loge, J.H.; Kristensen, V.N.; Dumeaux, V.; Fosså, S.D.; Børresen-Dale, A.L.; Edvardsen, H. The genetics and epigenetics of fatigue. PM&R 2010, 2, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Klages, K.L.; Berlin, K.S.; Cook, J.L.; Merchant, T.E.; Wise, M.S.; Mandrell, B.N.; Conklin, H.M.; Crabtree, V.M. Health-related quality of life, obesity, fragmented sleep, fatigue, and psychosocial problems among youth with craniopharyngioma. Psychooncology 2021, 31, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Penson, A.; Walraven, I.; Bronkhorst, E.; Maurice-Stam, H.; Grootenhuis, M.A.; Van der Heiden-van der Loo, M.; Tissing, W.J.E.; Van der Pal, H.J.H.; De Vries, A.C.H.; Bresters, D.; et al. The Impact of Cancer-Related Fatigue on HRQOL in Survivors of Childhood Cancer: A DCCSS LATER Study. Cancers 2022, 14, 2851. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Mock, V.; Atkinson, A.; Barsevick, A.; Cella, D.; Cimprich, B.; Cleeland, C.; Donnelly, J.; Eisenberger, M.A.; Escalante, C.; Hinds, P.; et al. NCCN Practice Guidelines for Cancer-Related Fatigue. Oncology 2000, 14, 151–161. [Google Scholar]
- Muller, H.L. The Diagnosis and Treatment of Craniopharyngioma. Neuroendocrinology 2020, 110, 753–766. [Google Scholar] [CrossRef]
- Muller, H.L. Management of Acquired Hypothalamic Obesity After Childhood-Onset Craniopharyngioma-A Narrative Review. Biomedicines 2025, 13, 1016. [Google Scholar] [CrossRef]
- Kolen, E.R.; Horvai, A.; Perry, V.; Gupta, N. Congenital craniopharyngioma: A role for imaging in the prenatal diagnosis and treatment of an uncommon tumor. Fetal Diagn. Ther. 2003, 18, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Krauchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocr. 2003, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Pickering, L.; Jennum, P.; Gammeltoft, S.; Poulsgaard, L.; Feldt-Rasmussen, U.; Klose, M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2014, 170, 873–884. [Google Scholar] [CrossRef]
- Joustra, S.D.; Thijs, R.D.; van den Berg, R.; van Dijk, M.; Pereira, A.M.; Lammers, G.J.; van Someren, E.J.; Romijn, J.A.; Biermasz, N.R. Alterations in diurnal rhythmicity in patients treated for nonfunctioning pituitary macroadenoma: A controlled study and literature review. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2014, 171, 217–228. [Google Scholar] [CrossRef]
- Gallopin, T.; Fort, P.; Eggermann, E.; Cauli, B.; Luppi, P.H.; Rossier, J.; Audinat, E.; Muhlethaler, M.; Serafin, M. Identification of sleep-promoting neurons in vitro. Nature 2000, 404, 992–995. [Google Scholar] [CrossRef]
- Donlea, J.M.; Alam, M.N.; Szymusiak, R. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr. Opin. Neurobiol. 2017, 44, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Ono, D.; Yamanaka, A. Hypothalamic regulation of the sleep/wake cycle. Neurosci. Res. 2017, 118, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Greco, M.A.; Shiromani, P.; Saper, C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 2000, 20, 3830–3842. [Google Scholar] [CrossRef]
- Jurkowlaniec, E.; Trojniar, W.; Tokarski, J. Daily pattern of EEG activity in rats with lateral hypothalamic lesions. J. Physiol. Pharmacol. 1994, 45, 399–411. [Google Scholar]
- Nishino, S.; Ripley, B.; Overeem, S.; Lammers, G.J.; Mignot, E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000, 355, 39–40. [Google Scholar] [CrossRef]
