LLM-Enhanced Multimodal Framework for Drug–Drug Interaction Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug–Drug Interaction Data
2.2. BioBERT Embedding
2.3. SSP: Structural Similarity Profile
2.4. PSP: Protein Similarity Profile
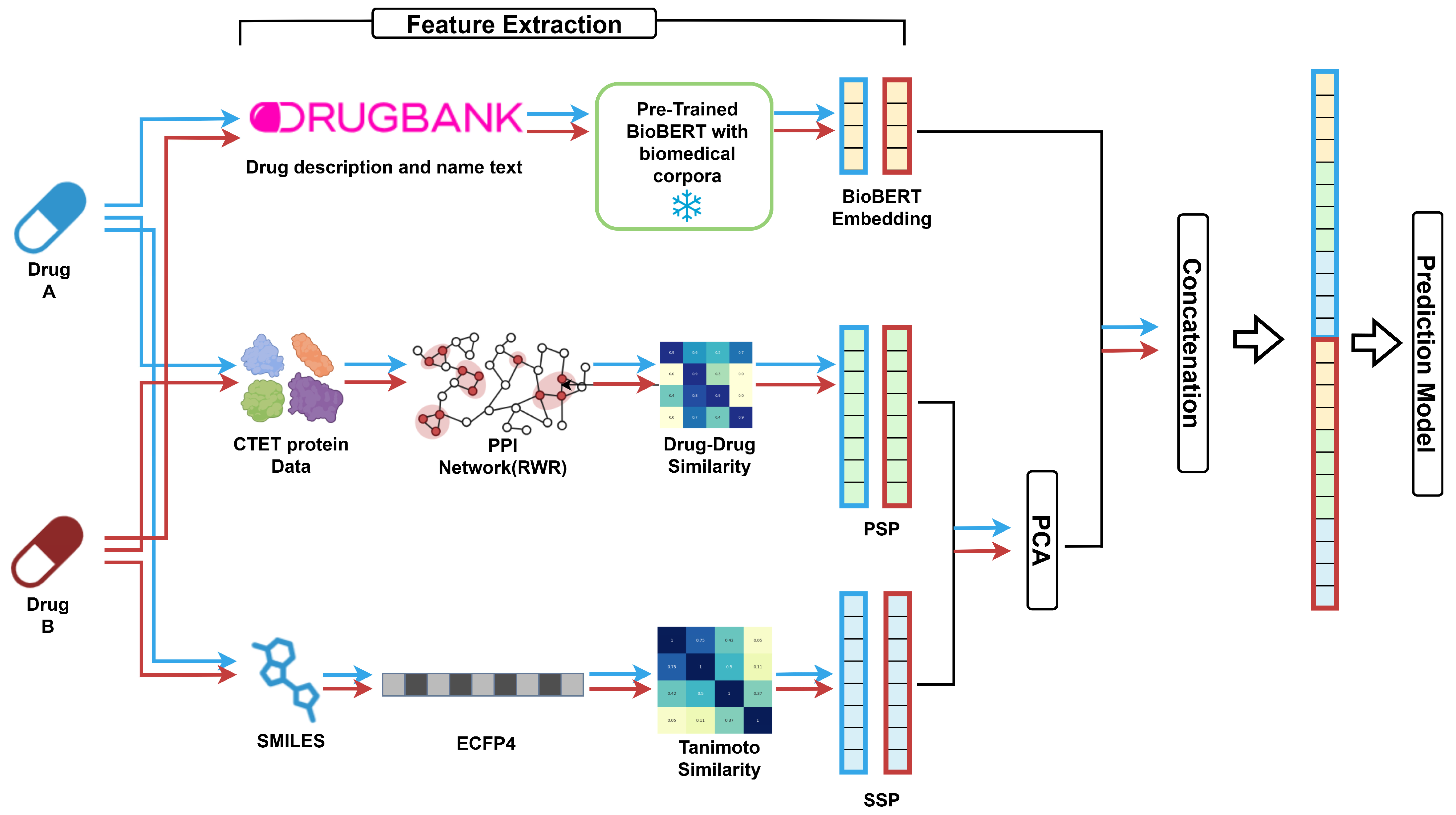
2.5. Model Architecture: Feature Processing and DDI Prediction
- PCA was applied to SSP and PSP to reduce dimensionality while preserving ≥ 95% of the variance. In contrast, applying PCA to BioBERT embeddings generally degraded performance in preliminary experiments and we therefore used the original 768-dimensional vectors, suggesting that their semantic information was critical for prediction.
- After feature extraction, the selected representations were concatenated into a single feature vector (Figure 1). Before any dimensionality reduction, the input vector was passed through a projection layer—a learnable linear map with the same input and output size as the concatenated feature. The projection layer re-weights and linearly mixes feature dimensions to mitigate cross-modal scale/variance mismatches and map them into a better-aligned joint space before compression. The projection output was then fed to a hidden layer, followed by an output layer that mapped the hidden dimension to logits for the 79 DDI classes.
- The network architecture was determined using a two-stage hyperparameter optimization process. First, grid search was used to identify the number of hidden layers (1–5). We then employed Optuna [33] to tune the hidden dimension, dropout rate, and learning rate for each feature combination. The final architecture consisted of a projection layer for feature re-weighting/alignment, followed by a hidden layer for dimensionality reduction, and an output layer for classification. ReLU activation and dropout were applied after each linear transformation to improve generalization.
