Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series
Abstract
1. Introduction
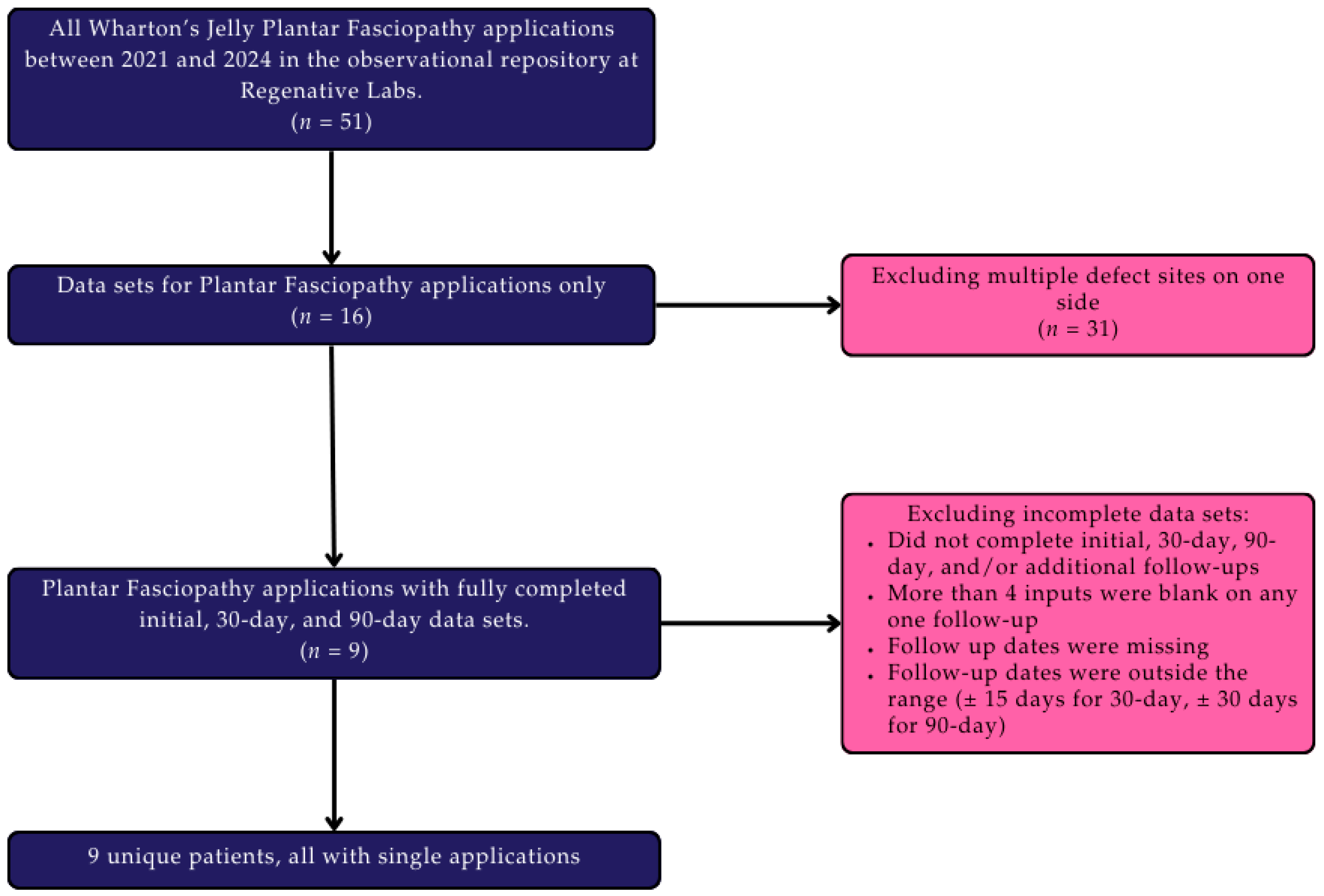
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Patient Care Procedures
2.4. Data Analysis
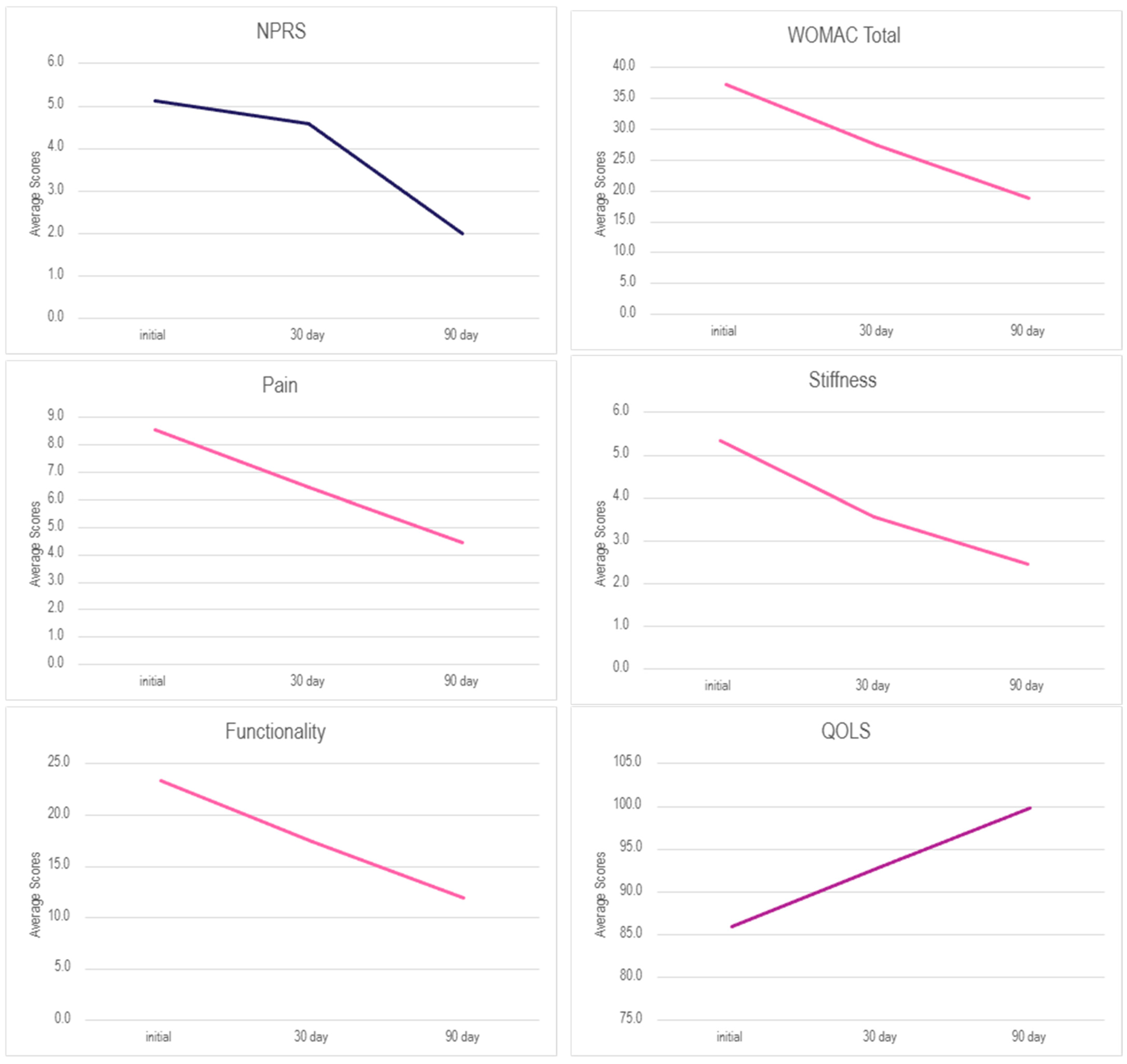
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trojian, T.; Tucker, A.K. Plantar Fasciitis. Am. Fam. Physician 2019, 99, 744–750. [Google Scholar] [CrossRef]
- Mørk, M.; Soberg, H.L.; Hoksrud, A.F.; Heide, M.; Groven, K.S. The struggle to stay physically active-A qualitative study exploring experiences of individuals with persistent plantar fasciopathy. J. Foot Ankle Res. 2023, 16, 20. [Google Scholar] [CrossRef]
- Di Caprio, F.; Gigli, M.; Ponziani, L. Plantar fasciitis: A literature review. J. Orthop. Trauma Rehabil. 2025. [Google Scholar] [CrossRef]
- Landorf, K.B. Plantar heel pain and plantar fasciitis. BMJ Clin. Evid. 2015, 2015, 1111. [Google Scholar] [PubMed Central]
- Monteagudo, M.; de Albornoz, P.M.; Gutierrez, B.; Tabuenca, J.; Álvarez, I. Plantar fasciopathy: A current concepts review. EFORT Open Rev. 2018, 3, 485–493. [Google Scholar] [CrossRef]
- Latt, L.D.; Jaffe, D.E.; Tang, Y.; Taljanovic, M.S. Evaluation and Treatment of Chronic Plantar Fasciitis. Foot Ankle Orthop. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Goff, J.D.; Crawford, R. Diagnosis and treatment of plantar fasciitis. Am. Fam. Physician 2011, 84, 676–682. [Google Scholar]
- Liu, P.; Chen, Q.; Yang, K.; Cai, F. Prevalence, characteristics, and associated risk factors of plantar heel pain in americans: The cross-sectional NHANES study. J. Orthop. Surg. Res. 2024, 19, 805. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.L.; Ma, Y.T.; Wu, W.; Zheng, Y.J. Correction: Evaluation of the efficacy of trigger points combined with extracorporeal shock waves in the treatment of plantar fasciitis: Heel temperature and plantar pressure. BMC Musculoskelet. Disord. 2024, 25, 546. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.T.; How, C.H.; Tan, B. Management of plantar fasciitis in the outpatient setting. Singap. Med. J. 2016, 57, 168–171. [Google Scholar] [CrossRef]
