The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System
Abstract
1. Introduction
2. Existing Methods to Simulate Microgravity
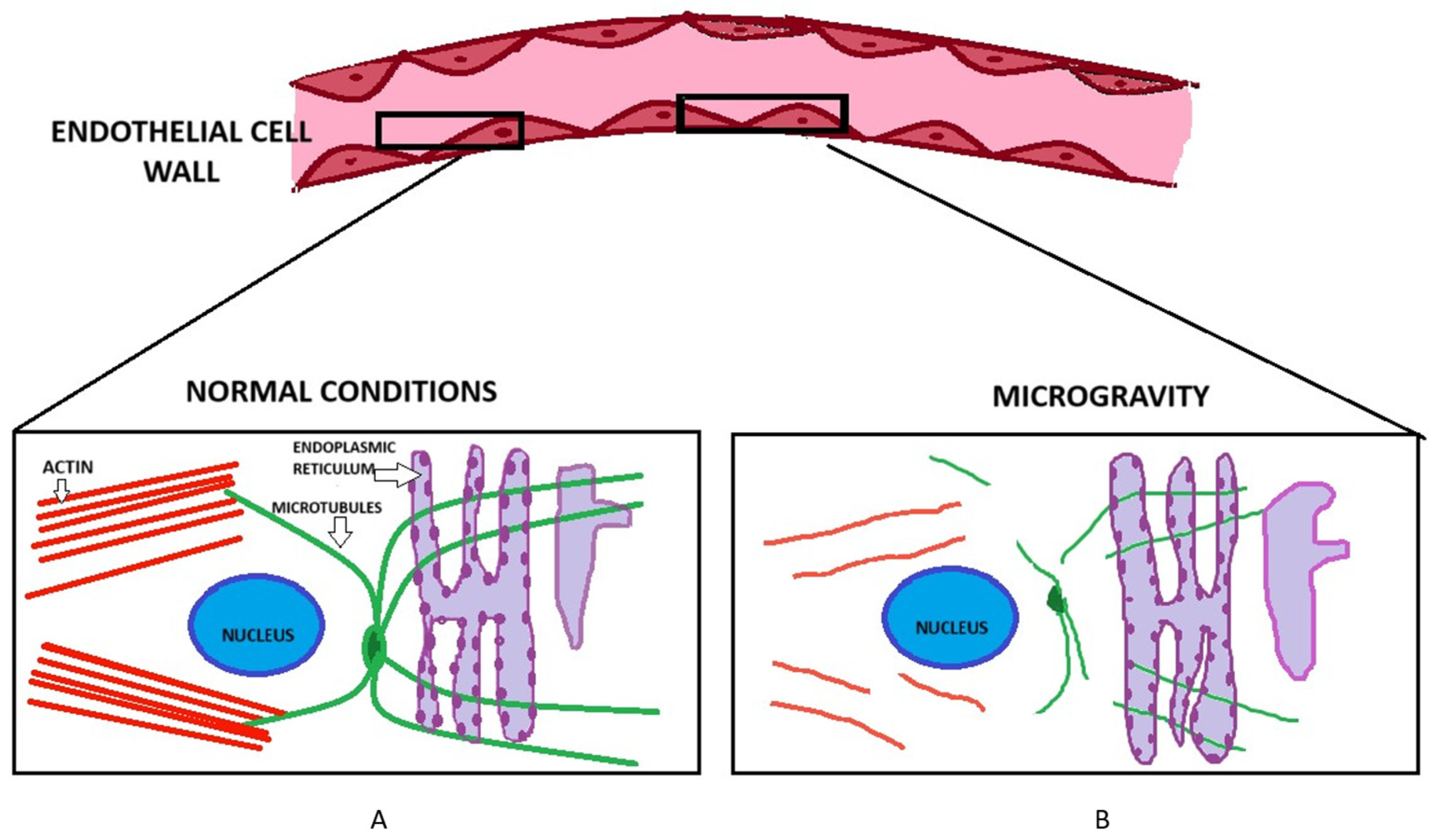
3. Impact of Microgravity on the Actin Cytoskeleton and Permeability of Endothelial Cells
4. Effect of Microgravity on Migration of Endothelial Cells
5. Effect of Microgravity on Maturation of Cardiomyocytes and Progenitor Stem Cells
6. Effect of Microgravity on Coagulation
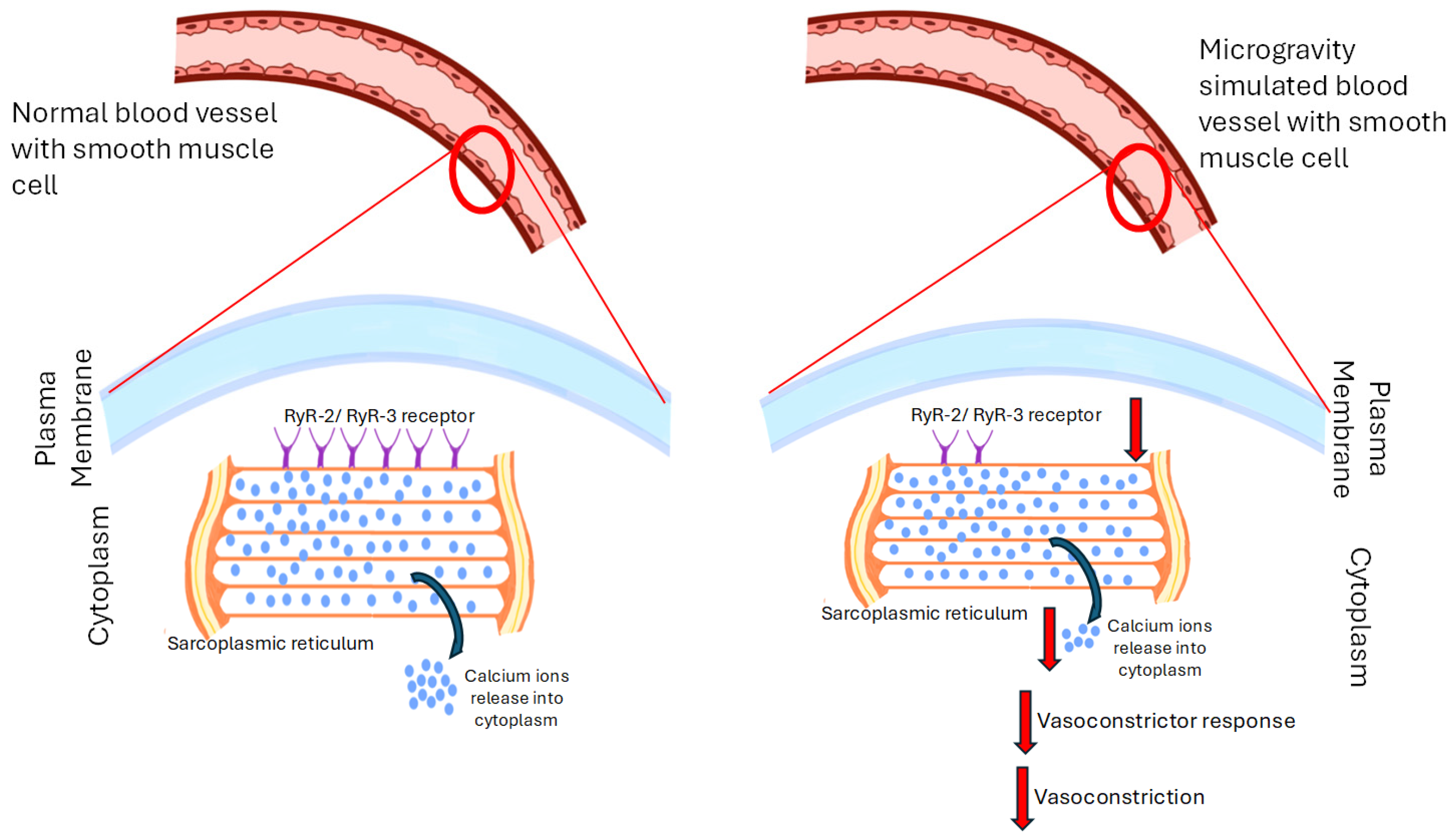
7. Simulated Microgravity’s Effects on Vasoconstriction
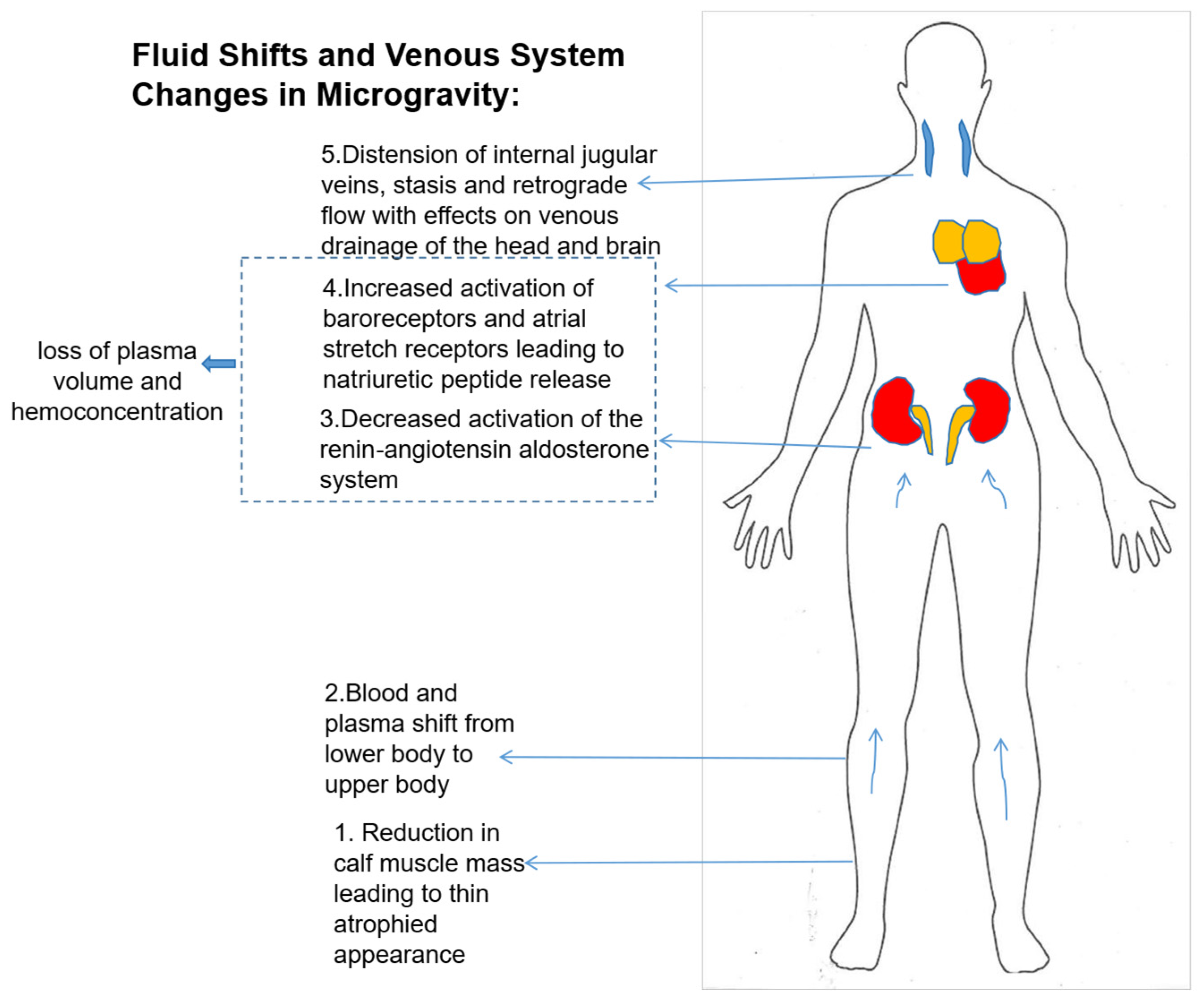
8. Effect of Microgravity on Blood Volume and Hematologic Changes
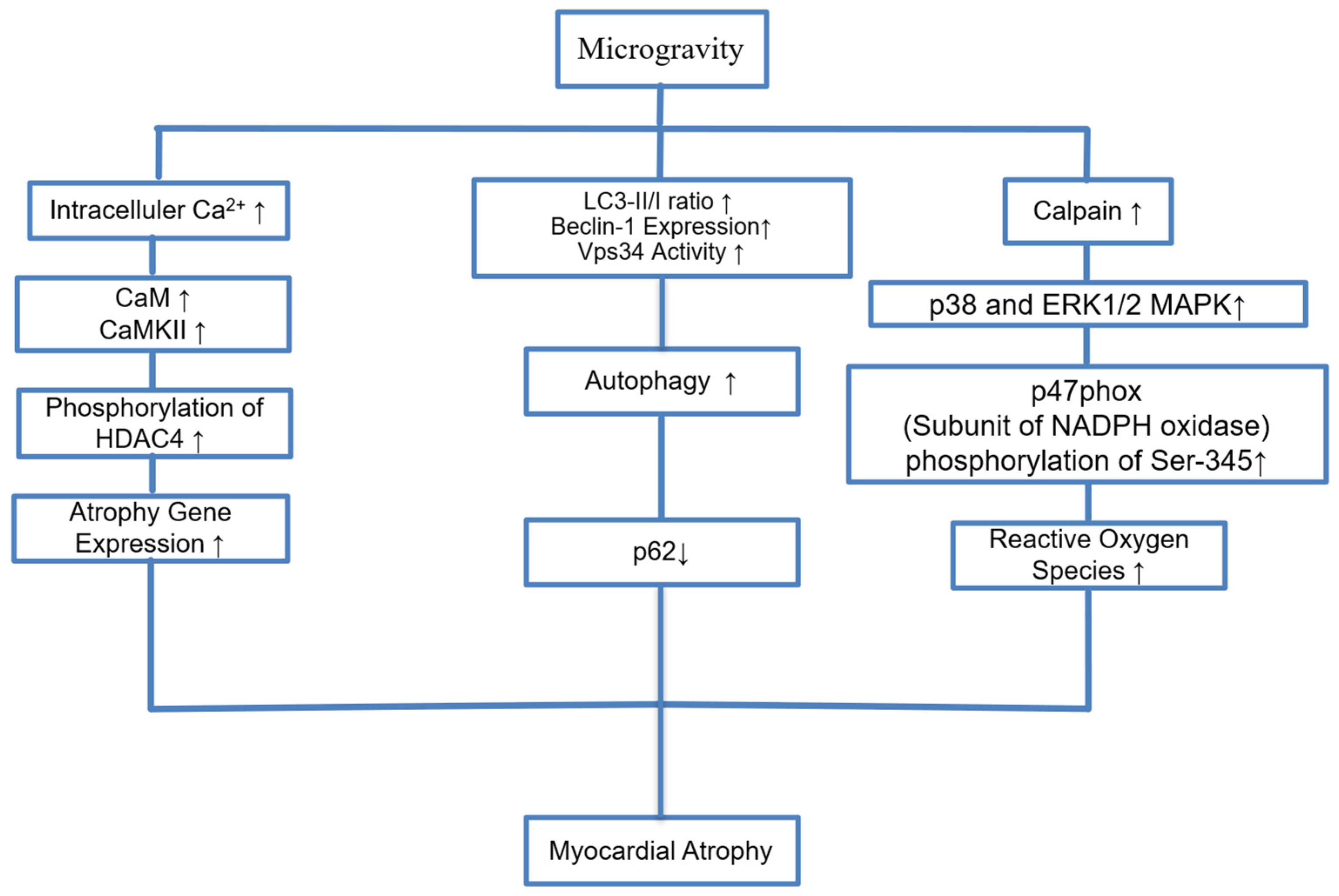
9. Myocardial Atrophy and Endothelial Dysfunction
10. Microgravity and Changes in Cardiac Dimensions
11. Several Countermeasures Are Being Investigated to Offset the Detrimental Effects of Microgravity on Human Physiology
12. Future Perspectives
13. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| MARCKS | myristoylated alanine-rich C kinase substrate |
| MLP-1 | MARCKS-like Protein-1 |
| HU | Hindlimb unweighting |
| HDT | Head down tilt |
| HSCs | Hematopoietic stem cells |
| IJV | Internal jugular vein |
| IST | International space station |
| MCA | Middle cerebral arteries |
| MSA | Mesenteric small arteries |
| TS | Tail suspended |
| mRNA | Messenger RNA |
| NE | Norepinephrine |
| NK | Natural killer cells |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| KCl | Potassium chloride |
| PRI | Platelet reactivity index |
| SATB2 | Special AT rich sequence binding protein 2 |
| VGCC | Voltage-gated calcium channels |
| VWF | Von Willibrand factor |
References
- Bonanni, R.; Cariati, I.; Marini, M.; Tarantino, U.; Tancredi, V. Microgravity and Musculoskeletal Health: What Strategies Should Be Used for a Great Challenge? Life 2023, 13, 1423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, H.; Yang, H.; Jiang, C.; Shi, J.; Chen, R.A.; Huang, Q.; Shao, D. Microgravity and immune cells. J. R. Soc. Interface 2023, 20, 20220869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baran, R.; Marchal, S.; Garcia Campos, S.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, L.; Li, H.; Cao, T.; Qu, L.; Zhang, L.; Fan, G.C.; Greer, P.A.; Li, J.; Jones, D.L.; Peng, T. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and ERK1/2 MAPK pathways. J. Biol. Chem. 2020, 295, 16840–16851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ling, S.; Li, Y.; Zhong, G.; Zheng, Y.; Xu, Q.; Zhao, D.; Sun, W.; Jin, X.; Li, H.; Li, J.; et al. Myocardial CKIP-1 Overexpression Protects from Simulated Microgravity-Induced Cardiac Remodeling. Front. Physiol. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Zhong, G.; Zhou, Y.; Yang, Y.; Tan, Y.; Li, Y.; Gao, X.; Sun, W.; Li, J.; Jin, X.; et al. Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity. Cell Prolif. 2020, 53, e12783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.N.; Zhang, L.F.; Ma, J. Simulated microgravity enhances vasoconstrictor responsiveness of rat basilar artery. J. Appl. Physiol. 2001, 90, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Cialdai, F.; Monici, M.; Morbidelli, L. The impact of microgravity and hypergravity on endothelial cells. Biomed. Res. Int. 2015, 2015, 434803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, Y.J.; Jeong, A.J.; Kim, M.; Lee, C.; Ye, S.K.; Kim, S. Time-averaged simulated microgravity (taSMG) inhibits proliferation of lymphoma cells, L-540 and HDLM-2, using a 3D clinostat. Biomed. Eng. Online 2017, 16, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishimura, Y. Technology using simulated microgravity. Regen. Ther. 2023, 24, 318–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rah, B.; Shafarin, J.; Qaisar, R.; Karim, A.; Hamad, M.; Muhammad, J.S. Mouse