Low-Molecular-Weight Heparin in Preeclampsia: Effects on Biomarkers and Prevention: A Narrative Review
Abstract
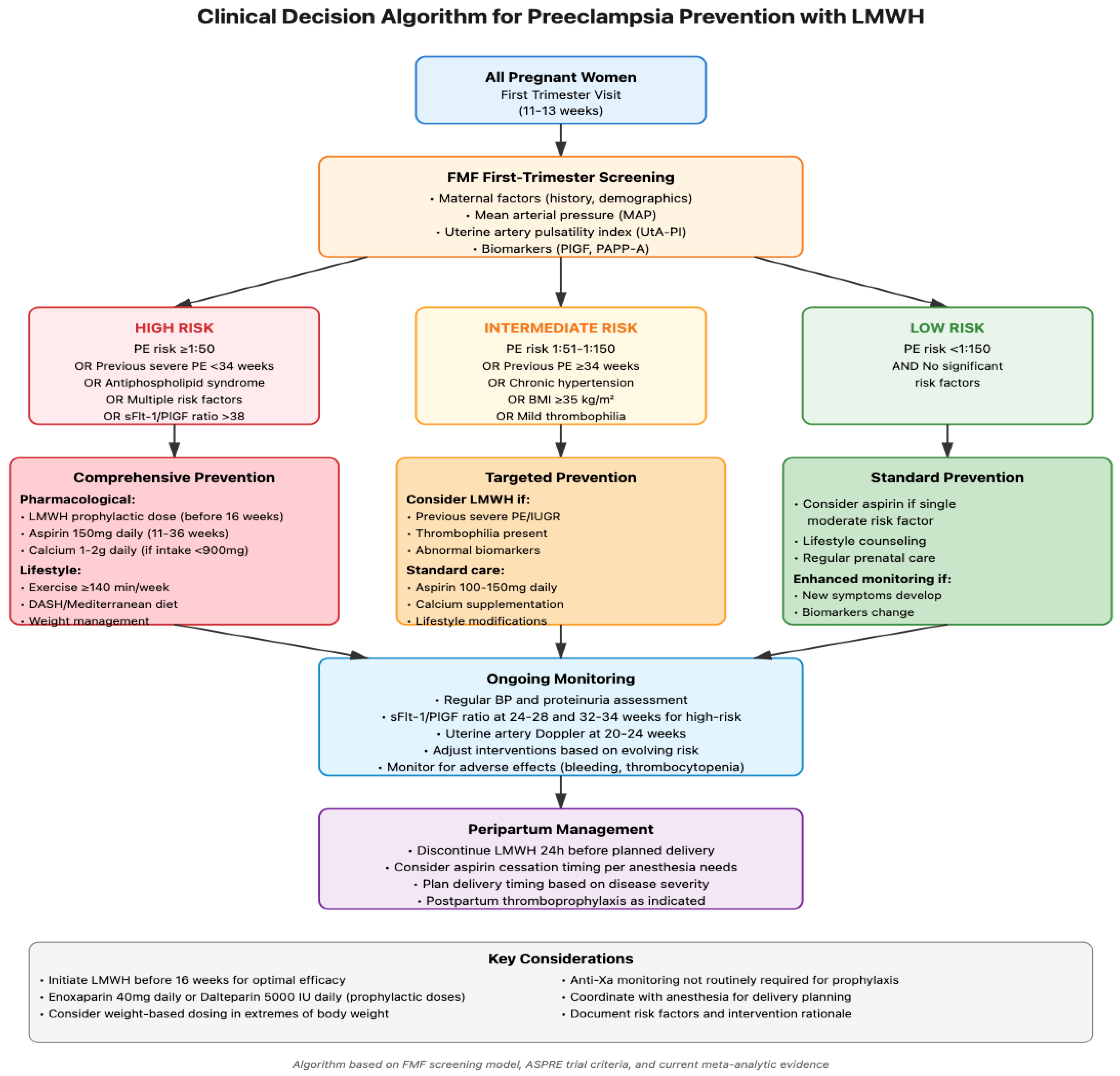
1. Introduction
1.1. Clinical Classification and Disease Manifestations
1.2. Risk Stratification and Predictive Modeling
1.3. Current Prevention Approaches
1.3.1. Pharmacological Strategies
1.3.2. Lifestyle Modifications
1.4. LMWH as a Preventive Measure
2. Pathophysiological Foundations of Preeclampsia
2.1. Placental Origins and Maternal Consequences
2.2. Biomarker Profiles in Preeclampsia
2.2.1. Angiogenic Balance Markers
2.2.2. Inflammatory Mediators
2.2.3. Endothelial Activation Markers
2.2.4. Hemostatic System Markers
2.2.5. Placental-Specific Markers
3. Pharmacology of Low-Molecular-Weight Heparin in Pregnancy
3.1. LMWH Preparations and Properties
3.2. Pharmacokinetics and Pharmacodynamics in Pregnancy
3.3. Safety Profile in Pregnancy
4. Effects of LMWH on Preeclampsia Biomarkers
4.1. Impact on Angiogenic Factors
4.2. Effects on Inflammatory Markers
4.3. Influence on Coagulation Parameters
4.4. Effects on Placental Development and Function
5. LMWH for Prevention of Preeclampsia: Clinical Evidence
5.1. Randomized Controlled Trials
5.2. Meta-Analyses and Systematic Reviews
5.3. Subgroup Analyses and Specific Populations
5.3.1. Previous Placenta-Mediated Complications
5.3.2. Thrombophilia Status
5.3.3. Timing of Intervention
6. Integration with Other Preventive Strategies
6.1. LMWH and Low-Dose Aspirin
6.2. LMWH and Calcium Supplementation
6.3. LMWH and Lifestyle Interventions
6.4. Risk-Stratified Combination Approaches
6.5. Timing and Sequencing Considerations
6.6. Safety Considerations in Combination Therapy
7. Guidelines and Current Recommendations
International Guidelines
8. Limitations and Areas for Future Directions
8.1. Current Evidence Limitations and Research Gaps
8.2. Ethical and Cost-Effectiveness Considerations
8.3. Priority Areas for Future Research
8.3.1. Biomarker-Guided Trial Design
8.3.2. Dose-Optimization and Timing Studies
8.3.3. Novel Combination Therapies
8.3.4. Long-Term Outcome Assessment
8.3.5. Standardization Initiatives
8.4. Implementation Challenges and Solutions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physicians |
| ACOG | American College of Obstetricians and Gynecologists |
| ADMA | Asymmetric dimethylarginine |
| APS | Antiphospholipid syndrome |
| ASA | Aspirin |
| BID | Twice daily |
| BMI | Body mass index |
| CI | Confidence interval |
| CRP | C-reactive protein |
| DASH | Dietary Approaches to Stop Hypertension |
| FGR | Fetal growth restriction |
| FMF | Fetal Medicine Foundation |
| HDP | Hypertensive disorders of pregnancy |
| HIT | Heparin-induced thrombocytopenia |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IGFBP-1 | Insulin-like growth factor binding protein-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| ISSHP | International Society for the Study of Hypertension in Pregnancy |
| ISTH | International Society on Thrombosis and Haemostasis |
| IU | International units |
| IUGR | Intrauterine growth restriction |
| LMWH | Low-molecular-weight heparin |
| MMP | Matrix metalloproteinase |
| NLR | Neutrophil-to-lymphocyte ratio |
| OD | Once daily |
| OR | Odds ratio |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAPP-A | Pregnancy-associated plasma protein A |
| PE | Preeclampsia |
| PI | Pulsatility index |
| PlGF | Placental growth factor |
| PLR | Platelet-to-lymphocyte ratio |
| PP13 | Placental protein 13 |
| RCT | Randomized controlled trial |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| RR | Relative risk |
| SB | Stillbirth |
| sEng | Soluble endoglin |
| sFlt-1 | Soluble fms-like tyrosine kinase-1 |
| SGA | Small for gestational age |
| SII | Systemic immune inflammation index |
| sICAM-1 | Soluble intercellular adhesion molecule-1 |
| SOGC | Society of Obstetricians and Gynaecologists of Canada |
| TF | Tissue factor |
| TFPI | Tissue factor pathway inhibitor |
| TNF-α | Tumor necrosis factor-alpha |
| tPA | Tissue plasminogen activator |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VTE | Venous thromboembolism |
References
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Latest Advances in understanding preeclampsia. Science 2005, 308, 1592–1594. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Brenner, B. Haemostatic changes in pregnancy. Thromb. Res. 2004, 114, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.J.J.; Lijfering, W.M.; Middeldorp, S. Current and future candidates for anticoagulant prophylaxis and treatment of venous thromboembolism in pregnancy. Semin. Thromb. Hemost. 2020, 46, 932–941. [Google Scholar]
- Gray, E.; Mulloy, B.; Barrowcliffe, T.W. Heparin and low-molecular-weight heparin. Thromb. Haemost. 2008, 99, 807–818. [Google Scholar]
- Li, W.; McIntyre, T.M.; Silverstein, R.L. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013, 1, 50–55. [Google Scholar] [CrossRef]
- Sobel, M.L.; Kingdom, J.; Drewlo, S. Angiogenic response of placental villi to heparin. Obstet. Gynecol. 2011, 117, 1375–1383. [Google Scholar] [CrossRef]
- Girardi, G.; Redecha, P.; Salmon, J.E. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat. Med. 2004, 10, 1222–1226. [Google Scholar]
- Rey, E.; Garneau, P.; David, M.; Gauthier, R.; Leduc, L.; Michon, N.; Morin, F.; Demers, C.; Kahn, S.R.; Magee, L.A.; et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: A pilot randomized controlled trial. J. Thromb. Haemost. 2009, 7, 58–64. [Google Scholar] [CrossRef]
- Lai, J.; Syngelaki, A.; Nicolaides, K.H.; von Dadelszen, P.; Magee, L.A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 2021, 224, 518.e1–518.e11. [Google Scholar] [CrossRef] [PubMed]
- Masini, G.; Foo, L.F.; Tay, J.; Wilkinson, I.B.; Valensise, H.; Gyselaers, W.; Lees, C.C. Preeclampsia has 2 phenotypes that require different treatment strategies. Am. J. Obstet. Gynecol. 2022, 227, 114–115. [Google Scholar] [CrossRef]
- Gibbone, E.; Huluta, I.; Wright, A.; Nicolaides, K.H.; Charakida, M. Maternal cardiac function at midgestation and development of preeclampsia. J. Am. Coll. Cardiol. 2022, 79, 52–62. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am. J. Obstet. Gynecol. 2013, 209, 544.e1–544.e12. [Google Scholar] [CrossRef] [PubMed]
- Ghulmiyyah, L.; Sibai, B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. A), 32–37. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019, CD004659. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Duley, L.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hy-pertensive disorders and related problems. Cochrane Database Syst. Rev. 2014, 2014, CD001059. [Google Scholar]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Mishra, G.D. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014, 12, 157. [Google Scholar] [CrossRef]