- After fixing the best hyperparameters, we trained from scratch for up to 300 epochs with Adam and cross-entropy loss. To ensure sufficient convergence, early stopping (patience = 20) was activated only after epoch 200, and the best validation-accuracy checkpoint was saved. Next, we resumed from this checkpoint and performed fine-tuning for up to 100 additional epochs with ReduceLROnPlateau (mode = max, factor = 0.1, patience = 5, min_lr = 1 × 10−6); early stopping (patience = 20) was also applied in this stage. Test metrics were reported from the best validation checkpoint of the fine-tuning stage. The overall MLP configuration is summarized in Supplementary Table S2, and detailed hyperparameters for each feature combination are provided in Supplementary Table S3. The dataset was split into training, validation, and test sets with a ratio of 0.64:0.16:0.20, using stratified sampling to preserve the distribution of all 79 interaction classes.
3. Results and Discussion
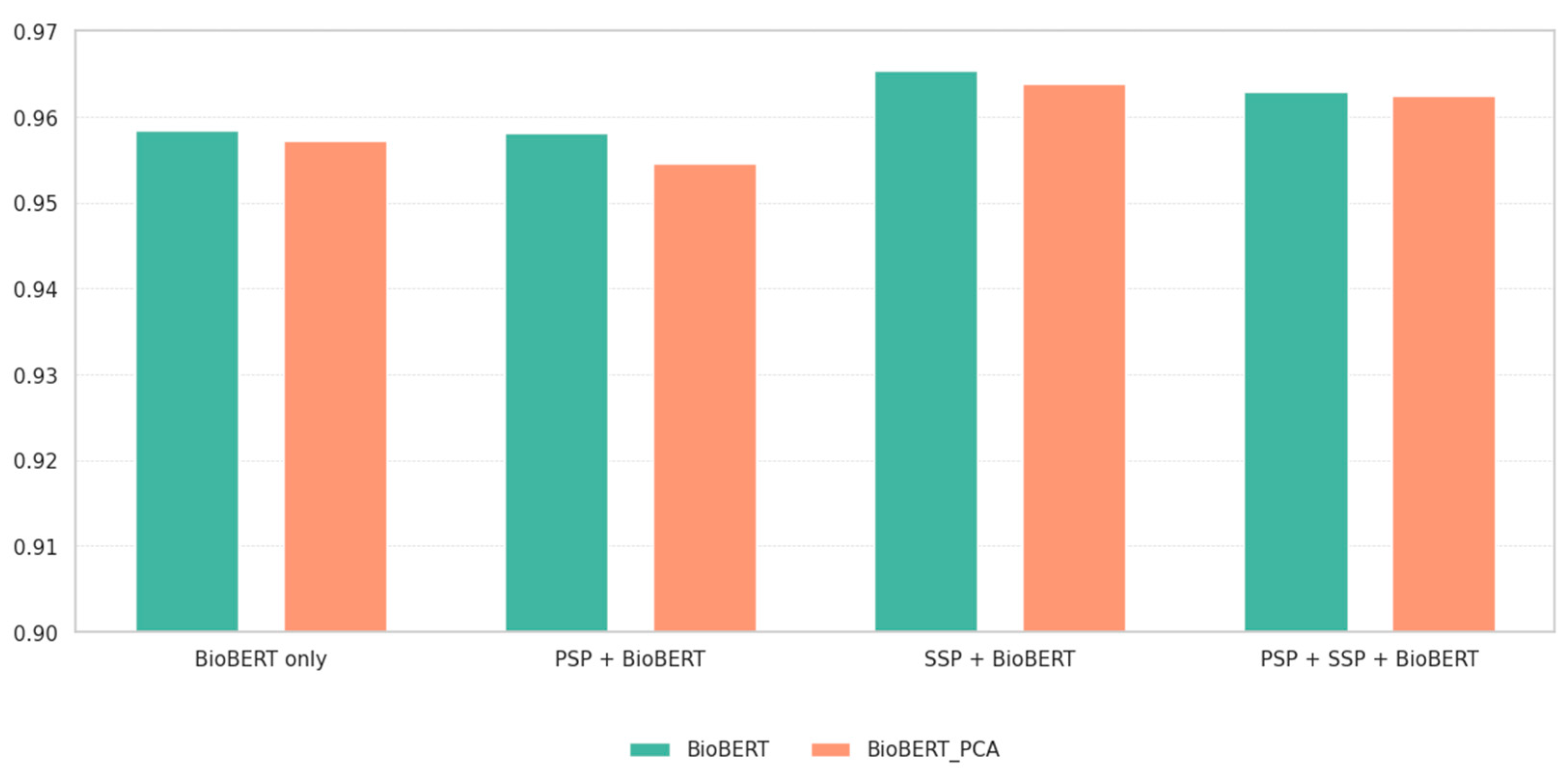
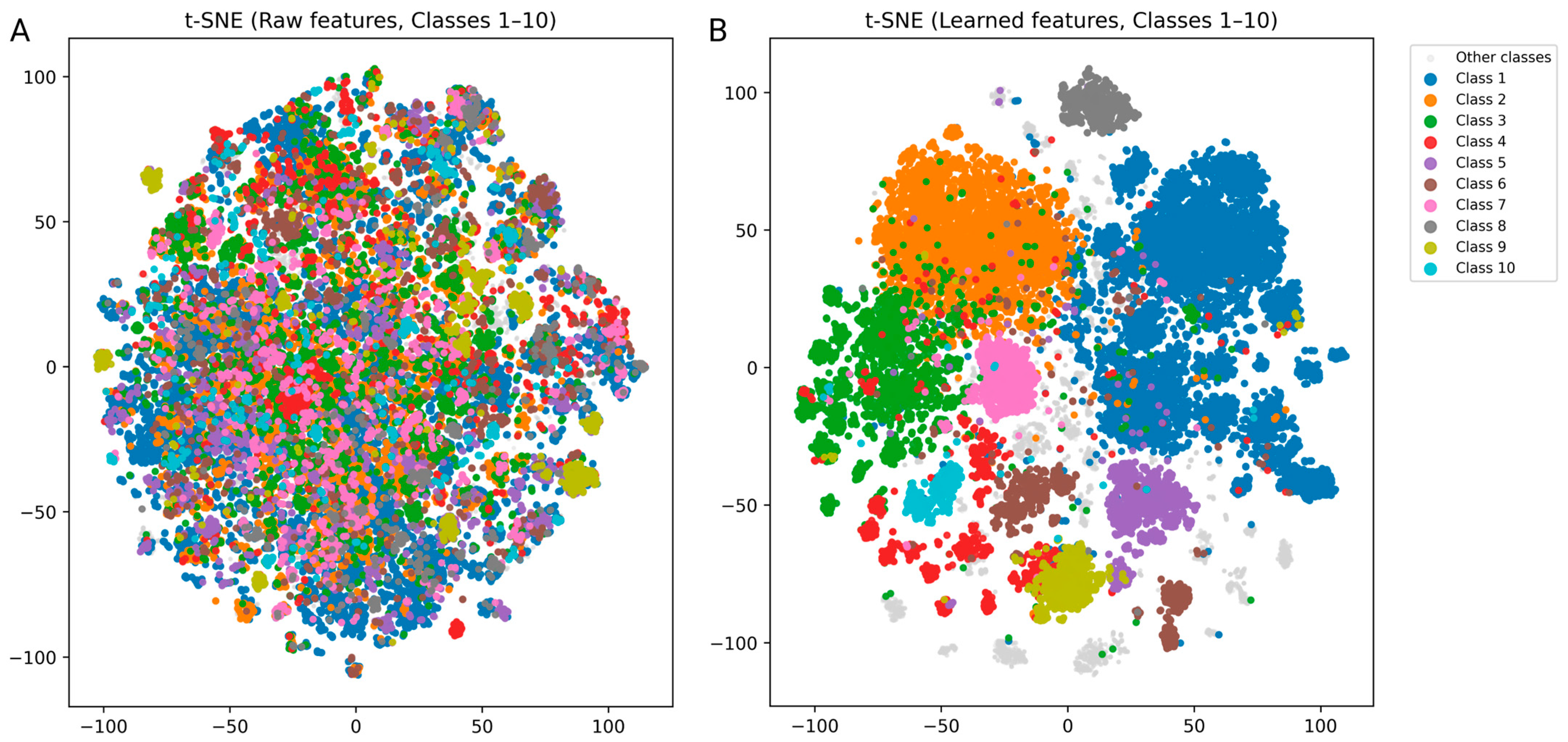
3.1. Performance Evaluation
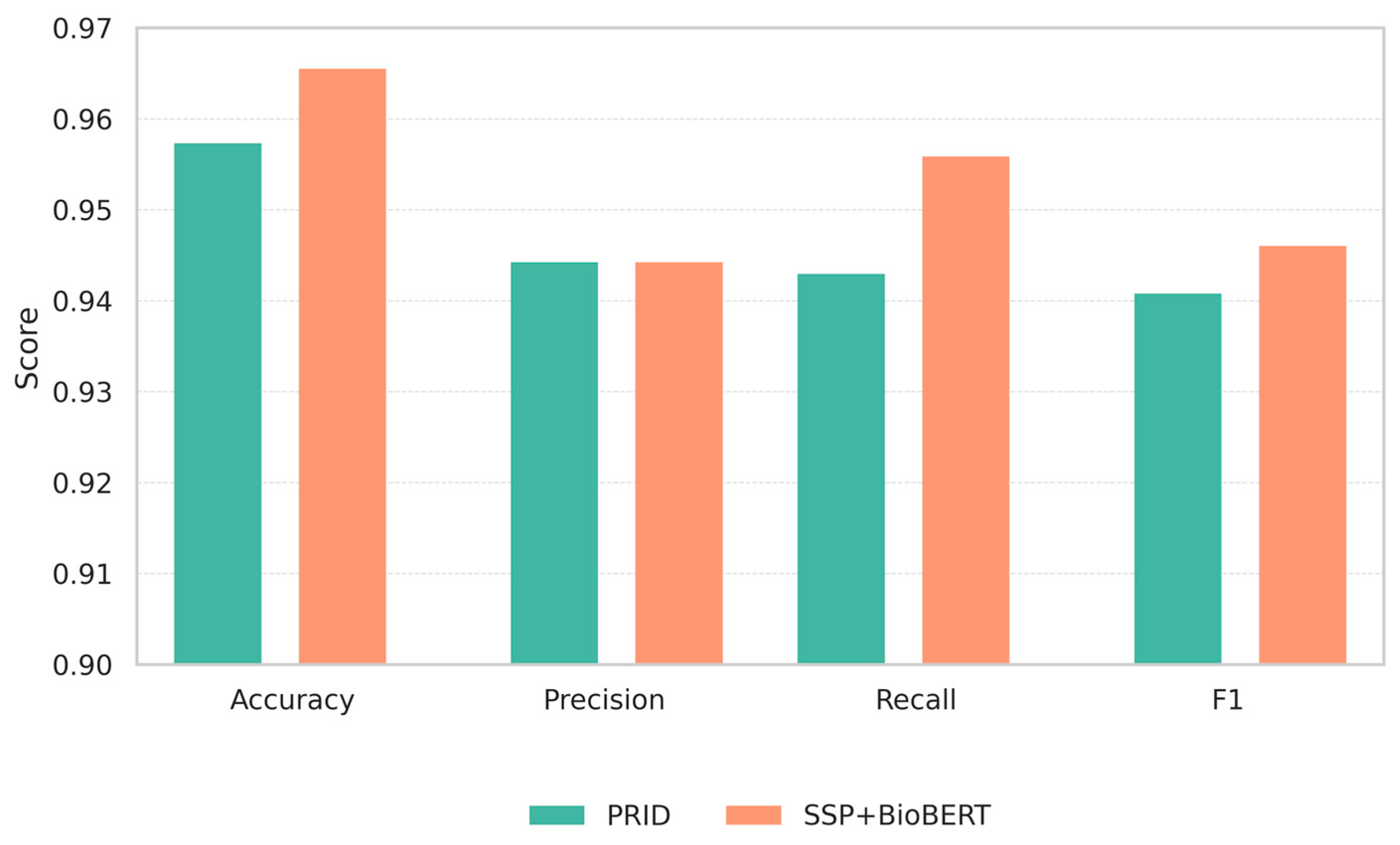
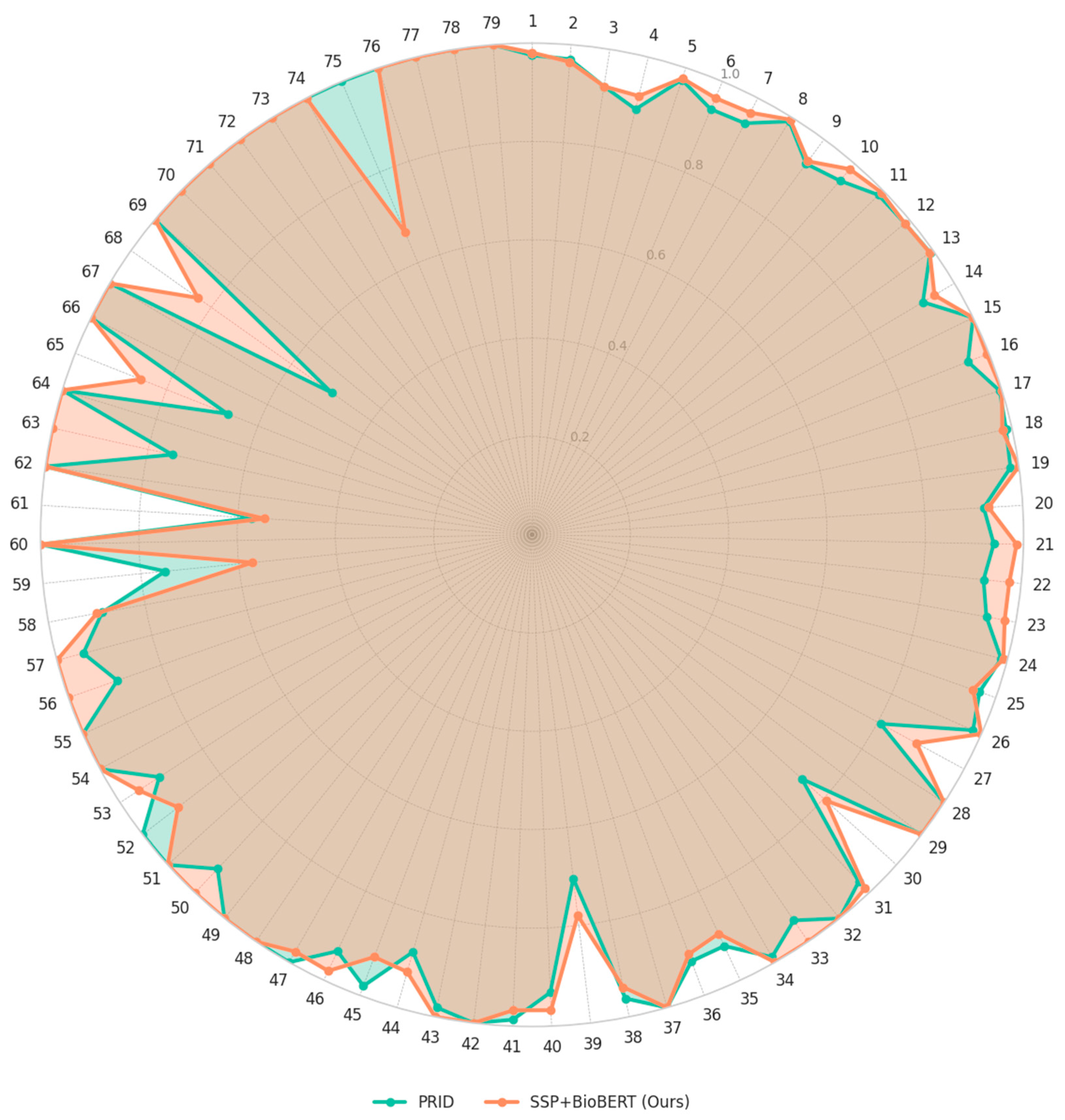
3.2. Comparison with Existing Methods
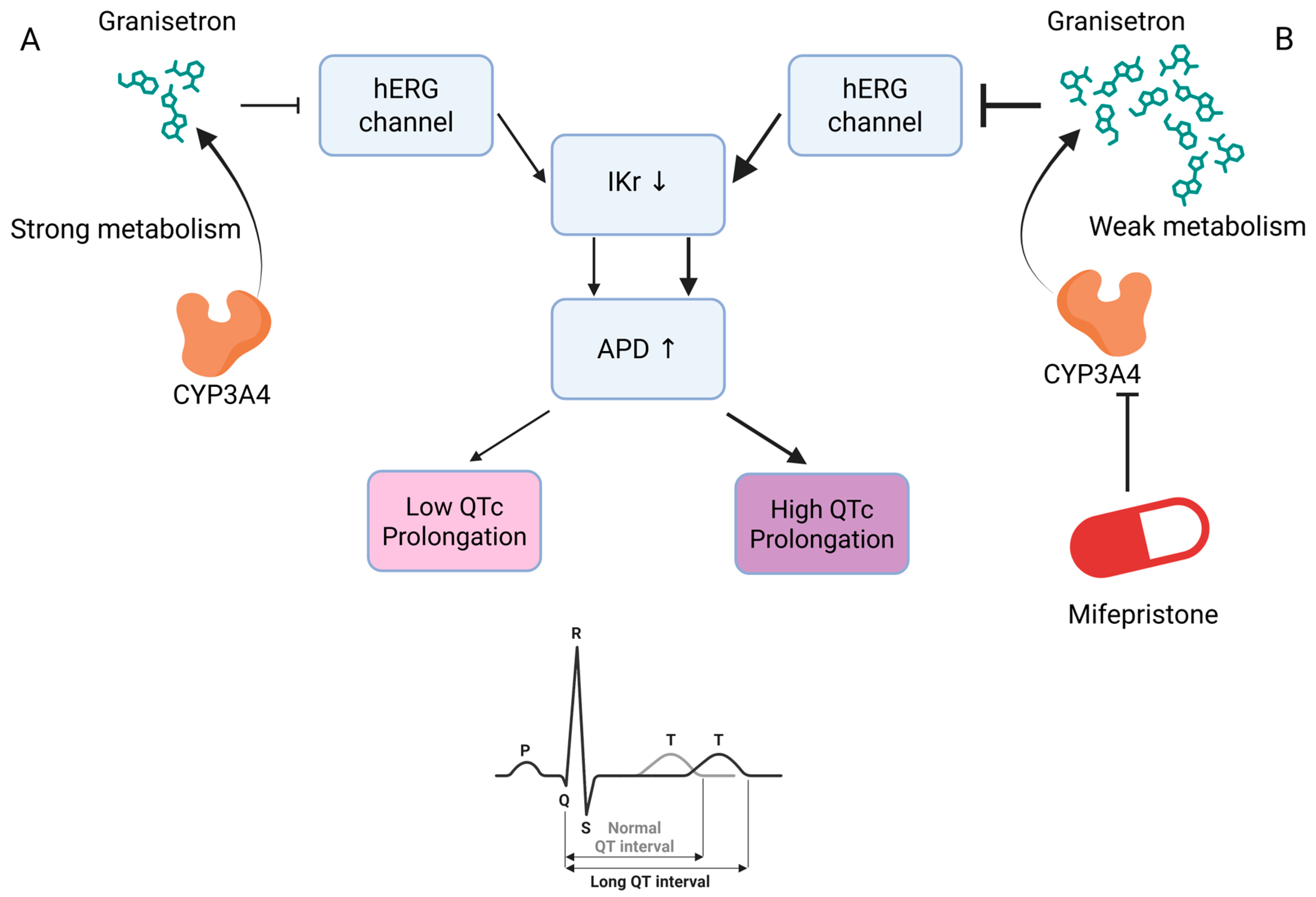
3.3. Clinical Implication of False Positive Cases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LLM | Large Language Model |
| CTET | Carrier, Transporter, Enzyme, and Target |
| DDI | Drug–drug Interaction |
| RWR | Random Walk with Restart |
| NLP | Natural Language Processing |
| PPI | Protein–protein Interaction |
| SMILES | Simplified Molecular Input Line Entry System |
| ECFP | Extended-Connectivity Fingerprint |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
References
- Nicholson, K.; Liu, W.; Fitzpatrick, D.; Hardacre, K.A.; Roberts, S.; Salerno, J.; Stranges, S.; Fortin, M.; Mangin, D. Prevalence of multimorbidity and polypharmacy among adults and older adults: A systematic review. Lancet Healthy Longev. 2024, 5, e287–e296. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Brito-Dellan, N.; Font, C.; Butler, T.; Rojas-Hernandez, C.M.; Butler, T.; Escalante, C.; MASCC Hemostasis Study Group. Complexity and clinical significance of drug–drug interactions (DDIs) in oncology: Challenging issues in the care of patients regarding cancer-associated thrombosis (CAT). Support. Care Cancer 2022, 30, 8559–8573. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Song, Z.; Bai, J.; Wang, J. Prevalence and clinical significance of potential drug-drug interactions of antimicrobials in Intensive Care Unit patients: A retrospective study. BMC Pharmacol. Toxicol. 2025, 26, 104. [Google Scholar] [CrossRef]
- Tornio, A.; Filppula, A.M.; Niemi, M.; Backman, J.T. Clinical studies on drug–drug interactions involving metabolism and transport: Methodology, pitfalls, and interpretation. Clin. Pharmacol. Ther. 2019, 105, 1345–1361. [Google Scholar] [CrossRef]
- Cai, R.; Liu, M.; Hu, Y.; Melton, B.L.; Matheny, M.E.; Xu, H.; Duan, L.; Waitman, L.R. Identification of adverse drug-drug interactions through causal association rule discovery from spontaneous adverse event reports. Artif. Intell. Med. 2017, 76, 7–15. [Google Scholar] [CrossRef]
- Lu, C.; Di, L. In vitro and in vivo methods to assess pharmacokinetic drug–drug interactions in drug discovery and development. Biopharm. Drug Dispos. 2020, 41, 3–31. [Google Scholar] [CrossRef]
- Tang, C.; Prueksaritanont, T. Use of in vivo animal models to assess pharmacokinetic drug-drug interactions. Pharm. Res. 2010, 27, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Temple, R.; Throckmorton, D.; Lesko, L. Drug interaction studies: Study design, data analysis, and implications for dosing and labeling. Clin. Pharmacol. Ther. 2007, 81, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, S.; Shao, B.; Zeng, X.; Wang, T.; Liu, T.-Y. DSN-DDI: An accurate and generalized framework for drug–drug interaction prediction by dual-view representation learning. Brief. Bioinform. 2023, 24, 1–12. [Google Scholar] [CrossRef]
- Yan, C.; Duan, G.; Zhang, Y.; Wu, F.-X.; Pan, Y.; Wang, J. Predicting drug-drug interactions based on integrated similarity and semi-supervised learning. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020, 19, 168–179. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Yin, P.-W.; Wu, X.-M.; Han, J.-X. Prediction of the drug–drug interaction types with the unified embedding features from drug similarity networks. Front. Pharmacol. 2021, 12, 794205. [Google Scholar] [CrossRef]