- Mignot, E.; Zeitzer, J.; Pizza, F.; Plazzi, G. Sleep Problems in Narcolepsy and the Role of Hypocretin/Orexin Deficiency. Front. Neurol. Neurosci. 2021, 45, 103–116. [Google Scholar] [CrossRef]
- Muller, H.L. Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: Review of the literature and perspectives. Int. J. Endocrinol. 2010, 2010, 519607. [Google Scholar] [CrossRef]
- Khan, R.B.; Merchant, T.E.; Sadighi, Z.S.; Bello, M.S.; Lu, Z.; Sykes, A.; Wise, M.S.; Crabtree, V.M.; Zabrowski, J.; Simmons, A.; et al. Prevalence, risk factors, and response to treatment for hypersomnia of central origin in survivors of childhood brain tumors. J. Neuro-Oncol. 2018, 136, 379–384. [Google Scholar] [CrossRef]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 2259. [Google Scholar] [CrossRef]
- Mandrell, B.N.; Guo, Y.; Li, Y.; Hancock, D.; Caples, M.; Ashford, J.M.; Merchant, T.E.; Conklin, H.M.; Crabtree, V.M. Internalizing Symptoms and Their Impact on Patient-Reported Health-Related Quality of Life and Fatigue among Patients with Craniopharyngioma During Proton Radiation Therapy. Children 2024, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, T.; Kramer, M.; Alessi, C.; Friedman, L.; Boehlecke, B.; Brown, T.; Coleman, J.; Kapur, V.; Lee-Chiong, T.; Owens, J.; et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An american academy of sleep medicine report. Sleep 2006, 29, 1415–1419. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Ward, S.D.; Sheldon, S.H.; Shiffman, R.N.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, e714–e755. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Cordani, R.; Veneruso, M.; Napoli, F.; Milanaccio, C.; Verrico, A.; Consales, A.; Cataldi, M.; Fava, D.; Di Iorgi, N.; Maghnie, M.; et al. Sleep disturbances in craniopharyngioma: A challenging diagnosis. J. Neurol. 2021, 268, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Dement, W.C.; Mitler, M.M.; Roth, T.; Westbrook, P.R.; Keenan, S. Guidelines for the multiple sleep latency test (MSLT): A standard measure of sleepiness. Sleep 1986, 9, 519–524. [Google Scholar] [CrossRef]
- Tachibana, N.; Taniike, M.; Okinaga, T.; Ripley, B.; Mignot, E.; Nishino, S. Hypersomnolence and increased REM sleep with low cerebrospinal fluid hypocretin level in a patient after removal of craniopharyngioma. Sleep Med. 2005, 6, 567–569. [Google Scholar] [CrossRef]
- Marcus, C.L.; Trescher, W.H.; Halbower, A.C.; Lutz, J. Secondary narcolepsy in children with brain tumors. Sleep 2002, 25, 435–439. [Google Scholar] [CrossRef]
- Mandrell, B.N.; LaRosa, K.; Hancock, D.; Caples, M.; Sykes, A.; Lu, Z.; Wise, M.S.; Khan, R.B.; Merchant, T.E.; McLaughlin-Crabtree, V. Predictors of narcolepsy and hypersomnia due to medical disorder in pediatric craniopharyngioma. J. Neuro-Oncol. 2020, 148, 307–316. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Lee-Chiong, T.; Alessi, C.; Friedman, L.; Aurora, R.N.; Boehlecke, B.; Brown, T.; Chesson, A.L., Jr.; Kapur, V.; Maganti, R.; et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007, 30, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Killeffer, F.A.; Stern, W.E. Chronic effects of hypothalamic injury. Report of a case of near total hypothalamic destruction resulting from removal of a craniopharyngioma. Arch. Neurol. 1970, 22, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Poretti, A.; Grotzer, M.A.; Ribi, K.; Schonle, E.; Boltshauser, E. Outcome of craniopharyngioma in children: Long-term complications and quality of life. Dev. Med. Child Neurol. 2004, 46, 220–229. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Biermasz, N.R.; Pereira, A.M.; van Kralingen, K.W.; Dekkers, O.M.; Rabe, K.F.; Smit, J.W.; Romijn, J.A. Patients cured from craniopharyngioma or nonfunctioning pituitary macroadenoma (NFMA) suffer similarly from increased daytime somnolence despite normal sleep patterns compared to healthy controls. Clin. Endocrinol. 2008, 69, 769–774. [Google Scholar] [CrossRef]