- Qiu, C.; Coughlin, K.B.; Frederick, I.O.; Sorensen, T.K.; Williams, M.A. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am. J. Hypertens. 2008, 21, 903–909. [Google Scholar] [PubMed]
- Frederick, I.O.; Williams, M.A.; Dashow, E.; Kestin, M.; Zhang, C.; Leisenring, W.M. Dietary fiber, potassium, magnesium and calcium in relation to the risk of preeclampsia. J. Reprod. Med. 2005, 50, 332–344. [Google Scholar] [PubMed]
- Borgen, I.; Aamodt, G.; Harsem, N.; Haugen, M.; Meltzer, H.M.; Brantsæter, A.L. Maternal sugar consumption and risk of preeclampsia in nulliparous Norwegian women. Eur. J. Clin. Nutr. 2012, 66, 920–925. [Google Scholar] [CrossRef]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The mediterranean diet and cardiovascular health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Baroutis, D.; Kalampokas, T.; Katsianou, E.; Psarris, A.; Daskalakis, G.; Panoulis, K.; Eleftheriades, M. The Role of the Mediterranean Diet in Assisted Reproduction: A Literature Review. Nutrients 2024, 16, 2807. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213 (Suppl. 4), S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. The immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. Diagnosis and management of gestational hypertension and preeclampsia. Obstet. Gynecol. 2003, 102, 181–192. [Google Scholar]
- Many, A.; Schreiber, L.; Rosner, S.; Lessing, J.B.; Eldor, A.; Kupferminc, M.J. Pathologic features of the placenta in women with severe pregnancy complications and thrombophilia. Obstet. Gynecol. 2001, 98, 1041–1044. [Google Scholar]
- Erez, O.; Mastrolia, S.A.; Thachil, J. Disseminated intravascular coagulation in pregnancy: Insights in pathophysiology, diagnosis and management. Am. J. Obstet. Gynecol. 2015, 213, 452–463. [Google Scholar] [CrossRef]
- Redecha, P.; Franzke, C.W.; Ruf, W.; Mackman, N.; Girardi, G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J. Clin. Investig. 2008, 118, 3453–3461. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.-H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.-I.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Ozler, A.; Turgut, A.; Sak, M.E.; Evsen, M.S.; Soydinc, H.E.; Evliyaoglu, O.; Gul, T. Serum levels of neopterin, tumor necrosis factor-alpha and Interleukin-6 in preeclampsia: Relationship with disease severity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1707–1712. [Google Scholar]
- Szarka, A.; Rigo, J.; Lázár, L.; Bekő, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Ramma, W.; Ahmed, A. Is inflammation the cause of pre-eclampsia? Biochem. Soc. Trans. 2011, 39, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, F.; Schlüssel, M.M.; Vaz, J.S.; Franco-Sena, A.B.; Pinto, T.J.P.; Bastos, F.I.; Adegboye, A.R.A.; Kac, G. C-reactive protein and later preeclampsia: Systematic review and meta-analysis taking into account the weight status. J. Hypertens. 2013, 31, 16–26. [Google Scholar] [CrossRef]
- Mannaerts, D.; Heyvaert, S.; De Cordt, C.; Macken, C.; Loos, C.; Jacquemyn, Y. Are neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and/or mean platelet volume (MPV) clinically useful as predictive parameters for preeclampsia? J. Matern. Fetal Neonatal Med. 2019, 32, 1412–1419. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J. Hum. Hypertens. 2012, 26, 236–241. [Google Scholar] [CrossRef]
- Saleh, L.; Verdonk, K.; Visser, W.; van den Meiracker, A.H.; Danser, A.H.J. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther. Adv. Cardiovasc. Dis. 2016, 10, 282–293. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Yoshimatsu, J.; Espinoza, J.; Kim, Y.M.; Park, K.; Kalache, K.; Edwin, S.; Bujold, E.; Gomez, R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J. Matern. Fetal Neonatal Med. 2002, 12, 19–27. [Google Scholar]
- Speer, P.D.; Powers, R.W.; Frank, M.P.; Harger, G.; Markovic, N.; Roberts, J.M. Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with small-for-gestational-age infants. Am. J. Obstet. Gynecol. 2008, 198, 112.e1–112.e7. [Google Scholar] [CrossRef] [PubMed]
- Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Redman, C.W.; Sargent, I.L.; Wang, Y.-L. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS ONE 2013, 8, e56754. [Google Scholar] [CrossRef] [PubMed]
- Cadroy, Y.; Grandjean, H.; Pichon, J.; Desprats, R.; Berrebi, A.; Fournié, A.; Boneu, B. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br. J. Obstet. Gynaecol. 1993, 100, 416–420. [Google Scholar] [CrossRef]
- Dusse, L.M.; Rios, D.R.; Pinheiro, M.B.; Cooper, A.J.; Lwaleed, B.A. Pre-eclampsia: Relationship between coagulation, fibrinolysis and inflammation. Clin. Chim. Acta 2011, 412, 17–21. [Google Scholar] [CrossRef]
- Schjetlein, R.; Haugen, G.; Wisløff, F. Markers of intravascular coagulation and fibrinolysis in preeclampsia: Association with intrauterine growth retardation. Acta Obstet. Gynecol. Scand. 1997, 76, 541–546. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Hoppensteadt, D.; Than, N.G.; Fareed, J.; Mazaki-Tovi, S.; Espinoza, J.; Chaiworapongsa, T.; Kim, S.-S.; Yoon, B.H.; et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J. Matern. Neonatal Med. 2008, 21, 855–869. [Google Scholar] [CrossRef]
- Pinheiro, M.B.; Gomes, K.B.; Dusse, L.M. Fibrinolytic system in preeclampsia. Clin. Chim. Acta 2013, 416, 67–71. [Google Scholar] [CrossRef]
- Macey, M.; Bevan, S.; Alam, S.; Verghese, L.; Agrawal, S.; Beski, S.; Thuraisingham, R.; MacCallum, P. Platelet activation and endogenous thrombin potential in pre-eclampsia. Thromb. Res. 2010, 125, e76–e81. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Sammar, M.; Chefetz, I.; Neumaier-Wagner, P.; Bartz, C.; Meiri, H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn. Ther. 2008, 24, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Maiz, N.; Valencia, C.; Plasencia, W.; Nicolaides, K.H. First-trimester maternal serum pregnancy-associated plasma pro-tein-A and pre-eclampsia. Ultrasound Obstet. Gynecol. 2009, 33, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Zaravinos, A.; Maiz, N.; Spandidos, D.A.; Nicolaides, K.H. First-trimester maternal plasma cell-free fetal DNA and preeclampsia. Am. J. Obstet. Gynecol. 2009, 201, 472.e1–472.e7. [Google Scholar] [CrossRef]
- Salomon, C.; Guanzon, D.; Scholz-Romero, K.; Longo, S.; Correa, P.; Illanes, S.E.; Rice, G.E. Placental exosomes as early biomarker of preeclampsia: Potential role of exosomal microRNAs across gestation. J. Clin. Endocrinol. Metab. 2017, 102, 3182–3194. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef]