- Zhu, J.; Che, C.; Jiang, H.; Xu, J.; Yin, J.; Zhong, Z. SSF-DDI: A deep learning method utilizing drug sequence and substructure features for drug–drug interaction prediction. BMC Bioinform. 2024, 25, 39. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Atas, H.; Martin, M.J.; Cetin-Atalay, R.; Atalay, V.; Dogan, T. Recent applications of deep learning and machine intelligence on in silico drug discovery: Methods, tools and databases. Brief. Bioinform. 2019, 20, 1878–1912. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning improves prediction of drug–drug and drug–food interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E4304–E4311. [Google Scholar] [CrossRef]
- Wang, H.; Lian, D.; Zhang, Y.; Qin, L.; Lin, X. Gognn: Graph of graphs neural network for predicting structured entity interactions. arXiv 2020, arXiv:2005.05537. [Google Scholar] [CrossRef]
- Seo, J.; Jung, H.; Ko, Y. PRID: Prediction model using RWR for interactions between drugs. Pharmaceutics 2023, 15, 2469. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ding, Y.; Long, S.; Poon, J.; Han, S.C. Ddi-mug: Multi-aspect graphs for drug-drug interaction extraction. Front. Digit. Health 2023, 5, 1154133. [Google Scholar] [CrossRef]
- Qi, H.; Li, X.; Zhang, C.; Zhao, T. Improving drug-drug interaction prediction via in-context learning and judging with large language models. Front. Pharmacol. 2025, 16, 1589788. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, X.; Qiu, Y.; Xia, J.; Zhang, W.; Liu, S. A multimodal deep learning framework for predicting drug–drug interaction events. Bioinformatics 2020, 36, 4316–4322. [Google Scholar] [CrossRef]
- Jin, Q.; Xie, J.; Huang, D.; Zhao, C.; He, H. Msff-ma-ddi: Multi-source feature fusion with multiple attention blocks for predicting drug–drug interaction events. Comput. Biol. Chem. 2024, 108, 108001. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Y.; Zhang, L.; Chu, Y.; Liu, Y.; Fang, Y.; Jiang, M.; Wang, Q.; Zhao, B.; Xiong, Y. MDF-SA-DDI: Predicting drug–drug interaction events based on multi-source drug fusion, multi-source feature fusion and transformer self-attention mechanism. Brief. Bioinform. 