- Manley, P.E.; McKendrick, K.; McGillicudy, M.; Chi, S.N.; Kieran, M.W.; Cohen, L.E.; Kothare, S.; Michael Scott, R.; Goumnerova, L.C.; Sun, P.; et al. Sleep dysfunction in long term survivors of craniopharyngioma. J. Neuro-Oncol. 2012, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.K.; Woods, C.; Fleming, M.; Rogers, B.; Behan, L.A.; O’Sullivan, E.P.; Kane, T.; Agha, A.; Smith, D.; Costello, R.W.; et al. Somnolence in adult craniopharyngioma patients is a common, heterogeneous condition that is potentially treatable. Clin. Endocrinol. 2011, 74, 750–755. [Google Scholar] [CrossRef]
- Crabtree, V.M.; Klages, K.L.; Sykes, A.; Wise, M.S.; Lu, Z.; Indelicato, D.; Merchant, T.E.; Avent, Y.; Mandrell, B.N. Sensitivity and Specificity of the Modified Epworth Sleepiness Scale in Children With Craniopharyngioma. J. Clin. Sleep Med. 2019, 15, 1487–1493. [Google Scholar] [CrossRef]
- Pickering, L.; Klose, M.; Feldt-Rasmussen, U.; Jennum, P. Polysomnographic findings in craniopharyngioma patients. Sleep Breath. 2017, 21, 975–982. [Google Scholar] [CrossRef]
- Snow, A.; Gozal, E.; Malhotra, A.; Tiosano, D.; Perlman, R.; Vega, C.; Shahar, E.; Gozal, D.; Hochberg, Z.; Pillar, G. Severe hypersomnolence after pituitary/hypothalamic surgery in adolescents: Clinical characteristics and potential mechanisms. Pediatrics 2002, 110, e74. [Google Scholar] [CrossRef]
- Niel, K.; LaRosa, K.N.; Klages, K.L.; Merchant, T.E.; Wise, M.S.; Witcraft, S.M.; Hancock, D.; Caples, M.; Mandrell, B.N.; Crabtree, V.M. Actigraphy versus Polysomnography to Measure Sleep in Youth Treated for Craniopharyngioma. Behav. Sleep Med. 2020, 18, 589–597. [Google Scholar] [CrossRef]
- Jacola, L.M.; Conklin, H.M.; Scoggins, M.A.; Ashford, J.M.; Merchant, T.E.; Mandrell, B.N.; Ogg, R.J.; Curtis, E.; Wise, M.S.; Indelicato, D.J.; et al. Investigating the Role of Hypothalamic Tumor Involvement in Sleep and Cognitive Outcomes Among Children Treated for Craniopharyngioma. J. Pediatr. Psychol. 2016, 41, 610–622. [Google Scholar] [CrossRef]
- Yang, L.; Xie, S.; Tang, B.; Wu, X.; Tong, Z.; Fang, C.; Ding, H.; Bao, Y.; Zheng, S.; Hong, T. Hypothalamic injury patterns after resection of craniopharyngiomas and correlation to tumor origin: A study based on endoscopic observation. Cancer Med. 2020, 9, 8950–8961. [Google Scholar] [CrossRef]
- Mann-Markutzyk, L.V.; Beckhaus, J.; Ozyurt, J.; Mehren, A.; Friedrich, C.; Muller, H.L. Daytime sleepiness and health-related quality of life in patients with childhood-onset craniopharyngioma. Sci. Rep. 2025, 15, 9407. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, N.; Brufani, C.; Warner, J.T.; Adams, C.B.; Richards, P.; Ansorge, O.; Shine, B.; Turner, H.E.; Wass, J.A. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. 2005, 62, 397–409. [Google Scholar] [CrossRef]
- Rose, M.L.; Bajaj, B.V.M.; Jimenez, R.; Dennehy, S.; Allison, K.; Wiltsie, L.; Ebb, D.; MacDonald, S.M.; Tarbell, N.J.; Yock, T.I. Quality of life in pediatric patients treated with adjuvant proton radiation for craniopharyngiomas. J. Neuro-Oncol. 2025, 175, 153–164. [Google Scholar] [CrossRef]