- Lim, W. Using low molecular weight heparin in special patient populations. J. Thromb. Thrombolysis 2010, 29, 233–240. [Google Scholar] [CrossRef] [PubMed]
- James, A.H. Prevention and management of venous thromboembolism in pregnancy. Am. J. Med. 2007, 120 (Suppl. 2), S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Sanson, B.J.; Lensing, A.W.; Prins, M.H.; Ginsberg, J.S.; Barkagan, Z.S.; Lavenne-Pardonge, E.; Brenner, B.; Dulitzky, M.; Nielsen, J.D.; Boda, Z.; et al. Safety of low-molecular-weight heparin in pregnancy: A systematic review. Thromb. Haemost. 1999, 81, 668–672. [Google Scholar] [CrossRef]
- Casele, H.L.; Laifer, S.A.; Woelkers, D.A.; Venkataramanan, R. Changes in the pharmacokinetics of the low-molecular-weight heparin enoxaparin sodium during pregnancy. Am. J. Obstet. Gynecol. 1999, 181 Pt 1, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; McLintock, C.; Taylor, R.S.; North, R.A. Prophylactic and therapeutic enoxaparin during pregnancy: Indications, outcomes and monitoring. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 123–128. [Google Scholar] [CrossRef]
- Norris, L.A.; Bonnar, J.; Smith, M.P.; Steer, P.J.; Savidge, G. Low molecular weight heparin (tinzaparin) therapy for moderate risk thromboprophylaxis during pregnancy: A pharmacokinetic study. Thromb. Haemost. 2004, 92, 791–796. [Google Scholar]
- Smith, M.P.; Norris, L.A.; Steer, P.J.; Savidge, G.F.; Bonnar, J. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am. J. Obstet. Gynecol. 2004, 190, 495–501. [Google Scholar] [CrossRef]
- Rey, E.; Rivard, G.E. Prophylaxis and treatment of thromboembolic diseases during pregnancy with dalteparin. Int. J. Gynecol. Obstet. 2000, 71, 19–24. [Google Scholar] [CrossRef]
- Nelson-Piercy, C.; Powrie, R.; Borg, J.Y.; Rodger, M.; Talbot, D.J.; Stinson, J.; Greer, I.A. Tinzaparin use in pregnancy: An international, retrospective study of the safety and efficacy profile. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 293–299. [Google Scholar] [CrossRef]
- Bleker, S.M.; Eerenberg, E.S.; Smits, L.J.M.; van Doorn, H.C.; Middeldorp, S. Low-molecular-weight heparin dosing in pregnant women: How can we reach agreement? Res Pract Thromb. Haemost. 2020, 4, 62–65. [Google Scholar]
- Cruz, M.; Fernández-Alonso, A.M.; Rodríguez, I.; Garrigosa, L.; Cao, A.; Carretero, P.; González-Paredes, A. Postcesarean thromboprophylaxis with two different regimens of bemiparin. Obstet. Gynecol. Int. 2011, 2011, 548327. [Google Scholar] [CrossRef]
- James, A.H.; Bates, S.M.; Bauer, K.A.; Branch, W.; Mann, K.; Paidas, M.; Silverman, N.; Konkle, B.A. Management of hereditary antithrombin deficiency in pregnancy. Thromb. Res. 2017, 157, 41–45. [Google Scholar] [CrossRef]
- van Lennep, J.E.R.; Meijer, E.; Klumper, F.J.C.M.; Middeldorp, J.M.; Bloemenkamp, K.W.M.; Middeldorp, S. Prophylaxis with low-dose low-molecular-weight-heparin during pregnancy and postpartum: Is it effective? J. Thromb. Haemost. 2011, 9, 473–480. [Google Scholar] [CrossRef]
- Lebaudy, C.; Hulot, J.; Amoura, Z.; Costedoat-Chalumeau, N.; Serreau, R.; Ankri, A.; Conard, J.; Cornet, A.; Dommergues, M.; Piette, J.; et al. Changes in enoxaparin pharmacokinetics during pregnancy and implications for antithrombotic therapeutic strategy. Clin. Pharmacol. Ther. 2008, 84, 370–377. [Google Scholar] [CrossRef]
- McDonnell, B.P.; Glennon, K.; McTiernan, A.; O’cOnnor, H.D.; Kirkham, C.; Kevane, B.; Donnelly, J.C.; Ni Áinle, F. Adjustment of therapeutic LMWH to achieve specific target anti-FXa activity does not affect outcomes in pregnant patients with venous thromboembolism. J. Thromb. Thrombolysis 2017, 43, 105–111. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tokunaga, N.; Sugimura, M.; Kanayama, N.; Terao, T. Predictive values of coagulation/fibrinolysis parameters for the termination of pregnancy complicated by severe preeclampsia. Semin. Thromb. Hemost. 2001, 27, 137–142. [Google Scholar] [CrossRef]
- Rodger, M.A.; Hague, W.M.; Kingdom, J.; Kahn, S.R.; Karovitch, A.; Sermer, M.; Clement, A.M.; Coat, S.; Chan, W.S.; Said, J.; et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): A multinational open-label randomised trial. Lancet 2014, 384, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- McLintock, C.; Brighton, T.; Chunilal, S.; Dekker, G.; McDonnell, N.; McRae, S.; Muller, P.; Tran, H.; Walters, B.N.J.; Young, L.; et al. Recommendations for the prevention of pregnancy-associated venous thrombo-embolism. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 3–13. [Google Scholar] [CrossRef]
- Thourani, V.H.; Brar, S.S.; Kennedy, T.P.; Thornton, L.R.; Watts, J.A.; Ronson, R.S.; Zhao, Z.-Q.; Sturrock, A.L.; Hoidal, J.R.; Vinten-Johansen, J. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am. J. Physiol Heart. Circ. Physiol. 2000, 278, H2084–H2093. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, S.; Ortiz, A.S.; Veglia, M.; Tersigni, C.; Di Simone, N. Low molecular weight heparin in obstetric care: A review of the literature. Reprod. Sci. 2011, 18, 602–613. [Google Scholar] [CrossRef]
- Di Simone, N.; Di Nicuolo, F.; Sanguinetti, M.; Ferrazzani, S.; D’ALessio, M.; Castellani, R.; Bompiani, A.; Caruso, A. Low-molecular weight heparin induces in vitro trophoblast invasiveness: Role of matrix metalloproteinases and tissue inhibitors. Placenta 2007, 28, 298–304. [Google Scholar] [CrossRef]
- Clark, P. The role of low molecular weight heparin in pregnancy: Beyond the usual indications. Thromb. Res. 2008, 123 (Suppl. 1), S21–S29. [Google Scholar]
- Lepercq, J.; Conard, J.; Borel-Derlon, A.; Darmon, J.Y.; Boudignat, O.; Francoual, C.; Priollet, P.; Cohen, C.; Yvelin, N.; Schved, J.F.; et al. Venous thromboembolism during pregnancy: A retrospective study of enoxaparin safety in 624 pregnancies. Br. J. Obstet. Gynaecol. 2001, 108, 1134–1140. [Google Scholar] [CrossRef]
- Greer, I.A.; Nelson-Piercy, C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: A systematic review of safety and efficacy. Blood 2005, 106, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Hohlfeld, P.; Spertini, F.; Hayoz, D.; Schapira, M.; Duchosal, M.A. Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 2006, 108, 1569–1570. [Google Scholar] [CrossRef][Green Version]
- Linkins, L.A.; Dans, A.L.; Moores, L.K.; Bona, R.; Davidson, B.L.; Schulman, S.; Crowther, M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guide-lines. Chest 2012, 141 (Suppl. 2), e495S–e530S. [Google Scholar] [CrossRef]
- Lefkou, E.; Khamashta, M.; Hampson, G.; Hunt, B. Low-molecular-weight heparin-induced osteoporosis and osteoporotic fractures: A myth or an existing entity? Lupus 2010, 19, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, E.; Kopp, S.; McBane, R.D.; Perlas, A.; Leffert, L.; Horlocker, T. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines. Reg. Anesth. Pain Med. 2018, 43, 263–309. [Google Scholar]