hindlimb unloading, as a model of simulated microgravity, leads to dysregulated iron homeostasis in liver and skeletal muscle cells. Life Sci. Space Res. 2025, 45, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sanavandi, H.; Guo, W. A magnetic levitation based low-gravity simulator with an unprecedented large functional volume. NPJ Microgravity 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammer, B.E.; Kidder, L.S.; Williams, P.C.; Xu, W.W. Magnetic Levitation of MC3T3 Osteoblast Cells as a Ground-Based Simulation of Microgravity. Microgravity Sci. Technol. 2009, 21, 311–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, A.R.; Ryoo, S.; Bugaj, L.; Attarzadeh, D.O.; Thiyagarajan, S.; Chen, K.; Attwater, S.; Abbot, B.; Li, D.; Champion, H.C.; et al. Early changes in vasoreactivity after simulated microgravity are due to an upregulation of the endothelium-dependent nitric oxide/cGMP pathway. Eur. J. Appl. Physiol. 2010, 110, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Xu, C.; Liu, J.; Fan, Q.; Gai, Y.; Zhao, S.; Wu, X.; Mi, T.; Wang, J.; et al. Comprehensive analysis of transcriptomics and metabolomics to understand tail-suspension-induced myocardial injury in rat. Front. Cardiovasc. Med. 2022, 9, 1074257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Xie, Q.; Xin, B.M.; Liu, J.L.; Liu, Y.; Li, Y.Z.; Wang, J.P. Inhibition of autophagy recovers cardiac dysfunction and atrophy in response to tail-suspension. Life Sci. 2015, 121, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pan, Y.; Tan, Y.; Wang, Y.; Sun, X. PINK1-Dependent Mitophagy Reduced Endothelial Hyperpermeability and Cell Migration Capacity Under Simulated Microgravity. Front. Cell Dev. Biol. 2022, 10, 896014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, M.; Wang, H.; Liu, Z.; Lin, L.; Wang, L.; Xie, M.; Li, D.; Zhang, J.; Zhang, R. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-kappaB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity. FASEB J. 2020, 34, 10835–10849. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Wakeham, D.J.; Thomas, J.D.; Abdullah, S.M.; Platts, S.; Bungo, M.W.; Levine, B.D. Cardiac Effects of Long-Duration Space Flight. J. Am. Coll. Cardiol. 2023, 82, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Xing, C.Y.; Zhang, J.X.; Zhou, J.H.; Li, Y.C.; Yang, H.Y.; Zhang, P.F.; Zhang, W.; Huang, Y.; Long, J.G.; et al. Time-restricted feeding alleviates cardiac dysfunction induced by simulated microgravity via restoring cardiac FGF21 signaling. FASEB J. 2020, 34, 15180–15196. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ling, S.; Zhao, D.; Li, Y.; Zhong, G.; Guo, M.; Li, Y.; Yang, L.; Du, J.; Zhou, Y.; et al. Panax quinquefolium saponin attenuates cardiac remodeling induced by simulated microgravity. Phytomedicine 2019, 56, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, J.; Li, J.; Ling, S.; Du, R.; Xing, W.; Li, Y.; Sun, W.; Li, Y.; Fan, Y.; et al. miR-199a-3p mitigates simulated microgravity-induced cardiac remodeling by targeting MEF2C. FASEB J. 2025, 39, e70331. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Fan, Y.; Sun, A.; Jia, X.; Deng, X. Simulated microgravity exposure modulates the phenotype of cultured vascular smooth muscle cells. Cell Biochem. Biophys. 2013, 66, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Higashibata, A.; Imamizo-Sato, M.; Seki, M.; Yamazaki, T.; Ishioka, N. Influence of simulated microgravity on the activation of the small GTPase Rho involved in cytoskeletal formation—Molecular cloning and sequencing of bovine leukemia-associated guanine nucleotide exchange factor. BMC Biochem. 