- Bischoff, M.; Khalil, D.A.; Frisch, S.; Backer, C.M.; Peters, S.; Friedrich, C.; Tippelt, S.; Kortmann, R.D.; Bison, B.; Muller, H.L.; et al. Outcome After Modern Proton Beam Therapy in Childhood Craniopharyngioma: Results of the Prospective Registry Study KiProReg. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 137–148. [Google Scholar] [CrossRef]
- Friedrich, C.; Boekhoff, S.; Bischoff, M.; Beckhaus, J.; Sowithayasakul, P.; Calaminus, G.; Eveslage, M.; Valentini, C.; Bison, B.; Harrabi, S.B.; et al. Outcome after proton beam therapy versus photon-based radiation therapy in childhood-onset craniopharyngioma patients-results of KRANIOPHARYNGEOM 2007. Front. Oncol. 2023, 13, 1180993. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Q.; Tian, H.; Wang, M.; Lin, R.; Bai, J.; Wang, D.; Dong, M. Proton beam therapy for craniopharyngioma: A systematic review and meta-analysis. Radiat. Oncol. 2024, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Poiset, S.J.; Song, A.; In Yoon, H.; Huang, J.; Jain, S.; Palmer, J.D.; Matsui, J.K.; Cappelli, L.; Mazza, J.M.; Ali, A.S.; et al. Long-Term Outcomes of Surgery and Radiation Treatment for Adult Patients with Craniopharyngioma. World Neurosurg. 2024, 187, e852–e859. [Google Scholar] [CrossRef]
- Partin, R.E.; Wogksch, M.D.; Dhaduk, R.; Ashford, J.M.; Indelicato, D.J.; Conklin, H.M.; Merchant, T.E.; Ness, K.K. Physical function, body mass index, and fitness outcomes in children, adolescents, and emerging adults with craniopharyngioma from proton therapy through five years of follow-up. J. Neuro-Oncol. 2022, 159, 713–723. [Google Scholar] [CrossRef]
- Madan, R.; Pitts, J.; Patterson, M.C.; Lloyd, R.; Keating, G.; Kotagal, S. Secondary Narcolepsy in Children. J. Child Neurol. 2021, 36, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.; Barateau, L.; Jaussent, I.; Vandi, S.; Antelmi, E.; Mignot, E.; Dauvilliers, Y.; Plazzi, G.; MonBo Study, G. Validation of Multiple Sleep Latency Test for the diagnosis of pediatric narcolepsy type 1. Neurology 2019, 93, e1034–e1044. [Google Scholar] [CrossRef]
- Mignot, E.; Lammers, G.J.; Ripley, B.; Okun, M.; Nevsimalova, S.; Overeem, S.; Vankova, J.; Black, J.; Harsh, J.; Bassetti, C.; et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 2002, 59, 1553–1562. [Google Scholar] [CrossRef]
- Sakuta, K.; Nakamura, M.; Komada, Y.; Yamada, S.; Kawana, F.; Kanbayashi, T.; Inoue, Y. Possible mechanism of secondary narcolepsy with a long sleep time following surgery for craniopharyngioma. Intern. Med. 2012, 51, 413–417. [Google Scholar] [CrossRef]
- O’Gorman, C.S.; Simoneau-Roy, J.; Pencharz, P.; MacFarlane, J.; MacLusky, I.; Narang, I.; Adeli, K.; Daneman, D.; Hamilton, J. Sleep-disordered breathing is increased in obese adolescents with craniopharyngioma compared with obese controls. J. Clin. Endocrinol. Metab. 2010, 95, 2211–2218. [Google Scholar] [CrossRef]
- Lipton, J.; Megerian, J.T.; Kothare, S.V.; Cho, Y.J.; Shanahan, T.; Chart, H.; Ferber, R.; Adler-Golden, L.; Cohen, L.E.; Czeisler, C.A.; et al. Melatonin deficiency and disrupted circadian rhythms in pediatric survivors of craniopharyngioma. Neurology 2009, 73, 323–325. [Google Scholar] [CrossRef]