- Leffert, L.; Butwick, A.; Carvalho, B.; Arendt, K.; Bates, S.M.; Friedman, A.; Horlocker, T.; Houle, T.; Landau, R.; Dubois, H.; et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth. Analg. 2018, 126, 928–944. [Google Scholar] [CrossRef]
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), e691S–e736S. [Google Scholar] [CrossRef]
- Nelson, S.M.; Greer, I.A. Heparin in obstetrics—Clinical applications. BJOG 2021, 128, 517–527. [Google Scholar]
- Carroll, T.Y.; Mulla, M.J.; Han, C.S.; Brosens, J.J.; Chamley, L.W.; Giles, I.; Pericleous, C.; Rahman, A.; Sfakianaki, A.K.; Paidas, M.J.; et al. Modulation of trophoblast angiogenic factor secretion by antiphospholipid antibodies is not reversed by heparin. Am. J. Reprod. Immunol. 2011, 66, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Searle, J.; Mockel, M.; Gwosc, S.; Datwyler, S.A.; Qadri, F.; Albert, G.I.; Holert, F.; Isbruch, A.; Klug, L.; Muller, D.N.; et al. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro—Brief report. Arter. Thromb. Vasc. Biol. 2011, 31, 2972–2974. [Google Scholar] [CrossRef]
- Girardi, G. Can statins prevent pregnancy complications? J. Reprod. Immunol. 2014, 101–102, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Carrier, M.; Le Gal, G.; Martinelli, I.; Perna, A.; Rey, É.; de Vries, J.I.P.; Gris, J.-C. Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood 2014, 123, 822–828. [Google Scholar] [CrossRef]
- Abheiden, C.; Van Hoorn, M.; Hague, W.; Kostense, P.; van Pampus, M.; de Vries, J. Does low-molecular-weight heparin influence fetal growth or uterine and umbilical arterial Doppler in women with a history of early-onset uteroplacental insufficiency and an inheritable thrombophilia? Secondary randomised controlled trial results. BJOG Br. J. Obstet. Gynaecol. 2016, 123, 797–805. [Google Scholar] [CrossRef]
- Groom, K.M.; McCowan, L.M.; Mackay, L.K.; Lee, A.C.; Said, J.M.; Kane, S.C.; Walker, S.P.; van Mens, T.E.; Hannan, N.J.; Tong, S.; et al. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: A randomized trial. Am. J. Obstet. Gynecol. 2017, 216, 296.e1–296.e14. [Google Scholar] [CrossRef]
- Hochart, H.; Jenkins, P.V.; Smith, O.P.; White, B. Low-molecular weight and unfractionated heparins induce a downregulation of inflammation: Decreased levels of proinflammatory cytokines and nuclear factor-kappaB in LPS-stimulated human monocytes. Br. J. Haematol. 2006, 133, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Young, E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar] [CrossRef]
- DiSimone, N.; Caliandro, D.; Castellani, R.; Ferrazzani, S.; DeCarolis, S.; Caruso, A. Low-molecular weight heparin restores in-vitro trophoblast invasiveness and differentiation in presence of immunoglobulin G fractions obtained from patients with antiphospholipid syndrome. Hum. Reprod. 1999, 14, 489–495. [Google Scholar] [CrossRef]
- Downing, L.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Greenfield, L.J.; Wakefield, T.W. Low-dose low–molecular-weight heparin is anti-inflammatory during venous thrombosis. J. Vasc. Surg. 1998, 28, 848–854. [Google Scholar] [CrossRef]
- Page, C.F. One explanation of the asthma paradox: Inhibition of natural anti-inflammatory mechanism by beta 2-agonists. Lancet 1991, 337, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, T.E.; Levine, M.N.; Hirsh, J.; Horsewood, P.; Roberts, R.S.; Gent, M.; Kelton, J.G. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N. Engl. J. Med. 1995, 332, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- de Swiet, M.; Ward, P.D.; Fidler, J.; Horsman, A.; Katz, D.; Letsky, E.; Peacock, M.; Wise, P.H. Prolonged heparin therapy in pregnancy causes bone demineralization. Br. J. Obstet. Gynaecol. 1983, 90, 1129–1134. [Google Scholar] [CrossRef]
- Martinelli, I.; Ruggenenti, P.; Cetin, I.; Pardi, G.; Perna, A.; Vergani, P.; Acaia, B.; Facchinetti, F.; La Sala, G.B.; Bozzo, M.; et al. Heparin in pregnant women with previous placenta-mediated pregnancy complications: A prospective, randomized, multicenter, controlled clinical trial. Blood 2012, 119, 3269–3275. [Google Scholar] [CrossRef]
- de Jong, P.G.; Kaandorp, S.; Di Nisio, M.; Goddijn, M.; Middeldorp, S.; Cochrane Pregnancy and Childbirth Group. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst. Rev. 2014, 2014, CD004734. [Google Scholar] [CrossRef]
- Simes, J.; Becattini, C.; Agnelli, G.; Eikelboom, J.W.; Kirby, A.C.; Mister, R.; Prandoni, P.; Brighton, T.A. Aspirin for the prevention of recurrent venous thromboembolism: The INSPIRE collabo-ration. Circulation 2014, 130, 1062–1071. [Google Scholar] [PubMed]
- Tanjung, M.T.; Siddik, H.D.; Hariman, H.; Koh, S.C. Coagulation and fibrinolysis in preeclampsia and neonates. Clin. Appl. Thromb. Hemost. 2005, 11, 467–473. [Google Scholar] [CrossRef]
- Quenby, S.; Mountfield, S.M.; Cartwright, J.E.; Whitley, G.S.J.; Vince, G. Effects of low-molecular-weight and unfractionated heparin on trophoblast function. Obstet. Gynecol. 2004, 104, 354–361. [Google Scholar] [CrossRef]
- Tersigni, C.; Marana, R.; Santamarìa, A.; Castellani, R.; Scambia, G.; Di Simone, N. In vitro evidences of heparin’s effects on embryo implantation and trophoblast development. Reprod. Sci. 2012, 19, 454–462. [Google Scholar] [CrossRef]
- Bose, P.; Black, S.; Kadyrov, M.; Weissenborn, U.; Neulen, J.; Regan, L.; Huppertz, B. Heparin and aspirin attenuate placental apoptosis in vitro: Implications for early pregnancy failure. Am. J. Obstet. Gynecol. 2005, 192, 23–30. [Google Scholar] [CrossRef]
- Hills, F.A.; Abrahams, V.M.; González-Timón, B.; Francis, J.; Cloke, B.; Hinkson, L.; Rai, R.; Mor, G.; Regan, L.; Sullivan, M.; et al. Heparin prevents programmed cell death in human trophoblast. Mol. Hum. Reprod. 2006, 12, 237–243. [Google Scholar] [CrossRef]
- Laliberté, F.; Dea, K.; Duh, M.S.; Kahler, K.H.; Rolli, M.; Lefebvre, P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause 2011, 18, 1052–1059. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Zhang, W.; Wang, Q. Analysis of perinatal coagulation function in preeclampsia. Medicine 2021, 100, e26271. [Google Scholar] [CrossRef]
- Gris, J.C.; Chauleur, C.; Faillie, J.L.; Baer, G.; Marès, P.; Fabbro-Peray, P.; Quéré, I.; Lefrant, J.Y.; Haddad, B.; Dauzat, M. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae. The pilot randomised controlled NOH-AP trial. Thromb. Haemost. 2010, 104, 771–779. [Google Scholar] [PubMed]