2022, 23, bbab421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tong, K.; Jin, S.; Wang, S.; Yang, C.; Jiang, F. CNN-Siam: Multimodal siamese CNN-based deep learning approach for drug–drug interaction prediction. BMC Bioinform. 2023, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, K.; Zhang, C.; Glass, L.M.; Sun, J.; Xiao, C. SumGNN: Multi-typed drug interaction prediction via efficient knowledge graph summarization. Bioinformatics 2021, 37, 2988–2995. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Zang, T. CNN-DDI: A learning-based method for predicting drug–drug interactions using convolution neural networks. BMC Bioinform. 2022, 23, 88. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, W.; Kim, S.; Kim, D.; Kim, S.; So, C.H.; Kang, J. BioBERT: A pre-trained biomedical language representation model for biomedical text mining. Bioinformatics 2020, 36, 1234–1240. [Google Scholar] [CrossRef]
- Gu, Y.; Tinn, R.; Cheng, H.; Lucas, M.; Usuyama, N.; Liu, X.; Naumann, T.; Gao, J.; Poon, H. Domain-specific language model pretraining for biomedical natural language processing. ACM Trans. Comput. Healthc. (HEALTH) 2021, 3, 1–23. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; De Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F. The ChEMBL Database in 2023: A drug discovery platform spanning multiple bioactivity data types and time periods. Nucleic Acids Res. 2024, 52, D1180–D1192. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Valdeolivas, A.; Tichit, L.; Navarro, C.; Perrin, S.; Odelin, G.; Levy, N.; Cau, P.; Remy, E.; Baudot, A. Random walk with restart on multiplex and heterogeneous biological networks. Bioinformatics 2019, 35, 497–505. [Google Scholar] [CrossRef]
- Ko, Y.; Cho, M.; Lee, J.-S.; Kim, J. Identification of disease comorbidity through hidden molecular mechanisms. Sci. Rep. 2016, 6, 39433. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A next-generation hyperparameter optimization framework. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 2623–2631. [Google Scholar]