- Palm, L.; Nordin, V.; Elmqvist, D.; Blennow, G.; Persson, E.; Westgren, U. Sleep and wakefulness after treatment for craniopharyngioma in childhood; influence on the quality and maturation of sleep. Neuropediatrics 1992, 23, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, J.; Özyurt, J.; Mehren, A.; Friedrich, C.; Müller, H.L. Fatigue in patients with hypothalamic syndrome—A cross-sectional analysis of the German childhood-onset craniopharyyngioma cohort. EJC Paediatr. Oncol. 2024, 4, 100174. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Owens, J.; Alessi, C.; Boehlecke, B.; Brown, T.M.; Coleman, J., Jr.; Friedman, L.; Kapur, V.K.; Lee-Chiong, T.; Pancer, J.; et al. Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 2006, 29, 1277–1281. [Google Scholar] [CrossRef]
- Brasure, M.; Fuchs, E.; MacDonald, R.; Nelson, V.A.; Koffel, E.; Olson, C.M.; Khawaja, I.S.; Diem, S.; Carlyle, M.; Wilt, T.J.; et al. Psychological and Behavioral Interventions for Managing Insomnia Disorder: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann. Intern. Med. 2016, 165, 113–124. [Google Scholar] [CrossRef]
- Cuny, T.; Reynaud, R.; Raverot, G.; Coutant, R.; Chanson, P.; Kariyawasam, D.; Poitou, C.; Thomas-Teinturier, C.; Baussart, B.; Samara-Boustani, D.; et al. Diagnosis and management of children and adult craniopharyngiomas: A French Endocrine Society/French Society for Paediatric Endocrinology & Diabetes Consensus Statement. Ann. D’endocrinologie 2025, 86, 101631. [Google Scholar] [CrossRef]
- Gan, H.W.; Morillon, P.; Albanese, A.; Aquilina, K.; Chandler, C.; Chang, Y.C.; Drimtzias, E.; Farndon, S.; Jacques, T.S.; Korbonits, M.; et al. National UK guidelines for the management of paediatric craniopharyngioma. Lancet Diabetes Endocrinol. 2023, 11, 694–706. [Google Scholar] [CrossRef]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef]
- Ismail, D.; O’Connell, M.A.; Zacharin, M.R. Dexamphetamine use for management of obesity and hypersomnolence following hypothalamic injury. J. Pediatr. Endocrinol. Metab. 2006, 19, 129–134. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Kapur, V.K.; Brown, T.; Swick, T.J.; Alessi, C.; Aurora, R.N.; Boehlecke, B.; Chesson, A.L., Jr.; Friedman, L.; Maganti, R.; et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007, 30, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.M.; Bendel, A.E.; Neglia, J.P.; Moertel, C.L.; Mahowald, M. Sleep in children with neoplasms of the central nervous system: Case review of 14 children. Pediatrics 2003, 112, e46–e54. [Google Scholar] [CrossRef] [PubMed]
- Denzer, C.; Denzer, F.; Lennerz, B.S.; Vollbach, H.; Lustig, R.H.; Wabitsch, M. Treatment of Hypothalamic Obesity with Dextroamphetamine: A Case Series. Obes. Facts 2019, 12, 91–102. [Google Scholar] [CrossRef]
- Honegger, J.; Barocka, A.; Sadri, B.; Fahlbusch, R. Neuropsychological results of craniopharyngioma surgery in adults: A prospective study. Surg. Neurol. 1998, 50, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kalapurakal, J.A.; Goldman, S.; Hsieh, Y.C.; Tomita, T.; Marymont, M.H. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med. Pediatr. Oncol. 2003, 40, 214–218. [Google Scholar] [CrossRef]
- Ramanbhavana, V.S.; Vara Prasad, K.S. A Case Series of Craniopharyngioma: Epidemiological Study and Management Analysis at Tertiary Care Center. Asian J. Neurosurg. 2019, 14, 1196–1202. [Google Scholar] [CrossRef]
- Schultes, B.; Ernst, B.; Schmid, F.; Thurnheer, M. Distal gastric bypass surgery for the treatment of hypothalamic obesity after childhood craniopharyngioma. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2009, 161, 201–206. [Google Scholar] [CrossRef]