- Liu, S.; Ruan, X.; Schönfeld, F.A.; Xu, H.; Schneider, H.; Oelkers, W.H.; Krattenmacher, R.; Berger, U.; Keck, C.; Hommel, G. The anti-inflammatory effect of heparin and its derivatives: A focus on microvascular endothelial cells. J. Vasc. Res. 2002, 39, 202–210. [Google Scholar]
- Davenport, P.; Tipping, P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein e-deficient mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef]
- Maugeri, N.; Di Fabio, G.; Barbanti, M.; de Gaetano, G.; Donati, M.B.; Cerletti, C. Parnaparin inhibits leukocyte-platelet interactions and reduces inflammatory markers in a model of endotoxemia. J. Pharmacol. Exp. Ther. 2011, 337, 409–417. [Google Scholar]
- Gris, J.C.; Mercier, E.; Quéré, I.; Lavigne-Lissalde, G.; Cochery-Nouvellon, E.; Hoffet, M.; Ripart-Neveu, S.; Tailland, M.-L.; Dauzat, M.; Marès, P. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004, 103, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.I.; van Pampus, M.G.; Hague, W.M.; Bezemer, P.D.; Joosten, J.H.; FRUIT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J. Thromb. Haemost. 2012, 10, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Haddad, B.; Winer, N.; Chitrit, Y.; Houfflin-Debarge, V.; Chauleur, C.; Bages, K.; Tsatsaris, V.; Benachi, A.; Bretelle, F.; Gris, J.C.; et al. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: A randomized controlled trial. Obstet. Gynecol. 2016, 128, 1053–1063. [Google Scholar] [CrossRef]
- Martinelli, I.; Maino, A.; Abbattista, M.; Bucciarelli, P.; Mannucci, P.M.; Rosendaal, F.R.; Dentali, F. Duration of low-molecular-weight heparin for prevention of recurrences of pregnancy loss in women with inherited thrombophilia: A multicenter randomized controlled trial. Blood 2017, 130, 103. [Google Scholar]
- Roberge, S.; Demers, S.; Nicolaides, K.H.; Bureau, M.; Côté, S.; Bujold, E. Prevention of pre-eclampsia by low-molecular-weight heparin in addition to aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 548–553. [Google Scholar] [CrossRef]
- McLaughlin, K.; Scholten, R.R.; Kingdom, J.C.; Floras, J.S.; Parker, J.D.; Canavan, T.; Adamson, S.L.; Courtney, J.; Ray, J.G. Should maternal antiphospholipid antibodies affect the timing of delivery in women with hypertensive disorders of pregnancy? A population-based study. Br. J. Obstet. Gynaecol. 2022, 129, 1007–1015. [Google Scholar]
- Seidler, A.L.; Hunter, K.E.; Cheyne, S.; Ghersi, D.; Berlin, J.A.; Askie, L. A guide to prospective meta-analysis. BMJ 2019, 367, l5342. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef]
- Skeith, L.; Carrier, M.; Kaaja, R.; Martinelli, I.; Petroff, D.; Schleußner, E.; Laskin, C.A.; Rodger, M.A. A meta-analysis of low-molecular-weight heparin to prevent pregnancy loss in women with inherited thrombophilia. Blood 2016, 127, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; McLeod, A.; Windrim, R.C.; Kingdom, J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst. Rev. 2013, 2013, CD006780. [Google Scholar] [CrossRef] [PubMed]
- Saccone, G.; Berghella, V.; Maruotti, G.M.; Ghi, T.; Rizzo, G.; Simonazzi, G.; Rizzo, N.; Facchinetti, F.; Dall’aSta, A.; Visentin, S.; et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: The PREGNANTS study. Am. J. Obstet. Gynecol. 2017, 216, 525.e1–525.e12. [Google Scholar] [CrossRef]
- Maher, G.M.; O’keeffe, G.W.; Kenny, L.C.; Kearney, P.M.; Dinan, T.G.; Khashan, A.S. Hypertensive disorders of pregnancy and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e018313. [Google Scholar] [CrossRef]
- Zhang, T.; Ye, X.; Zhu, T.; Xiao, X.; Liu, Y.; Wei, X.; Liu, Y.; Wu, C.; Guan, R.; Li, X.; et al. Antithrombotic treatment for recurrent miscarriage: Bayesian network meta-analysis and systematic review. Medicine 2015, 94, e1732. [Google Scholar] [CrossRef]
- Rodger, M.A.; Gris, J.-C.; De Vries, J.I.P.; Martinelli, I.; Rey, E.; Schleussner, E.; Middeldorp, S.; Kaaja, R.; Langlois, N.J.; Ramsay, T.; et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet 2016, 388, 2629–2641. [Google Scholar] [PubMed]
- Lin, Y.; Xu, J.; Wu, L.; Chen, Y.; Zhang, M.; Wang, L.; Liu, X.; Huang, S.; Li, D.; Zhou, P. Low molecular weight heparin in the treatment of pregnant women with preeclampsia: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2023, 161, 22–33. [Google Scholar]
- Skeith, L.; Rodger, M.A. Prevention of pregnancy complications with low-dose aspirin plus heparin: More questions than answers. Expert Rev. Hematol. 2014, 7, 317–320. [Google Scholar]
- Ginsberg, J.S.; Kowalchuk, G.; Hirsh, J.; Brill-Edwards, P.; Burrows, R. Heparin Therapy During Pregnancy. Risks to the fetus and mother. Arch. Intern. Med. 1989, 149, 2233–2236. [Google Scholar] [CrossRef]
- van Hoorn, M.E.; Hague, W.M.; van Pampus, M.G.; Bezemer, D.; de Vries, J.I. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: The FRUIT-RCT. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 168–173. [Google Scholar] [CrossRef]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous throm-boembolism. J. Thromb. Thrombolysis. 2016, 41, 92–128. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef]
- Staff, A.C.; Redman, C.W.G.; Williams, D.; Leeson, P.; Moe, K.; Thilaganathan, B.; Magnus, P.; Steegers, E.A.; Tsigas, E.Z.; Ness, R.B.; et al. Pregnancy and long-term maternal cardiovascular health: Progress through har-monization of research cohorts and biobanks. Hypertension 2016, 67, 251–260. [Google Scholar] [CrossRef]
- Roberts, J.M.; Druzin, M.; August, P.A.; Leeson, P.; Moe, K.; Thilaganathan, B.; Magnus, P.; Steegers, E.A.; Tsigas, E.Z.; Ness, R.B.; et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Henderson, J.T.; Whitlock, E.P.; O’cOnnor, E.; Senger, C.A.; Thompson, J.H.; Rowland, M.G. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2014, 160, 695–703. [Google Scholar] [CrossRef]
- Belizán, J.M.; Villar, J.; Gonzalez, L.; Campodonico, L.; Bergel, E. Calcium supplementation to prevent hypertensive disorders of pregnancy. N. Eng. J. Med. 1991, 325, 1399–1405. [Google Scholar] [CrossRef]
- Thangaratinam, S.; Langenveld, J.; Mol, B.W.; Khan, K.S. Prediction and primary prevention of pre-eclampsia. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 419–433. [Google Scholar] [CrossRef]
- Roberge, S.; Sibai, B.; McCaw-Binns, A.; Bujold, E. Low-dose aspirin in early gestation for prevention of preeclampsia and small-for-gestational-age neonates: Meta-analysis of large randomized trials. Am. J. Perinatol. 2016, 33, 781–785. [Google Scholar] [CrossRef]
- Costantine, M.M.; Cleary, K.; Hebert, M.F.; Ahmed, M.S.; Brown, L.M.; Ren, Z.; Easterling, T.R.; Haas, D.M.; Haneline, L.S.; Caritis, S.N.; et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: A pilot randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 214, 720.e1–720.e17. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Zhao, X.; Duan, D.; Dou, W.; Fu, W.; Chen, H.; Bo, Y.; Qiu, Y.; Chen, G.; et al. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-style Diet in Relation to Preeclampsia: A Case-Control Study. Sci. Rep. 2020, 10, 9078. [Google Scholar] [CrossRef]