2006, 7, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lu, D.; Chen, Q.; Long, M. Microgravity-Induced Alterations of Inflammation-Related Mechanotransduction in Endothelial Cells on Board SJ-10 Satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baio, J.; Martinez, A.F.; Silva, I.; Hoehn, C.V.; Countryman, S.; Bailey, L.; Hasaniya, N.; Pecaut, M.J.; Kearns-Jonker, M. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. NPJ Microgravity 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, F.; Wang, Y.C.; Hu, Z.B.; Xu, H.Y.; Sun, J.; Gao, Y.; Li, X.T.; Yang, C.B.; Xie, C.; Li, C.F.; et al. Simulated Microgravity Promotes Angiogenesis through RhoA-Dependent Rearrangement of the Actin Cytoskeleton. Cell Physiol. Biochem. 2017, 41, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Chen, Z.; Luo, Q.; Zhang, B.; Song, G. Simulated microgravity inhibits the migration of mesenchymal stem cells by remodeling actin cytoskeleton and increasing cell stiffness. Cytotechnology 2016, 68, 2235–2243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quynh Chi, H.N.; Nghia Son, H.; Chinh Chung, D.; Huan, L.D.; Hong Diem, T.; Long, L.T. Simulated microgravity reduces proliferation and reorganizes the cytoskeleton of human umbilical cord mesenchymal stem cells. Physiol. Res. 2020, 69, 897–906. [Google Scholar] [CrossRef]
- Kugelmann, D.; Waschke, J.; Radeva, M.Y. Adducin is involved in endothelial barrier stabilization. PLoS ONE 2015, 10, e0126213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moztarzadeh, S.; Radeva, M.Y.; Sepic, S.; Schuster, K.; Hamad, I.; Waschke, J.; Garcia-Ponce, A. Lack of adducin impairs the stability of endothelial adherens and tight junctions and may be required for cAMP-Rac1-mediated endothelial barrier stabilization. Sci. Rep. 2022, 12, 14940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machnicka, B.; Ponceau, A.; Picot, J.; Colin, Y.; Lecomte, M.C. Deficiency of alphaII-spectrin affects endothelial cell-matrix contact and migration leading to impairment of angiogenesis in vitro. Cell Mol. Biol. Lett. 2020, 25, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.; Bala, N.; Nguyen, V.L.; Kessler, L.; LaDisa, J.F., Jr.; Alli, A.A. Activity and function of the endothelial sodium channel is regulated by the effector domain of MARCKS-like protein 1 in mouse aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2025, 328, C1101–C1108. [Google Scholar] [CrossRef] [PubMed]
- Alli, A.A.; Bao, H.F.; Liu, B.C.; Yu, L.; Aldrugh, S.; Montgomery, D.S.; Ma, H.P.; Eaton, D.C. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am. J. Physiol. Ren. Physiol. 2015, 309, F456–F463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montgomery, D.S.; Yu, L.; Ghazi, Z.M.; Thai, T.L.; Al-Khalili, O.; Ma, H.P.; Eaton, D.C.; Alli, A.A. ENaC activity is regulated by calpain-2 proteolysis of MARCKS proteins. Am. J. Physiol. Cell Physiol. 2017, 313, C42–C53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alli, A.A.; Bao, H.F.; Alli, A.A.; Aldrugh, Y.; Song, J.Z.; Ma, H.P.; Yu, L.; Al-Khalili, O.; Eaton, D.C. Phosphatidylinositol phosphate-dependent regulation of Xenopus ENaC by MARCKS protein. Am. J. Physiol. Ren. Physiol. 2012, 303, F800–F811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, C.; Yue, Q.; Moseley, A.; Al-Khalili, O.; Wynne, B.M.; Ma, H.; Wang, L.; Eaton, D.C. Myristoylated alanine-rich C kinase substrate-like protein-1 regulates epithelial sodium channel activity in renal distal convoluted tubule cells. Am. J. Physiol. Cell Physiol. 