- van Santen, H.M.; van Schaik, J.; van Roessel, I.; Beckhaus, J.; Boekhoff, S.; Muller, H.L. Diagnostic criteria for the hypothalamic syndrome in childhood. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2023, 188, 214–225. [Google Scholar] [CrossRef]
- Irestorm, E.; Schouten-van Meeteren, A.Y.N.; van Gorp, M.; Twisk, J.W.R.; van Santen, H.M.; Partanen, M.; Grootenhuis, M.A.; van Litsenburg, R.R.L. The development of fatigue after treatment for pediatric brain tumors does not differ between tumor locations. Pediatr. Blood Cancer 2024, 71, e31028. [Google Scholar] [CrossRef]
- Lin, B.; Xiang, S.; Chen, J.; Jing, Y.; Ye, Z.; Zhang, Y.; Cao, X.; Yin, Z.; Qiao, N.; Zhou, X. Assessment of quality of life in patients with craniopharyngioma and identification of risk factors for compromised overall wellness. Arch. Endocrinol. Metab. 2023, 68, e230001. [Google Scholar] [CrossRef] [PubMed]
- Murata, J.; Sawamura, Y.; Ikeda, J.; Hashimoto, S.; Honma, K. Twenty-four hour rhythm of melatonin in patients with a history of pineal and/or hypothalamo-neurohypophyseal germinoma. J. Pineal Res. 1998, 25, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.S.; Naylor, M.W. Narcolepsy associated with lesions of the diencephalon. Neurology 1989, 39, 1505–1508. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Stakes, J.W.; Hobson, J.A. Transient cataplexy after removal of a craniopharyngioma. Neurology 1984, 34, 1372–1375. [Google Scholar] [CrossRef]
- Laudon, M.; Frydman-Marom, A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int. J. Mol. Sci. 2014, 15, 15924–15950. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.W. Management of Craniopharyngiomas in the Era of Molecular Oncological Therapies: Not a Panacea. J. Endocr. Soc. 2021, 5, bvab094. [Google Scholar] [CrossRef]
- Roth, C.L.; Zenno, A. Treatment of hypothalamic obesity in people with hypothalamic injury: New drugs are on the horizon. Front. Endocrinol. 2023, 14, 1256514. [Google Scholar] [CrossRef]
- Stec, N.E.; Barker, F.G., 2nd; Brastianos, P.K. Targeted treatment for craniopharyngioma. J. Neuro-Oncol. 2025, 172, 503–513. [Google Scholar] [CrossRef] [PubMed]
- De Alcubierre, D.; Gkasdaris, G.; Mordrel, M.; Joncour, A.; Briet, C.; Almairac, F.; Boetto, J.; Mouly, C.; Larrieu-Ciron, D.; Vasiljevic, A.; et al. BRAF and MEK inhibitor targeted therapy in papillary craniopharyngiomas: A cohort study. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2024, 191, 251–261. [Google Scholar] [CrossRef]
- Agosti, E.; Zeppieri, M.; Antonietti, S.; Piazza, A.; Ius, T.; Fontanella, M.M.; Fiorindi, A.; Panciani, P.P. Advancing Craniopharyngioma Management: A Systematic Review of Current Targeted Therapies and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 723. [Google Scholar] [CrossRef]
- Carley, D.W.; Prasad, B.; Reid, K.J.; Malkani, R.; Attarian, H.; Abbott, S.M.; Vern, B.; Xie, H.; Yuan, C.; Zee, P.C. Pharmacotherapy of Apnea by Cannabimimetic Enhancement, the PACE Clinical Trial: Effects of Dronabinol in Obstructive Sleep Apnea. Sleep 2018, 41, zsx184. [Google Scholar] [CrossRef] [PubMed]
- Faquih, T.; Potts, K.S.; Nagarajan, P.; Yu, B.; Kaplan, R.; Isasi, C.R.; Qi, Q.; Taylor, K.D.; Liu, P.Y.; Strausz, S.J.; et al. Steroid hormone biosynthesis and dietary related metabolites associated with excessive daytime sleepiness. eBioMedicine 2025, 119, 105881. [Google Scholar] [CrossRef] [PubMed]