- Arvizu, M.; Stuart, J.J.; Rich-Edwards, J.W.; Gaskins, A.J.; Rosner, B.; Chavarro, J.E. Prepregnancy adherence to dietary recommendations for the prevention of cardiovascular disease in relation to risk of hypertensive disorders of pregnancy. Am. J. Clin. Nutr. 2020, 112, 1429–1437. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef]
- Vesco, K.K.; Karanja, N.; King, J.C.; Gillman, M.W.; Leo, M.C.; Perrin, N.; McEvoy, C.T.; Eckhardt, C.L.; Smith, K.S.; Stevens, V.J. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity 2014, 22, 1989–1996. [Google Scholar] [CrossRef]
- Roberts, J.M.; Bodnar, L.M.; Patrick, T.E.; Powers, R.W. The role of obesity in preeclampsia. Pregnancy Hypertens. 2011, 1, 6–16. [Google Scholar] [CrossRef]
- Chatzi, L.; Mendez, M.; Garcia, R.; Roumeliotaki, T.; Ibarluzea, J.; Tardón, A.; Amiano, P.; Lertxundi, A.; Iñiguez, C.; Vioque, J.; et al. Mediterranean diet adherence during pregnancy and fetal growth: INMA (Spain) and RHEA (Greece) mother–child cohort studies. Br. J. Nutr. 2012, 107, 135–145. [Google Scholar] [CrossRef]
- Urech, C.; Fink, N.S.; Hoesli, I.; Wilhelm, F.H.; Bitzer, J.; Alder, J. Effects of relaxation on psychobiological wellbeing during pregnancy: A randomized controlled trial. Psychoneuroendocrinology 2010, 35, 1348–1355. [Google Scholar] [CrossRef]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PlGF ratio in the as-sessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its im-portance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008, 2008, CD004227. [Google Scholar] [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72 (Suppl. 1), 257S–264S. [Google Scholar] [CrossRef]
- Williamson, C.S. Nutrition in pregnancy. Nutr. Bull. 2006, 31, 28–59. [Google Scholar] [CrossRef]
- Middeldorp, S. New studies of low-molecular-weight heparin in pregnancy. Thromb. Res. 2015, 135 (Suppl. 1), S26–S29. [Google Scholar] [CrossRef]
- Fox, N.S.; Laughon, S.K.; Bender, S.D.; Saltzman, D.H.; Rebarber, A. Anti-factor Xa plasma levels in pregnant women receiving low molecular weight heparin thromboprophylaxis. Obstet. Gynecol. 2008, 112, 884–889. [Google Scholar] [CrossRef]
- Lynch, S.R. Interaction of iron with other nutrients. Nutr. Rev. 1997, 55, 102–110. [Google Scholar] [CrossRef]
- De Jesús, G.R.; Sciascia, S.; Andrade, D.; Barbhaiya, M.; Tektonidou, M.; Banzato, A.; Pengo, V.; Ji, L.; Meroni, P.L.; Ugarte, A.; et al. Factors associated with first thrombosis in patients presenting with obstetric antiphospholipid syndrome (APS) in theAPSAlliance for Clinical Trials and International Networking Clinical Database and Repository: A retrospective study. BJOG Br. J. Obstet. Gynaecol. 2019, 126, 656–661. [Google Scholar]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar]
- Ananth, C.V.; Lavery, J.A.; Vintzileos, A.M.; Skupski, D.W.; Varner, M.; Saade, G.; Biggio, J.; Williams, M.A.; Wapner, R.J.; Wright, J.D. Severe placental abruption: Clinical definition and associations with maternal complications. Am. J. Obstet. Gynecol. 2016, 214, 272.e1–272.e9. [Google Scholar] [CrossRef]
- Bauersachs, R.M.; Dudenhausen, J.; Faridi, A.; Fischer, T.; Fung, S.; Geisen, U.; Harenberg, J.; Herchenhan, E.; Keller, F.; Kemkes-Matthes, B.; et al. Risk stratification and heparin prophylaxis to prevent venous thromboembolism in pregnant women. Thromb. Haemost. 2007, 98, 1237–1245. [Google Scholar] [CrossRef]
- ACOG Practice Bulletin No. 222: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260.
- ACOG Practice Bulletin No. 197: Inherited Thrombophilias in Pregnancy. Obstet. Gynecol. 2018, 132, e18–e34.
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 2014, 36, 416–441. [Google Scholar] [CrossRef]
- Chan, W.-S.; Rey, E.; Kent, N.E.; Corbett, T.; David, M.; Douglas, M.J.; Gibson, P.S.; Magee, L.; Rodger, M.; Smith, R.E. Venous thromboembolism and antithrombotic therapy in pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 527–553. [Google Scholar] [CrossRef]
- Royal College of Obstetricians & Gynaecologists. Reducing the Risk of Venous Thromboembolism During Pregnancy and the Puerperium. In Green-Top Guideline No. 37a. RCOG; Royal College of Obstetricians & Gynaecologists: London, UK, 2015. [Google Scholar]
- Royal College of Obstetricians & Gynaecologists. The Investigation and Management of the Small-for-Gestational-Age Fetus. In Green-top Guideline No. 31. RCOG; Royal College of Obstetricians & Gynaecologists: London, UK, 2014. [Google Scholar]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Baumann Kreuziger, L.; Bounameaux, H.; Doerschug, K.C.; Geersing, G.J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Executive summary: Antithrombotic Therapy for VTE Disease: Second Update of the CHEST Guideline and Expert Panel Report. Chest 2021, 160, 2247–2259. [Google Scholar]
- Scheres, L.J.; Bistervels, I.M.; Middeldorp, S. Everything the clinician needs to know about evidence-based anticoagulation in pregnancy. Blood Rev. 2019, 33, 82–97. [Google Scholar] [CrossRef]
- Monreal, M.; Monreal, L.; Lavin, S.; Lafoz, E.; Anglés, A.; Monasterio, J. Heparin-related osteoporosis in rats. A comparative study between unfractionated heparin and a low molecular weight heparin. Haemostasis 1990, 4, 115. [Google Scholar]
- Mclintock, C.; Brighton, T.; Chunilal, S.; Dekker, G.; Mcdonnell, N.; Mcrae, S.; Muller, P.; Tran, H.; Walters, B.N.; Young, L. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 14–22. [Google Scholar] [CrossRef]
- Ginsberg, J.S.; Bates, S.M.; Kowalchuk, G. The effects of unfractionated heparin on maternal and fetal hemostasis. Thromb. Haemost. 1989, 61, 301–304. [Google Scholar]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 2012, 122, 43–52. [Google Scholar] [CrossRef]
- Chappell, L.C.; Duckworth, S.; Seed, P.T.; Griffin, M.; Myers, J.; Mackillop, L.; Simpson, N.; Waugh, J.; Anumba, D.; Kenny, L.C.; et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation 2013, 128, 2121–2131. [Google Scholar] [CrossRef]
- Dielis, A.W.J.H.; Castoldi, E.; Spronk, H.M.H.; VAN Oerle, R.; Hamulyák, K.; Ten Cate, H.; Rosing, J. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J. Thromb. Haemost. 2008, 6, 125–131. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Korzeniewski, S.J.; Cortez, J.M.; Pappas, A.; Tarca, A.L.; Chaemsaithong, P.; Dong, Z.; Yeo, L.; Hassan, S.S. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: A prospective study. J. Matern. Fetal Neonatal Med. 2014, 27, 132–144. [Google Scholar] [CrossRef]