2020, 319, C589–C604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.P.; Huang, L.; Tian, X.; Liang, F.Q.; Wei, J.C.; Zhang, X.; Li, S.; Zhang, Q.H. Knockdown of ezrin suppresses the migration and angiogenesis of human umbilical vein endothelial cells in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Effects of Mechanical Stress on Endothelial Cells In Situ and In Vitro. Int. J. Mol. Sci. 2023, 24, 16518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janmaleki, M.; Pachenari, M.; Seyedpour, S.M.; Shahghadami, R.; Sanati-Nezhad, A. Impact of Simulated Microgravity on Cytoskeleton and Viscoelastic Properties of Endothelial Cell. Sci. Rep. 2016, 6, 32418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, F.; Wang, Y.C.; Zhao, T.Z.; Zhang, S.; Du, T.Y.; Yang, C.B.; Li, Y.H.; Sun, X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS ONE 2012, 7, e40365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Li, C.; Wang, R.; Zhao, X.; Pan, Y.; Zhang, Q.; Li, S.; Fan, J.; Wang, Y.; Sun, X. PIEZO1 Promotes the Migration of Endothelial Cells via Enhancing CXCR4 Expression under Simulated Microgravity. Int. J. Mol. Sci. 2024, 25, 7254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cotrupi, S.; Ranzani, D.; Maier, J.A. Impact of modeled microgravity on microvascular endothelial cells. Biochim. Biophys. Acta 2005, 1746, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shi, Y.; Qiu, C.; Zhao, J.; Gong, Y.; Nie, C.; Wu, B.; Yang, Y.; Wang, F.; Luo, L. Effects of Simulated Microgravity on Ultrastructure and Apoptosis of Choroidal Vascular Endothelial Cells. Front. Physiol. 2020, 11, 577325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Versari, S.; Villa, A.; Bradamante, S.; Maier, J.A. Alterations of the actin cytoskeleton and increased nitric oxide synthesis are common features in human primary endothelial cell response to changes in gravity. Biochim. Biophys. Acta 2007, 1773, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Manganini, M.; Maier, J.A. Transforming growth factor beta2 inhibition of hepatocyte growth factor-induced endothelial proliferation and migration. Oncogene 2000, 19, 124–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.F.; Fishman, M.C.; et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudic, R.D.; Shesely, E.G.; Maeda, N.; Smithies, O.; Segal, S.S.; Sessa, W.C. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Investig. 1998, 101, 731–736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jha, R.; Wu, Q.; Singh, M.; Preininger, M.K.; Han, P.; Ding, G.; Cho, H.C.; Jo, H.; Maher, K.O.; Wagner, M.B.; et al. Simulated Microgravity and 3D Culture Enhance Induction, Viability, Proliferation and Differentiation of Cardiac Progenitors from Human Pluripotent Stem Cells. Sci. Rep. 2016, 6, 30956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forghani, P.; Rashid, A.; Armand, L.C.; Wolfson, D.; Liu, R.; Cho, H.C.; Maxwell, J.T.; Jo, H.; Salaita, K.; Xu, C. Simulated microgravity improves maturation of cardiomyocytes derived from human induced pluripotent stem cells. Sci. Rep. 2024, 14, 2243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez Garzon, N.A.; Pinzon-Fernandez, M.V.; Saavedra, T.J.; Nati-Castillo, H.A.; Arias-Intriago, M.; Salazar-Santoliva, C.; Izquierdo-Condoy, J.S. Microgravity and Cellular Biology: Insights into Cellular Responses and Implications for Human Health. Int. J. Mol. Sci. 2025, 26, 3058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, D.; Song, J.; Ling, S.; Niu, S.; Lu, L.; Cui, Z.; Li, Y.; Hao, S.; Zhong, G.; Qi, Z.; et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019, 33, 6904–6918. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tian, H.; Zhang, J.; Qian, J.; Li, L.; Shi, L.; Zhao, Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays. FASEB J. 2019, 33, 5903–5913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, P.; Piatkowski, B.T.; Cherukuri, Y.; Asmann, Y.W.; Zubair, A.C. Impact of spaceflight on gene expression in cultured human mesenchymal stem/stromal cells. PLoS ONE 2025, 20, e0315285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pala, R.; Cruciani, S.; Manca, A.; Garroni, G.; El Faqir, M.A.; Lentini, V.; Capobianco, G.; Pantaleo, A.; Maioli, M. Mesenchymal Stem Cell Behavior under Microgravity: From Stress Response to a Premature Senescence. Int. J. Mol. Sci. 2023, 24, 7753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaka, S.; Carpo, N.; Tran, V.; Cepeda, C.; Espinosa-Jeffrey, A. Space Microgravity Alters Neural Stem Cell Division: Implications for Brain Cancer Research on Earth and in Space. Int. J. Mol. Sci. 2022, 23, 14320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, M.; Wang, Y.; Yang, M.; Liu, Y.; Qu, B.; Ye, Z.; Liang, W.; Sun, X.; Luo, Z. The effects and mechanisms of clinorotation on proliferation and differentiation in bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2015, 460, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, K.; Yamada, Y.; Yamada, M.; Yokoyama, Y.; Fujiwara, H.; Yoshida, K.; Yoshida, K.; Toda, M.; Jinzaki, M. Posture-induced changes in the vessels of the head and neck: Evaluation using conventional supine CT and upright CT. Sci. Rep. 2020, 10, 16623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, D.S.; Lee, S.M.; Matz, T.P.; Westby, C.M.; Scott, J.M.; Stenger, M.B.; Platts, S.H. Internal jugular pressure increases during parabolic flight. Physiol. Rep. 2016, 4, e13068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marshall-Goebel, K.; Laurie, S.S.; Alferova, I.V.; Arbeille, P.; Aunon-Chancellor, S.M.; Ebert, D.J.; Lee, S.M.C.; Macias, B.R.; Martin, D.S.; Pattarini, J.M.; et al. Assessment of Jugular Venous Blood Flow Stasis and Thrombosis During Spaceflight. JAMA Netw. Open 2019, 2, e1915011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marshall-Goebel, K.; Lee, S.M.C.; Lytle, J.R.; Martin, D.S.; Miller, C.A.; Young, M.; Laurie, S.S.; Macias, B.R. Jugular venous flow dynamics during acute weightlessness. J. Appl. Physiol. 2024, 136, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, J.N.; Hedge, E.T.; Greaves, D.K.; Robertson, A.D.; Nahas, H.; Yu, A.C.H.; Petersen, L.G.; Au, J.S. Characterization of internal jugular vein region-specific distension and flow patterns during progressive volume shifting. J. Appl. Physiol. 2024, 137, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.C.; Martin, D.S.; Miller, C.A.; Scott, J.M.; Laurie, S.S.; Macias, B.R.; Mercaldo, N.D.; Ploutz-Snyder, L.; Stenger, M.B. Venous and Arterial Responses to Partial Gravity. Front. Physiol. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Gonzalez, F.J.; Ziccardi, M.R.; McCauley, M.D. Virchow’s Triad and the Role of Thrombosis in COVID-Related Stroke. Front. Physiol. 2021, 12, 769254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herault, S.; Fomina, G.; Alferova, I.; Kotovskaya, A.; Poliakov, V.; Arbeille, P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur. J. Appl. Physiol. 2000, 81, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Arbeille, P.; Zuj, K.A.; Macias, B.R.; Ebert, D.J.; Laurie, S.S.; Sargsyan, A.E.; Martin, D.S.; Lee, S.M.C.; Dulchavsky, S.A.; Stenger, M.B.; et al. Lower body negative pressure reduces jugular and portal vein volumes and counteracts the elevation of middle cerebral vein velocity during long-duration spaceflight. J. Appl. Physiol. 2021, 131, 1080–1087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Limper, U.; Tank, J.; Ahnert, T.; Maegele, M.; Grottke, O.; Hein, M.; Jordan, J. The thrombotic risk of spaceflight: Has a serious problem been overlooked for more than half of a century? Eur. Heart J. 2021, 42, 97–100. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Wenthe, A. Managing Hemostasis in Space. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Kashirina, D.N.; Percy, A.J.; Pastushkova, L.K.; Borchers, C.H.; Kireev, K.S.; Ivanisenko, V.A.; Kononikhin, A.S.; Nikolaev, E.N.; Larina, I.M. The molecular mechanisms driving physiological changes after long duration space flights revealed by quantitative analysis of human blood proteins. BMC Med. Genom. 