- Radford, A.; Kim, J.W.; Hallacy, C.; Ramesh, A.; Goh, G.; Agarwal, S.; Sastry, G.; Askell, A.; Mishkin, P.; Clark, J. Learning transferable visual models from natural language supervision. In Proceedings of the International Conference on Machine Learning, Online, 18–24 July 2021; pp. 8748–8763. [Google Scholar]
- Chen, T.; Kornblith, S.; Norouzi, M.; Hinton, G. A simple framework for contrastive learning of visual representations. In Proceedings of the International Conference on Machine Learning, Online, 13–18 July 2020; pp. 1597–1607. [Google Scholar]
- Vadlapatla, R.K.; Patel, M.; Paturi, D.K.; Pal, D.; Mitra, A.K. Clinically relevant drug–drug interactions between antiretrovirals and antifungals. Expert Opin. Drug Metab. Toxicol. 2014, 10, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.; Stiber, J.A.; Perfect, J.R.; Johnson, M.D. Real-world implications of QT prolongation in patients receiving voriconazole and amiodarone. J. Antimicrob. Chemother. 2019, 74, 228–233. [Google Scholar] [CrossRef]
- Amariles, P.; Rivera-Cadavid, M.; Ceballos, M. Clinical Relevance of Drug Interactions in People Living with Human Immunodeficiency Virus on Antiretroviral Therapy—Update 2022: Systematic Review. Pharmaceutics 2023, 15, 2488. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, J.; Baldwin, S.; Smith, G.; Ayrton, A.; Clarke, S.; Chenery, R. Characterisation of the cytochrome P450 enzymes involved in the in vitro metabolism of granisetron. Br. J. Clin. Pharmacol. 1994, 38, 557–566. [Google Scholar] [CrossRef]
- He, K.; Woolf, T.F.; Hollenberg, P.F. Mechanism-based inactivation of cytochrome P-450-3A4 by mifepristone (RU486). J. Pharmacol. Exp. Ther. 1999, 288, 791–797. [Google Scholar] [CrossRef]
- Beedham, C.; Miceli, J.J.; Obach, R.S. Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J. Clin. Psychopharmacol. 2003, 23, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Preskorn, S.; Hochfeld, M.; Meng, X. A thorough QTc study of 3 doses of iloperidone including metabolic inhibition via CYP2D6 and/or CYP3A4 and a comparison to quetiapine and ziprasidone. J. Clin. Psychopharmacol. 2013, 33, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ebert, U.; Thong, N.; Oertel, R.; Kirch, W. Effects of rifampicin and cimetidine on pharmacokinetics and pharmacodynamics of lamotrigine in healthy subjects. Eur. J. Clin. Pharmacol. 