- De Carolis, S.; Botta, A.; Santucci, S.; Salvi, S.; Moresi, S.; Di Pasquo, E.; Del Sordo, G.; Martino, C. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus 2012, 21, 776–778. [Google Scholar] [CrossRef]
- Empson, M.; Lassere, M.; Craig, J.; Scott, J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst. Rev. 2005, 2005, CD002859. [Google Scholar] [CrossRef]
- Ruffatti, A.; Tonello, M.; Visentin, M.S.; Bontadi, A.; Hoxha, A.; De Carolis, S.; Botta, A.; Salvi, S.; Nuzzo, M.; Rovere-Querini, P.; et al. Risk factors for pregnancy failure in patients with anti-phospholipid syndrome treated with conventional therapies: A multicentre, case-control study. Arthritis Rheum. 2011, 50, 1684–1689. [Google Scholar] [CrossRef]

| Category | Biomarkers | Alteration in Preeclampsia | Potential Clinical Utility | References |
|---|---|---|---|---|
| Angiogenic Factors | sFlt-1 | Increased | Prediction, diagnosis, prognosis | [45,46,47] |
| PlGF | Decreased | Prediction, diagnosis, prognosis | [45,46,47] | |
| sFlt-1/PlGF ratio | Increased | Prediction, diagnosis, rule-out | [46,47] | |
| sEng | Increased | Prediction, severity assessment | [48,49] | |
| Inflammatory Markers | TNF-α | Increased | Pathophysiological understanding | [50,51] |
| IL-6 | Increased | Prediction, severity assessment | [50,51] | |
| IL-1β | Increased | Pathophysiological understanding | [22,51] | |
| CRP | Increased | Risk assessment | [52,53,54] | |
| NLR, PLR | Increased | Simple screening tools | [54] | |
| Endothelial Dysfunction | Endothelin-1 | Increased | Severity assessment | [55,56] |
| sICAM-1, VCAM-1 | Increased | Pathophysiological understanding | [57] | |
| ADMA | Increased | Risk stratification | [58] | |
| Endothelial microparticles | Increased | Severity assessment | [59] | |
| Coagulation and Fibrinolysis | Thrombin–antithrombin complexes | Increased | Hypercoagulability assessment | [60,61] |
| D-dimer | Increased | Hypercoagulability assessment | [60,61] | |
| TF, TFPI | Altered levels | Pathophysiological understanding | [62] | |
| PAI-1 | Increased | Fibrinolytic capacity assessment | [63] | |
| Platelet activation markers | Increased | Platelet function assessment | [64] | |
| Placental-Derived Factors | PP13 | Altered levels | Early prediction | [65] |
| PAPP-A | Decreased (1st trimester) | First-trimester screening | [66] | |
| Cell-free fetal DNA | Increased | Prediction, severity assessment | [67] | |
| Placental extracellular vesicles | Altered profile | Emerging biomarkers | [68] |
| LMWH | Mean Molecular Weight (Daltons) | Anti-Xa:Anti-IIa Ratio | Half-Life (h) | Dosing in Pregnancy Prevention | Dosing in Pregnancy Treatment | References |
|---|---|---|---|---|---|---|
| Enoxaparin | 4500 | 3.8:1 | 4.5–7 | 40 mg OD or 20 mg OD (weight < 50 kg) | 1 mg/kg BID or 1.5 mg/kg OD | [74,75] |
| Dalteparin | 6000 | 2.7:1 | 3–5 | 5000 IU OD | 100 IU/kg BID or 200 IU/kg OD | [76,77] |
| Tinzaparin | 6500 | 1.9:1 | 3–4 | 4500 IU OD | 175 IU/kg OD | [78,79] |
| Nadroparin | 4300 | 3.6:1 | 3.5–4 | 2850 IU OD | 85.5 IU/kg BID or 171 IU/kg OD | [80] |
| Bemiparin | 3600 | 8:1 | 5–6 | 2500–3500 IU OD | 115 IU/kg OD | [81] |
| Category | Biomarker | LMWH Effect | Proposed Mechanism | References |
|---|---|---|---|---|
| Angiogenic Factors | sFlt-1 | Decrease | Binding and neutralization, reduced placental production | [103,104,106] |
| PlGF | Increase | Protection from sFlt-1 antagonism, enhanced production | [104,106,107] | |
| sFlt-1/PlGF ratio | Decrease | Combined effect on sFlt-1 and PlGF | [107] | |
| sEng | Decrease/No effect | Variable effects reported | [103,108] | |
| Inflammatory Markers | TNF-α | Decrease | Inhibition of production, enhanced clearance | [111,128] |
| IL-6 | Decrease | Reduced production by trophoblasts and immune cells | [111,128] | |
| CRP | Decrease | General anti-inflammatory effect | [13] | |
| Complement activation | Decrease | Inhibition of alternative pathway | [128] | |
| Leukocyte adhesion molecules | Decrease | Reduced expression on endothelial cells | [110,111] | |
| Coagulation and Fibrinolysis | Thrombin generation | Decrease | Enhanced antithrombin activity | [116,117] |
| D-dimer | Decrease | Reduced fibrin formation and degradation | [117] | |
| Tissue factor activity | Decrease | Direct inhibition, increased TFPI | [118] | |
| PAI-1 | Decrease | Enhanced clearance, reduced production | [119,120] | |
| tPA activity | Increase | Reduced PAI-1 inhibition | [119] | |
| Placental Development | MMPs | Increase | Enhanced expression and activity | [122,123] |
| Trophoblast apoptosis | Decrease | Anti-apoptotic signaling | [124] | |
| Placental VEGF expression | Increase | Stimulation of production, protection from degradation | [126,127] | |
| Placental vascularization | Increase | Pro-angiogenic effects, reduced ischemia | [126,127] |
| Study (Year) | Population | Sample Size | Intervention | Control | Initiation | Primary Outcome | Preeclampsia Results | Other Outcomes | Confounders |
|---|---|---|---|---|---|---|---|---|---|
| Rey et al. (2009) [14] | Previous severe PE/IUGR/abruption/SB | 110 | Dalteparin 5000 IU/day + ASA | ASA | <16 weeks | Composite: PE/IUGR/abruption/SB | PE: 2.8% vs. 31.3% (p < 0.001) | Significant reduction in composite outcome | Small sample size, open-label design |
| Gris et al. (2004) [129] | Thrombophilia + previous loss | 160 | Enoxaparin 40 mg/day | ASA 100 mg/day | <8 weeks | Live birth | PE: 0% vs. 10.0% (p = 0.01) | Significant improvement in live birth rate | Selected population with thrombophilia |
| de Vries et al. (2012) [130] FRUIT | Previous early HDP + thrombophilia | 139 | Nadroparin 3800 IU/day + ASA | ASA | <12 weeks | Recurrent HDP < 34 weeks | No significant difference in recurrent PE | Earlier onset of recurrent HDP in control group | Heterogeneous thrombophilia types |
| Rodger et al. (2014) [87] TIPPS | Previous PE/IUGR/abruption/SB or thrombophilia | 292 | Dalteparin 5000 IU/day | No LMWH | <21 weeks | Composite: PE/IUGR/VTE/SB | No significant difference in PE | No significant difference in composite outcome | Mixed population with/without thrombophilia |
| Haddad et al. (2016) [131] HEPEPE | Previous severe PE | 224 | Enoxaparin 40 mg/day | No LMWH | 12–16 weeks | Preeclampsia | PE: 10.4% vs. 18.9% (p = 0.09) | No significant differences in secondary outcomes | Late initiation of intervention |
| Groom et al. (2017) [107] EPPI | High risk by screening + abnormal uterine artery | 149 | Enoxaparin 40 mg/day | Standard care | 12–14 weeks | PE/SGA < 5th percentile | PE: 8.0% vs. 22.2% (p = 0.02) | Significant reduction in preterm birth | Doppler-guided selection |