| CP pts/ Total pts | Age at Study | CO CP/ Total CP | Study Design | Methods, Instruments | Sleep Disturbances | Authors/Year of Publication |
|---|---|---|---|---|---|---|
| 2/2 | 19, 12 | 2/2 | Case series | MSLT, PSG | Patient 1: SDB, narcolepsy Patient 2: SDB, parasomnia | Cordani et al., 2021 [76] |
| 70/70 | 6–20 | 70/70 | Cross-sectional | ESS | EDS by M-ESS: 28.8%; EDS by MSLT: 81.8%; SDB: 5.7% | Crabtree et al., 2019 [88] |
| 28/28 | 19–67 | 7/28 | Case-control | Questionnaire | EDS by ESS: 25% sleep obstructive apnea: 46% | Crowley et al., 2011 [87] |
| 4/7 | 17 | 3/4 | Case series | MSLT, PSG | 1 patient: EDS, improvement with dextroamphetamine | Denzer et al., 2019 [118] |
| 13/13 | 17–76 | 1/13 | Cross-sectional | NA | Presurgical: 20%, postsurgical: 8% sleep problems (n.s.) | Honegger et al., 1998 [119] |
| 9/12 | ♂: med. 20 ♀: med. 15 | 9/9 | Case series | MSLT, ESS, PSG | EDS, improvement with dexamphetamine: 8/12 | Ismail et al., 2006 [115] |
| 25/25 | 1–15 | 25/25 | Cross sectional | ESS | Sleep disorders (n.s.): 12% | Kalapurakal et al., 2003 [120] |
| 1/1 | 5–10 | 1/1 | Case report | Actigraphy | Disturbed sleep pattern (frequent falling asleep, reversal sleep rhythm) | Killeffer et al., 1970 [83] |
| 84/84 | 10.3 ± 4.3 | 84/84 | Cross-sectional | ESS | Correlation btw. hypothalamic involvement and BMI/EDS | Klages et al., 2021 [50] |
| 3/3 | 15–22 | 3/3 | Cross-sectional | MSLT, PSG | Nighttime activity, inappropriate daytime episodes of rest | Lipton et al., 2009 [107] |
| 3/10 | 6–16 | 3/3 | Retrospective | ESS | Narcolepsy type 2 | Madan et al., 2021 [102] |
| 98/98 | 3–20 | 98/98 | Cross-sectional | Questionnaire | EDS: 80%; hypersomnia: 45%; narcolepsy: 35%; OSA: 5% | Mandrell et al., 2020 [80] |
| 28/28 | 10–32 | 28/28 | Retrospective | MSLT, PSG | EDS: 43% (12/28); obstructive sleep apnea: 43% (3/7) | Manley et al., 2012 [86] |
| 1/3 | 5 | 1/1 | Case series | NA | Secondary narcolepsy | Marcus et al., 2002 [79] |
| 79/79 | 3.5–33.2 | 79/79 | Cross-sectional | MSLT, ESS, PSG | EDS: 35% (42% of severely obese) decreased nocturnal salivary melatonin levels | Müller et al., 2002 [36] |
| 79/79 | 6–33.2 | 79/79 | Cross-sectional | MSLT, PSG | EDS; reduced nocturnal melatonin levels | Müller et al., 2006 [35] |
| 50/50 | 3–20 | 50/50 | Cross-sectional | ESS | Hypersomnia: 50% | Niel et al., 2020 [91] |
| 78/78 | 6–20 | 78/78 | Cross-sectional | Actigraphy | EDS: 82% | Niel et al. 2021 [47] |
| 15/15 | 10–21 | 15/15 | Cross-sectional | ESS | SDB (AHI higher than controls) | O’Gorman et al., 2010 [106] |
| 10/10 | 7.1–22.9 | 10/10 | Cross-sectional | MSLT, PSG | Decreased rates of REM sleep, low sleep efficiency | Palm et al., 1992 [108] |
| 15/15 | 18–70 | 4/15 | Case-control | ESS | EDS, reduced sleep time and low midnight melatonin | Pickering et al., 2014 [59] |
| 7/7 | 21–68 | 1/7 | Case-control | Questionnaire | Hypersomnia: 57% | Pickering et al., 2017 [89] |
| 21/21 | <16 | 21/21 | Cross-sectional | MSLT, PSG | EDS: 29% | Poretti et al., 2004 [84] |
| 41/41 | 1–59 | ~ 50% | Retrospective/prospective | NA | Preoperative sleep disorders (n.s.): 15% | Ramanbhavana et al., 2019 [121] |
| 1/1 | 19 | 1/1 | Case report | MSLT, ESS, PSG | Secondary narcolepsy | Sakuta et al., 2012 [105] |
| 1/1 | 29 | 1/1 | Case report | MSLT, PSG | OSAS | Schultes et al., 2009 [122] |
| 3/5 | 11–19 | 3/3 | Cross-sectional | ESS | EDS | Snow et al., 2002 [90] |
| 1/1 | 11 | 1/1 | Case report | Actigraphy | Secondary narcolepsy | Tachibana et al., 2005 [78] |