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elahi, M.M.; Witt, A.N.; Pryzdial, E.L.G.; McBeth, P.B. Thrombotic triad in microgravity. Thromb. Res. 2024, 233, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Vaquer, S.; Mazzolai, L.; Roberts, L.N.; Pavela, J.; Watanabe, M.; Weerts, G.; Green, D.A. The effect of microgravity on the human venous system and blood coagulation: A systematic review. Exp. Physiol. 2021, 106, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Twomey, L.; Navasiolava, N.; Robin, A.; Bareille, M.P.; Gauquelin-Koch, G.; Beck, A.; Larcher, F.; Meade-Murphy, G.; Sheridan, S.; Maguire, P.B.; et al. A dry immersion model of microgravity modulates platelet phenotype, miRNA signature, and circulating plasma protein biomarker profile. Sci. Rep. 2021, 11, 21906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, K.; Wang, Y.; Yan, R.; Shi, Q.; Wang, Z.; Yuan, Y.; Cheng, H.; Li, S.; Fan, Y.; Zhuang, F. Effects of microgravity and hypergravity on platelet functions. Thromb. Haemost. 2009, 101, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Behnke, B.J.; Stabley, J.N.; McCullough, D.J.; Davis, R.T., 3rd; Dominguez, J.M., 2nd; Muller-Delp, J.M.; Delp, M.D. Effects of spaceflight and ground recovery on mesenteric artery and vein constrictor properties in mice. FASEB J. 2013, 27, 399–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stabley, J.N.; Dominguez, J.M., 2nd; Dominguez, C.E.; Mora Solis, F.R.; Ahlgren, J.; Behnke, B.J.; Muller-Delp, J.M.; Delp, M.D. Spaceflight reduces vasoconstrictor responsiveness of skeletal muscle resistance arteries in mice. J. Appl. Physiol. 2012, 113, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Sangha, D.S.; Han, S.; Purdy, R.E. Simulated microgravity upregulates an endothelial vasoconstrictor prostaglandin. J. Appl. Physiol. 2001, 91, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Gao, F.; Bai, Y.G.; Bao, J.X.; Huang, X.F.; Ma, J.; Zhang, L.F. Contrasting effects of simulated microgravity with and without daily -Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. J. Appl. Physiol. 2009, 107, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Lampe, L.; Wienhold, K.; Meyer, G.; Baisch, F.; Maass, H.; Hollmann, W.; Rost, R. Effects of simulated microgravity (HDT) on blood fluidity. J. Appl. Physiol. 1992, 73, 1366–1369. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Jia, H.; Wang, Y.F.; Song, J.P. Cardiovascular adaptations and pathological changes induced by spaceflight: From cellular mechanisms to organ-level impacts. Mil. Med. Res. 2024, 11, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perhonen, M.A.; Franco, F.; Lane, L.D.; Buckey, J.C.; Blomqvist, C.G.; Zerwekh, J.E.; Peshock, R.M.; Weatherall, P.T.; Levine, B.D. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 2001, 91, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Hargens, A.R.; Bhattacharya, R.; Schneider, S.M. Space physiology VI: Exercise, artificial gravity, and countermeasure development for prolonged space flight. Eur. J. Appl. Physiol. 2013, 113, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Zuckerman, J.H.; Pawelczyk, J.A. Cardiac atrophy after bed-rest deconditioning: A nonneural mechanism for orthostatic intolerance. Circulation 1997, 96, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Videbaek, R.; Norsk, P. Atrial distension in humans during microgravity induced by parabolic flights. J. Appl. Physiol. 