- Landorf, K.B.; Keenan, A.M.; Herbert, R.D. Effectiveness of foot orthoses to treat plantar fasciitis: A randomized trial. Arch. Intern. Med. 2006, 166, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.W.; Yoon, Y.S.; Yoon, M.K.; Choi, S.G.; Kim, D.W.; Jang, H.Y. Effectiveness of Shoe Rotation in Managing Plantar Fasciitis in Patients. J. Clin. Med. 2024, 13, 4624. [Google Scholar] [CrossRef]
- Nahin, R.L. Prevalence and Pharmaceutical Treatment of Plantar Fasciitis in United States Adults. J. Pain 2018, 19, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Yeo, J.; Lee, S.H.; Lee, Y.J.; Park, Y.; Goo, B.; Ha, I.H. Healthcare usage and cost for plantar fasciitis: A retrospective observational analysis of the 2010–2018 health insurance review and assessment service national patient sample data. BMC Health Serv. Res. 2023, 23, 546. [Google Scholar] [CrossRef]
- Buchanan, B.K.; Kushner, D. Plantar Fasciitis. PubMed. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431073/ (accessed on 5 August 2025).
- Ward, L.; Mercer, N.P.; Azam, M.T.; Hoberman, A.; Hurley, E.T.; Butler, J.J.; Ubillus, H.; Cronin, J.; Kennedy, J.G. Outcomes of Endoscopic Treatment for Plantar Fasciitis: A Systematic Review. Foot Ankle Spec. 2022, 18, 193–201. [Google Scholar] [CrossRef]
- Johnson-Lynn, S.; Cooney, A.; Ferguson, D.; Bunn, D.; Gray, W.; Coorsh, J.; Kakwani, R.; Townshend, D. A Feasibility Study Comparing Platelet-Rich Plasma Injection with Saline for the Treatment of Plantar Fasciitis Using a Prospective, Randomized Trial Design. Foot Ankle Spec. 2019, 12, 153–158. [Google Scholar] [CrossRef]
- Valotto, E., Jr.; Silva, S.B.; Henrique, L.; Muniz, L.H.G.; Evangelista, F.F.; Scremin, L.H.G.; Kobayashi, E.M.; Kimura, E. A randomized study comparing the effect of platelet-rich plasma and platelet-poor plasma for the treatment of plantar fasciitis. Res. Soc. Dev. 2023, 12, e7812943168. [Google Scholar] [CrossRef]
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental Tissues as Biomaterials in Regenerative Medicine. Biomed. Res. Int. 2022, 2022, 6751456. [Google Scholar] [CrossRef]
- Main, B.J.; Maffulli, N.; Valk, J.A.; Rodriguez, H.C.; Gupta, M.; El-Amin, S.F., III.; Gupta, A. Umbilical Cord-Derived Wharton’s Jelly for Regenerative Medicine Applications: A Systematic Review. Pharmaceuticals 2021, 14, 1090. [Google Scholar] [CrossRef]