2000, 56, 299–304. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, X.; Zhao, L.; Hu, M.; Gao, F. Clinical and pharmacological hallmarks of rifapentine’s use in diabetes patients with active and latent tuberculosis: Do we know enough? Drug Des. Dev. Ther. 2017, 11, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.K.; Nguyen, D.; Richards-Peterson, L.; Skordos, K.W. The metabolic drug-drug interaction profile of Dabrafenib: In vitro investigations and quantitative extrapolation of the P450-mediated DDI risk. Drug Metab. Dispos. 2014, 42, 1180–1190. [Google Scholar] [CrossRef]
- Rivasi, G.; Rafanelli, M.; Mossello, E.; Brignole, M.; Ungar, A. Drug-related orthostatic hypotension: Beyond anti-hypertensive medications. Drugs Aging 2020, 37, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Gish, E.C.; Miller, J.L.; Honey, B.L.; Johnson, P.N. Lofexidine, an α2-receptor agonist for opioid detoxification. Ann. Pharmacother. 2010, 44, 343–351. [Google Scholar] [CrossRef] [PubMed]
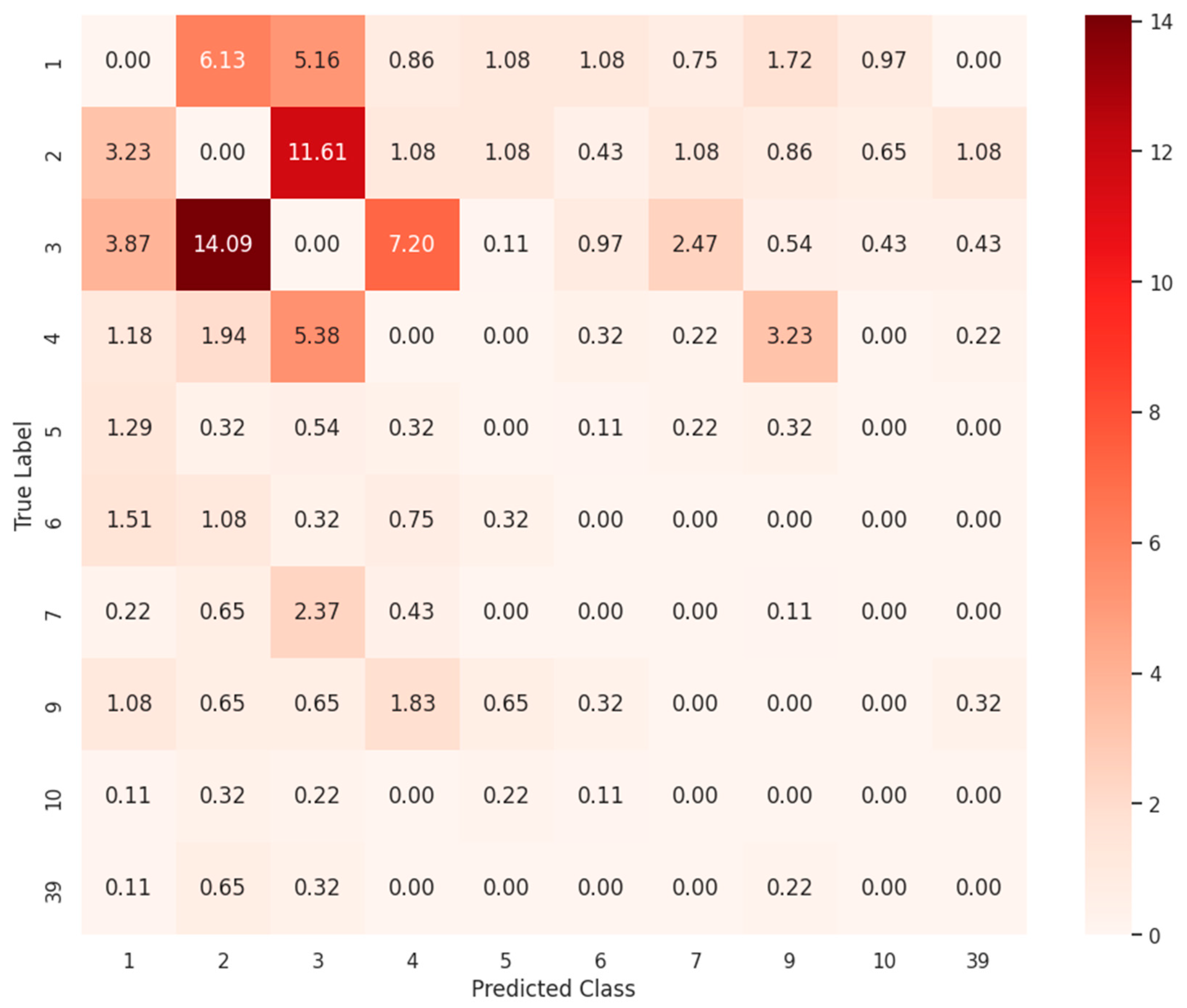
| Class | Description | Test Samples | Test Sample Ratio (%) | Misclassified Count | Misclassified Ratio (%) |
|---|---|---|---|---|---|
| 3 | The serum concentration of Drug b can be increased when it is combined with Drug a. | 4122 | 11.52 | 268 | 21.72 |
| 2 | The metabolism of Drug b can be decreased when combined with Drug a. | 6186 | 17.29 | 260 | 21.07 |
| 1 | The risk or severity of adverse effects can be increased when Drug a is combined with Drug b. | 11,583 | 32.38 | 152 | 12.32 |
| 4 | The serum concentration of Drug b can be decreased when it is combined with Drug a. | 1683 | 4.71 | 128 | 10.37 |
| 9 | The metabolism of Drug b can be increased when combined with Drug a. | 935 | 2.61 | 68 | 5.51 |
| 7 | Drug a may increase the QTc-prolonging activities of Drug b. | 1180 | 3.3 | 51 | 4.13 |
| 6 | The therapeutic efficacy of Drug b can be decreased when used in combination with Drug a. | 1488 | 4.16 | 37 | 3 |
| 5 | Drug a may increase the hypotensive activities of Drug b. | 1647 | 4.6 | 36 | 2.92 |
| 10 | Drug a may increase the anticoagulant activities of Drug b. | 631 | 1.76 | 20 | 1.62 |