| Martinelli et al. (2017) [132] HAPPY | Previous early PE/IUGR/abruption | 156 | Nadroparin 3800 IU/day | No LMWH | <14 weeks | Composite: PE/IUGR/abruption/SB | No significant difference in PE | No significant difference in composite outcome | Underpowered for individual outcomes |
| Roberge et al. (2016) [133] | FGR history or high risk by screening | 91 | Dalteparin 5000 IU/day + ASA | ASA | 11–14 weeks | Uterine artery PI at 22–24 weeks | PE: 2.1% vs. 17.0% (p = 0.03) | Significant improvement in uterine artery flow | Small sample size |
| McLaughlin et al. (2022) [134] HepASA | Previous placental syndrome | 380 | Dalteparin 5000 IU/day + ASA | ASA | 6–16 weeks | Composite: PE/IUGR/abruption/SB | No significant difference in PE | No significant difference in composite outcome | Broad inclusion criteria |
| Seidler et al. (2019) [135] | Severe PE/FGR history or high risk | 314 | Enoxaparin 40 mg/day | Standard care | 12–16 weeks | Composite adverse outcome | PE: 9.7% vs. 17.6% (p = 0.046) | Significant reduction in preterm birth < 37 weeks | Risk-stratified approach |
| Study (Year) | Included Studies | Population | Intervention | Comparison | Preeclampsia Outcome | Other Outcomes | Conclusions | Confounders |
|---|---|---|---|---|---|---|---|---|
| Dodd et al. (2013) [138] | 8 RCTs (963 women) | Previous PE/IUGR | LMWH +/− aspirin | Placebo/aspirin/standard care | RR 0.52 (95% CI 0.32–0.86) | Reduced IUGR (RR 0.54, 95% CI 0.32–0.91) | LMWH reduces recurrent PE and IUGR | Heterogeneity in study populations |
| Rodger et al. (2014) [105] | 6 RCTs (848 women) | Previous placenta-mediated complications | LMWH | No LMWH | RR 0.47 (95% CI 0.22–1.03) | Reduced composite outcome (RR 0.52, 95% CI 0.32–0.86) | LMWH reduces recurrent placenta-mediated complications | Variable thrombophilia status |
| Roberge et al. (2016) [133] | 8 RCTs (885 women) | High-risk pregnancies | LMWH +/− aspirin | Placebo/aspirin/standard care | RR 0.40 (95% CI 0.27–0.60) | Reduced severe PE (RR 0.39, 95% CI 0.26–0.58) | LMWH reduces PE and severe PE in high-risk women | Timing of initiation variable |
| Skeith et al. (2016) [137] Cochrane | 9 RCTs (979 women) | Previous placenta-mediated complications | LMWH | No LMWH | RR 0.46 (95% CI 0.29–0.73) | No significant reduction in other individual outcomes | LMWH may reduce PE in women with prior complications | Substantial trial heterogeneity |
| Saccone et al. (2017) [139] | 10 RCTs (1089 women) | Previous PE | LMWH +/− aspirin | Placebo/aspirin/standard care | LMWH + ASA vs. ASA: OR 0.53 (95% CI 0.28–0.99) | LMWH + ASA reduced composite adverse outcome vs. ASA | LMWH + ASA more effective than ASA alone for recurrent PE | Concurrent aspirin use variable |
| Maher et al. (2017) [140] | 4 RCTs (522 women) | Previous PE | LMWH + aspirin | Aspirin | RR 0.70 (95% CI 0.40–1.23) | No significant difference in other outcomes | No added benefit of LMWH over aspirin alone | Limited to aspirin comparison |
| Zhang et al. (2015) [141] | 11 RCTs (1115 women) | Previous placenta-mediated complications | LMWH +/− aspirin | No LMWH +/− aspirin | RR 0.42 (95% CI 0.28–0.62) | Reduced IUGR (RR 0.56, 95% CI 0.41–0.77) | LMWH reduces recurrent PE and IUGR | Dosing regimens varied |
| Rodger et al. (2016) [142] | 21 RCTs (2876 women) | Multiple high-risk groups | LMWH +/− aspirin | No LMWH +/− aspirin | All women: RR 0.63 (95% CI 0.46–0.87) | Reduced IUGR and placental abruption | LMWH effective for specific high-risk subgroups | Population heterogeneity |
| Lin et al. (2023) [143] | 14 RCTs (2451 women) | Previous PE/IUGR or high risk | LMWH +/− aspirin | Standard care/aspirin | RR 0.59 (95% CI 0.47–0.75) | Reduced IUGR and preterm birth | LMWH reduces PE and improves other outcomes | Study quality variable |
| Organization | Year | Recommendations | Population | Evidence Level |
|---|---|---|---|---|
| ACOG [175,176] | 2020 | No specific recommendation for LMWH for PE prevention | N/A | N/A |
| May consider LMWH in APS with previous adverse outcomes | APS + previous PE/IUGR | Low | ||
| SOGC [177,178] | 2019 | May consider LMWH in previous severe early-onset PE/IUGR | Previous PE/IUGR < 34 weeks | Moderate |
| May consider LMWH in thrombophilia with previous placental complications | Thrombophilia + previous PE/IUGR | Low | ||
| RCOG [179,180] | 2018 | Consider LMWH in APS with previous adverse outcomes | APS + previous PE/IUGR | Moderate |
| Not routinely recommended for PE prevention without thrombophilia | Previous PE without thrombophilia | Low | ||
| ISSHP [22] | 2021 | Does NOT recommend LMWH for PE prevention | All women | Strong |
| LMWH acceptable for other indications (e.g., thromboprophylaxis in APS) | Specific indications | Moderate | ||
| ISTH [181,182] | 2020 | May consider LMWH in previous severe placenta-mediated complications | Previous severe PE/IUGR | Low |
| Higher priority if thrombophilia present | Thrombophilia + previous PE/IUGR | Moderate | ||
| ACCP [183,184] | 2022 | Does not recommend routine LMWH for PE prevention | All women at risk for PE | Low |
| LMWH only if other indications for anticoagulation exist | Specific high-risk groups | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroutis, D.; Koukoumpanis, K.; Tzanis, A.A.; Theodora, M.; Rizogiannis, K.; Bairaktaris, D.; Manios, E.; Pergialiotis, V.; Alexopoulos, E.; Daskalakis, G. Low-Molecular-Weight Heparin in Preeclampsia: Effects on Biomarkers and Prevention: A Narrative Review. Biomedicines 2025, 13, 2337. https://doi.org/10.3390/biomedicines13102337
Baroutis D, Koukoumpanis K, Tzanis AA, Theodora M, Rizogiannis K, Bairaktaris D, Manios E, Pergialiotis V, Alexopoulos E, Daskalakis G. Low-Molecular-Weight Heparin in Preeclampsia: Effects on Biomarkers and Prevention: A Narrative Review. Biomedicines. 2025; 13(10):2337. https://doi.org/10.3390/biomedicines13102337
Chicago/Turabian StyleBaroutis, Dimitris, Konstantinos Koukoumpanis, Alexander A. Tzanis, Marianna Theodora, Konstantinos Rizogiannis, Dimitrios Bairaktaris, Efstathios Manios, Vasilios Pergialiotis, Evangelos Alexopoulos, and George Daskalakis. 2025. "Low-Molecular-Weight Heparin in Preeclampsia: Effects on Biomarkers and Prevention: A Narrative Review" Biomedicines 13, no. 10: 2337. https://doi.org/10.3390/biomedicines13102337
APA StyleBaroutis, D., Koukoumpanis, K., Tzanis, A. A., Theodora, M., Rizogiannis, K., Bairaktaris, D., Manios, E., Pergialiotis, V., Alexopoulos, E., & Daskalakis, G. (2025). Low-Molecular-Weight Heparin in Preeclampsia: Effects on Biomarkers and Prevention: A Narrative Review. Biomedicines, 13(10), 2337. https://doi.org/10.3390/biomedicines13102337