- Gupta, A.; El-Amin, S.F., 3rd; Levy, H.J.; Sze-Tu, R.; Ibim, S.E.; Maffulli, N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J. Orthop. Surg. Res. 2020, 15, 49. [Google Scholar] [CrossRef]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of Wharton’s jelly. Biol. Neonate 1997, 71, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Tamea, C.; Shou, J.; Okafor, A.; Sparks, J.; Dodd, R.; Woods, C.; Lambert, N.; Schulte, O.; Barrett, T. Safety and Efficacy of Wharton’s Jelly Connective Tissue Allograft for Rotator Cuff Tears: Findings from a Retrospective Observational Study. Biomedicines 2024, 12, 710. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Pennisi, F.; Maggi, S.; Veronese, N.; Soysal, P. Aging, longevity, and healthy aging: The public health approach. Aging Clin. Exp. Res. 2025, 37, 125. [Google Scholar] [CrossRef]
- Murtagh, S.; Touloumis, A.; Olivier, G.; Butterworth, J.; Hammerbeck, U. Physiotherapy Outcomes Are Associated With Shorter Waiting Times, More Treatment Sessions and Younger Age: Analysis of a Clinical Database. Musculoskelet. Care 2024, 22, e1924. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Cheng, B.; Zhang, N.; Zhao, Y.; Qin, X.; He, D.; Chu, X.; Shi, S.; Cai, Q.; et al. A prospective study of associations between accelerated biological aging and twenty musculoskeletal disorders. Commun. Med. 2024, 4, 266. [Google Scholar] [CrossRef]
- Ujala Malik Fatima, A.; Ahmad, E.; Taqi, S.Z.; Tahir, I.; Rehman, A. Prevalence of Plantar Fasciitis Pain and Its Association with Quality of Work Among Sales Promotion Persons at Supermarkets. J. Health Rehabil. Res. 2024, 4, 1–4. [Google Scholar] [CrossRef]
- Yin, M.C.; Yan, Y.J.; Tong, Z.Y.; Xu, C.Q.; Qiao, J.J.; Zhou, X.N.; Ye, J.; Mo, W. Development and Validation of a Novel Scoring System for Severity of Plantar Fasciitis. Orthop. Surg. 2020, 12, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mithu, M.M.; Haque, M.A.; Xiao, J.; Li, J.; Chen, S.; Wu, T. Comparative effectiveness of endoscopic plantar fasciotomy, needle knife therapy, and conventional painkillers in the treatment of plantar fasciitis: A real-world evidence study. J. Orthop. Surg. Res. 2024, 19, 633. [Google Scholar] [CrossRef]