| 39 | The serum concentration of the active metabolites of Drug b can be reduced when Drug b is used in combination with Drug a resulting in a loss in efficacy. | 65 | 0.18 | 19 | 1.54 |
| True Class | Predicted Class | Drug A | Drug B | Comment |
|---|---|---|---|---|
| 2 | 3 | Ketoconazole | Delavirdine | CYP3A4 inhibition by ketoconazole increases delavirdine serum levels [36]. |
| 2 | 3 | Voriconazole | Amiodarone | CYP3A4 inhibition by voriconazole increases amiodarone serum levels [37]. |
| 2 | 3 | Cobicistat | Ketamine | CYP3A4 inhibition by cobicistat increases oral ketamine serum levels [38]. |
| 3 | 7 | Mifepristone | Granisetron | CYP3A4 inhibition by mifepristone may increase the risk of QTc prolongation with granisetron [39,40]. |
| 3 | 7 | Mifepristone | Ziprasidone | CYP3A4 inhibition by mifepristone may increase the risk of QTc prolongation with ziprasidone [40,41]. |
| 3 | 7 | Mifepristone | Iloperidone | CYP3A4 inhibition by mifepristone may increase the risk of QTc prolongation with iloperidone [41,42]. |
| 9 | 4 | Rifampicin | Lamotrigine | UGT induction by rifampicin decreases lamotrigine serum levels [43]. |
| 9 | 4 | Rifapentine | Dabrafenib | CYP3A4/2C8 induction by rifapentine decreases dabrafenib serum levels [44,45]. |
| 41 | 5 | Silodosin | Lofexidine | Coadministration of the α1-blocker silodosin and the central α2-agonist lofexidine may increase the risk of hypotension through additive effects [46,47]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.; Ko, Y. LLM-Enhanced Multimodal Framework for Drug–Drug Interaction Prediction. Biomedicines 2025, 13, 2355. https://doi.org/10.3390/biomedicines13102355
Im S, Ko Y. LLM-Enhanced Multimodal Framework for Drug–Drug Interaction Prediction. Biomedicines. 2025; 13(10):2355. https://doi.org/10.3390/biomedicines13102355
Chicago/Turabian StyleIm, Song, and Younhee Ko. 2025. "LLM-Enhanced Multimodal Framework for Drug–Drug Interaction Prediction" Biomedicines 13, no. 10: 2355. https://doi.org/10.3390/biomedicines13102355
APA StyleIm, S., & Ko, Y. (2025). LLM-Enhanced Multimodal Framework for Drug–Drug Interaction Prediction. Biomedicines, 13(10), 2355. https://doi.org/10.3390/biomedicines13102355