1997, 83, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tahimic, C.G.T.; Almeida, E.A.C.; Globus, R.K. Spaceflight Modulates the Expression of Key Oxidative Stress and Cell Cycle Related Genes in Heart. Int. J. Mol. Sci. 2021, 22, 9088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandal, P.H.; Kim, D.; Fiebig, L.; Winnard, A.; Caplan, N.; Green, D.A.; Weber, T. Effectiveness of nutritional countermeasures in microgravity and its ground-based analogues to ameliorate musculoskeletal and cardiopulmonary deconditioning-A Systematic Review. PLoS ONE 2020, 15, e0234412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hesse, C.; Siedler, H.; Luntz, S.P.; Arendt, B.M.; Goerlich, R.; Fricker, R.; Heer, M.; Haefeli, W.E. Modulation of endothelial and smooth muscle function by bed rest and hypoenergetic, low-fat nutrition. J. Appl. Physiol. 2005, 99, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Platts, S.H.; Ziegler, M.G.; Waters, W.W.; Mitchell, B.M.; Meck, J.V. Midodrine prescribed to improve recurrent post-spaceflight orthostatic hypotension. Aviat. Space Environ. Med. 2004, 75, 554–556. [Google Scholar] [PubMed]
- Galcenko, K.; Bourdakou, M.M.; Spyrou, G.M. Exploring the Impact of Microgravity on Gene Expression: Dysregulated Pathways and Candidate Repurposed Drugs. Int. J. Mol. Sci. 2025, 26, 1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, R.; Tang, H.; Ding, C.; Rajbanshi, B.; Liu, Y.; Ma, D.; Duan, Z.; Qi, Y.; Dai, L.; Zhang, B.; et al. A Novel Piezo1 Agonist Promoting Mesenchymal Stem Cell Proliferation and Osteogenesis to Attenuate Disuse Osteoporosis. Small Sci. 2024, 4, 2400061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulavara, A.P.; Peters, B.T.; Miller, C.A.; Kofman, I.S.; Reschke, M.F.; Taylor, L.C.; Lawrence, E.L.; Wood, S.J.; Laurie, S.S.; Lee, S.M.C.; et al. Physiological and Functional Alterations after Spaceflight and Bed Rest. Med. Sci. Sports Exerc. 2018, 50, 1961–1980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Ref. No | Study | Model Used | Main Findings | Key Molecular Targets |
|---|---|---|---|---|
| [4] | Liang et al., 2020 | Tail-suspension was conducted to simulate microgravity in mice (WT and cardiomyocyte-specific Capns1 knockout) | Microgravity induced myocardial apoptosis, mitochondrial damage, and cardiac dysfunction; calpain activation plays a central role via MAPK pathways | Calpain, NADPH oxidase (p47phox, p67phox, Rac1), ERK1/2, p38 MAPK |
| [14] | White et al., 2010 | Rat hindlimb unloading (HL) model | Aute (3-day) simulated microgravity induces endothelial-dependent vascular hyporesponsiveness in aortic rings via upregulation of the NO/cGMP pathway. NOS-3 expression does not change, but phosphorylation increases at activating site (Ser1177) and decreases at inhibitory site (Ser495), enhancing NOS-3 activity. | NOS-3 (eNOS), phospho-NOS-3 (Ser1177, Ser495), HSP90, NO, cGMP |
| [15] | Liu et al., 2022 | Tail-suspension was conducted to simulate microgravity in rats | Microgravity induces myocardial atrophy and decreases cardiac function; transcriptomic and metabolomic profiling revealed significant molecular pathway alterations. | FoxO signaling, Mki67, Cdk1, Plk1, Ccna2, Cdc20, Top2a, Bub1, Ndc80, Ccnb2, Ttk; ADP ↓ (downregulated), L-glutamate ↑ (upregulated) |
| [16] | Liu et al., 2015 | Tail-suspension model in rats | Physical inactivity (tail-suspension) increases autophagic activity, leading to cardiac dysfunction and atrophy without inducing apoptosis. Autophagy inhibition (chloroquine) reverses myocardial atrophy and restores systolic function. | Autophagy pathway (LC3, p62, Beclin-1, Vps34, mTOR) |
| [17] | Li et al., 2022 | Human umbilical vein endothelial cells (HUVECs) exposed to 2-D clinostat-simulated microgravity in vitro | Simulated microgravity increased endothelial hyperpermeability and migration; PINK1-dependent mitophagy activation attenuated these effects | PINK1, Parkin, p62, Drp1, Mfn2, NLRP3 inflammasome, |