- Davis, J.M.; Purita, J.R.; Shou, J.; Barrett, T.C. Three-Dimensional Electron Microscopy of Human Umbilical Cord Tissue Allograft Pre and Post Processing: A Literature Comparison. J. Biomed. Res. Environ. Sci. 2022, 3, 934–940. Available online: https://www.jelsciences.com/articles/jbres1535.pdf (accessed on 2 July 2025). [CrossRef]
- Zhang, J. Research On Plantar Fasciitis. Stem Cells Res. Dev. Ther. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Alabau-Dasi, R.; Nieto-Gil, P.; Ortega-Avila, A.B.; Gijon-Nogueron, G. Variations in the Thickness of the Plantar Fascia After Training Based in Training Race. Pilot Study J. Foot Ankle Surg. 2022, 61, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Ranalletta, M.; Pasqualini, I.; Zicaro, J.P.; Paz, M.C.; Camino, P.; Piuzzi, N.S. Substantial Variability in Platelet-Rich Plasma Composition Is Based on Patient Age and Baseline Platelet Count. Arthrosc. Sports Med. Rehabil. 2023, 5, e853–e858. [Google Scholar] [CrossRef] [PubMed]
- Rahman, E.; Rao, P.; Abu-Farsakh, H.N.; Thonse, C.; Ali, I.; Upton, A.E.; Baratikkae, S.Y.; Carruthers, J.D.A.; Mosahebi, A.; Heidari, N.; et al. Systematic Review of Platelet-Rich Plasma in Medical and Surgical Specialties: Quality, Evaluation, Evidence, and Enforcement. J. Clin. Med. 2024, 13, 4571. [Google Scholar] [CrossRef]
- Russ, K.; Yoo, P.; Lambert, N.; Lee, A.; Shou, J.; Barrett, T. Regenerative Protocol for Knee Degeneration: A Case Study with Five-Year Follow-Up. Arch. Clin. Biomed. Res. 2025, 9, 275–279. [Google Scholar] [CrossRef]
| Age Range | BMI Range | ||
|---|---|---|---|
| 20–29 | 0 | Underweight (<18.5) | 0 |
| 30–39 | 0 | Healthy weight (18.5–24.9) | 1 |
| 40–49 | 0 | Overweight (25.0–29.9) | 2 |
| 50–59 | 1 | Obese (>30.0) | 2 |
| 60–69 | 1 | NA * | 3 |
| 70–79 | 4 | Mean BMI | 30.9 |
| 80–89 | 2 | Gender | |
| 90–99 | 0 | Male | 5 |
| Mean Age | 73 | Female | 4 |
| Scale | Initial—Day 30 | Initial—Day 90 |
|---|---|---|
| NPRS | 10.80% | 60.98% |
| WOMAC Total | 26.27% | 49.55% |
| Pain | 24.68% | 48.05% |
| Stiffness | 33.33% | 54.17% |
| Functionality | 25.24% | 49.05% |
| QOLS | 8.01% | 6.83% |
| Scale (Females) | n | Initial | n2 | 30 Day | n3 | 90 Day | Scale (Males) | n | Initial | n2 | 30 Day | n3 | 90 Day |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPRS | 3 | 6.33 | 3 | 5 | 3 | 2.33 | NPRS | 5 | 4.4 | 4 | 4.25 | 5 | 1.8 |