| 27/27 | 27–80 | 8/27 | Case-control | ESS | EDS: 33% | Van der Klaauw et al., 2008 [85] |
| 80/80 | 2–20 | 80/80 | Cross-sectional | MSLT, PSG | Poor sleep | Witcraft et al., 2022 [44] |
| 131/131 | 9–20 | 32/131 | Cross-sectional | ESS | Worse EDS in bilateral-HI group | Yang et al., 2020 [93] |
| 41/41 | 22 (13–45) | 41/41 | Cross-sectional | ESS, MFI-20 | No correlation btw. fatigue and EDS, physical + overall fatigue high in pts. with HI | Beckhaus et al., 2024 [109] |
| 119/119 | 22 (14–42) | 119/119 | Cross-sectional | ESS, PedQol | Negative correlation btw. ESS and QoL, EDS with posterior HI | Mann-Markutzyk et al., 2025 [94] |
| 88/336 | 14 (6–29) | 88/88 | Retrospective cohort study | Hypothalamic score [123] | Mild sleep disorder: 14% Severe sleep disorder: 10% | Van Roessel et al., 2025 [13] |
| 35/425 | 67 (16%) > 18 yrs | 35/35 | Retrospective cohort study | PedsQL, MFS | Fatigue not related to tumor location, worse in follow-up | Irestorm et al., 2024 [124] |
| 54/54 | 37.1 ± 15.5 | 0/54 | Retrospective cohort study | PSG, MSLT | Secondary narcolepsy:14% Hypersomnia: 26% | Dodet et al., 2023 [40] |
| 109/109 | 40 (28–56) | 0/109 | Retrospective cohort study | PSQI | Sleep disturbance: 47/109 (43%) | Lin et al., 2023 [125] |
| 62/62 | 11 ± 4.0 | 62/62 | Cross-sectional | MSLT, PSG | EDS: 76% | Jacola et al., 2016 [92] |
| Patient Cohort | Disturbance | Therapeutic Intervention | Effect/ Tolerability | Authors |
|---|---|---|---|---|
| 10, adult obese patients | EDS | Melatonin (6 mg) | EDS improved (10/10 patients) | Müller et al., 2006 [35] |
| 5, adult overweight/obese patients | EDS | Modafinil | EDS improved (4/5) (*one died before intervention) | Crowley et al., 2011 [87] |
| 1, 5-year-old child | Secondary narcolepsy | Modafinil (200 mg) Methylphenidate (20 mg) | Improvement (No prolonged FU available) | Marcus et al., 2002 [79] |
| 12, obese adolescent/young adult patients (9 with CP) | EDS | Dexamphetamine (5 mg twice daily) | EDS improved (8/12) Improved concentration and physical exercise tolerance (3/12) (1 discontinued for deteriorating health) | Ismail et al., 2006 [115] |
| 1, 17-year-old obese boy | EDS | Dextroamphetamine | EDS improved | Denzer et al., 2019 [118] |
| 7, adult overweight/obese patients | SDB EDS | NIV CPAP | EDS improved | Crowley et al., 2011 [87] |
| 2, adolescent patients | SBD EDS | NIV CPAP | Resolution of SBD, EDS not improved | Snow et al., 2002 [90] |
| 1 | EDS | Correction of sleep hygiene | EDS improved | Manley et al., 2012 [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, H.L. Sleep Disorders, Dysregulation of Circadian Rhythms, and Fatigue After Craniopharyngioma—A Narrative Review. Biomedicines 2025, 13, 2356. https://doi.org/10.3390/biomedicines13102356
Müller HL. Sleep Disorders, Dysregulation of Circadian Rhythms, and Fatigue After Craniopharyngioma—A Narrative Review. Biomedicines. 2025; 13(10):2356. https://doi.org/10.3390/biomedicines13102356
Chicago/Turabian StyleMüller, Hermann L. 2025. "Sleep Disorders, Dysregulation of Circadian Rhythms, and Fatigue After Craniopharyngioma—A Narrative Review" Biomedicines 13, no. 10: 2356. https://doi.org/10.3390/biomedicines13102356
APA StyleMüller, H. L. (2025). Sleep Disorders, Dysregulation of Circadian Rhythms, and Fatigue After Craniopharyngioma—A Narrative Review. Biomedicines, 13(10), 2356. https://doi.org/10.3390/biomedicines13102356