| [18] | Jiang et al., 2020 | Human umbilical vein endothelial cells (HUVECs) exposed to 2D clinorotation (72 h) | Simulated microgravity induces ER stress, which activates the iNOS/NO pathway. This promotes NF-κB activation and NLRP3 inflammasome assembly, leading to increased production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-8) and endothelial apoptosis. Pharmacologic inhibition of ER stress, iNOS, NF-κB, or NLRP3 reduces these effects. | CHOP and GRP78 (ER stress markers), iNOS and NO, NF-κB/IκB signaling, NLRP3 inflammasome, and pro-inflammatory cytokines (IL-6, TNF-α, IL-8, IL-1β). |
| [19] | Shibata et al., 2023 | 13 astronauts (MRI pre/post ~155 days aboard ISS; exercise countermeasures) | Despite reduced total cardiac work in microgravity, exercise countermeasures on the ISS preserved left and right ventricular mass and function. No significant reduction in LV or RV mass postflight; modest trends toward increased LV stroke volume and ejection fraction. | - |
| [20] | Wang et al., 2020 | Male rats under hindlimb unloading (HU) for 6 weeks with ad libitum or time-restricted feeding (TRF; 8 h/day) | Simulated microgravity (HU) caused LV dyssynchrony, reduced cardiac function, decreased PDH activity, and impaired glucose utilization. TRF preserved cardiac function and metabolism, enhanced cardiomyocyte contractility, and improved FGF21 signaling. Liver or cardiac FGF21/FGFR1 knockdown abolished TRF benefits. | FGF21, FGFR1, PDH |
| [21] | Sun et al., 2019 | Male rats in hindlimb unloading (HU) model for 8 weeks with/without Panax quinquefolium saponin (PQS) treatment | Simulated microgravity (HU) caused cardiac remodeling, impaired function, elevated serum cardiac injury markers, and increased cardiomyocyte apoptosis. PQS treatment reduced injury markers, improved cardiac structure and function, and decreased apoptosis via AMPK activation and inhibition of Erk1/2 and CaMKII/HDAC4 pathways. | AMPK, Erk1/2, CaMKII, HDAC4, CK-MB, cTnT, IMA, |
| [22] | Pan et al., 2025 | Mouse tail suspension and rhesus monkey bedrest models; cardiac-specific transgenic (TG) mice and AAV9-mediated overexpression | Simulated microgravity caused cardiac remodeling (fibrosis, smaller cardiomyocytes, reduced ejection fraction) and downregulated miR-199a-3p. Cardiac-specific overexpression of miR-199a-3p (transgenic mice or AAV9 delivery) mitigated remodeling and dysfunction by targeting and inhibiting MEF2C. | miR-199a-3p, MEF2C |
| Protein | Function | Reference (PMID) |
|---|---|---|
| adducin | Endothelial barrier stabilization | [30,31] |
| spectrin | Cell–matrix contact and migration | [32] |
| MARCKS/MLP1 | Adaptor protein between ion channels and membrane lipids | [33,34,35,36,37] |
| Ezrin | Linker protein between membrane proteins and actin cytoskeleton; migration; angiogenesis | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, Z.; Rafay, R.H.; Bala, N.; Dogan, Y.E.; Alli, A.A. The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System. Biomedicines 2025, 13, 2336. https://doi.org/10.3390/biomedicines13102336
Shahzad Z, Rafay RH, Bala N, Dogan YE, Alli AA. The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System. Biomedicines. 2025; 13(10):2336. https://doi.org/10.3390/biomedicines13102336
Chicago/Turabian StyleShahzad, Zenab, Ramish H. Rafay, Niharika Bala, Yunus E. Dogan, and Abdel A. Alli. 2025. "The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System" Biomedicines 13, no. 10: 2336. https://doi.org/10.3390/biomedicines13102336
APA StyleShahzad, Z., Rafay, R. H., Bala, N., Dogan, Y. E., & Alli, A. A. (2025). The Heart in Space: Effects of Microgravity on Different Cell Types and Their Functions in the Cardiovascular System. Biomedicines, 13(10), 2336. https://doi.org/10.3390/biomedicines13102336