| WOMAC Total | 4 | 48.25 | 4 | 36.25 | 4 | 26 | WOMAC Total | 5 | 28.4 | 5 | 20.4 | 5 | 13 |
| Pain | 4 | 11.5 | 4 | 9.75 | 4 | 6.75 | Pain | 5 | 6.2 | 5 | 3.8 | 5 | 2.6 |
| Stiffness | 4 | 6 | 4 | 3.75 | 4 | 3 | Stiffness | 5 | 4.8 | 5 | 3.4 | 5 | 2 |
| Functionality | 4 | 30.75 | 4 | 22.75 | 4 | 16.25 | Functionality | 5 | 17.4 | 5 | 13.2 | 5 | 8.4 |
| QOLS | 4 | 70.5 | 4 | 77.75 | 4 | 81.25 | QOLS | 5 | 98.4 | 5 | 105 | 4 | 102.5 |
| Scale (Females) | Initial—30 Day | Initial—90 Day | Scale (Males) | Initial—30 Day | Initial—90 Day |
|---|---|---|---|---|---|
| NPRS | 21% | 63% | NPRS | 3% | 59% |
| WOMAC Total | 25% | 46% | WOMAC Total | 28% | 54% |
| Pain | 15% | 41% | Pain | 39% | 58% |
| Stiffness | 38% | 50% | Stiffness | 29% | 58% |
| Functionality | 26% | 47% | Functionality | 24% | 52% |
| QOLS | 10% | 15% | QOLS | 7% | 4% |
| P2 | S2 | PF2 | W2 | Q2 | FNPRS | FP | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Valid | 9 | 9 | 9 | 9 | 9 | 8 | 9 | |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Mean | 6.4444 | 3.5556 | 17.4444 | 27.4444 | 92.8889 | 2.0000 | 4.4444 | ||
| Median | 4.0000 | 4.0000 | 12.0000 | 19.0000 | 95.0000 | 0.5000 | 2.0000 | ||
| Std. Deviation | 5.91843 | 2.96273 | 17.29242 | 24.92544 | 15.90161 | 2.87849 | 4.63980 | ||
| Skewness | 0.197 | −0.220 | 0.702 | 0.480 | 0.086 | 1.533 | 0.534 | ||
| Std. Error of Skewness | 0.717 | 0.717 | 0.717 | 0.717 | 0.717 | 0.752 | 0.717 | ||
| Kurtosis | −2.224 | −1.786 | −1.130 | −1.548 | −1.846 | 1.982 | −1.469 | ||
| Std. Error of Kurtosis | 1.400 | 1.400 | 1.400 | 1.400 | 1.400 | 1.481 | 1.400 | ||
| Minimum | 0.00 | 0.00 | 1.00 | 1.00 | 74.00 | 0.00 | 0.00 | ||
| Maximum | 14.00 | 7.00 | 46.00 | 66.00 | 112.00 | 8.00 | 12.00 | ||
| FS | FPF | FW | FQ | ||||||
| n | Valid | 9 | 9 | 9 | 8 | ||||
| Missing | 0 | 0 | 0 | 1 | |||||
| Mean | 2.4444 | 11.8889 | 18.7778 | 91.8750 | |||||
| Median | 2.0000 | 4.0000 | 11.0000 | 95.0000 | |||||
| Std. Deviation | 2.24227 | 15.14467 | 21.63202 | 18.23997 | |||||
| Skewness | 0.308 | 0.839 | 0.754 | −0.672 | |||||
| Std. Error of Skewness | 0.717 | 0.717 | 0.717 | 0.752 | |||||
| Kurtosis | −1.227 | −1.472 | −1.478 | −1.064 | |||||
| Std. Error of Kurtosis | 1.400 | 1.400 | 1.400 | 1.481 | |||||
| Minimum | 0.00 | 0.00 | 0.00 | 65.00 | |||||
| Maximum | 6.00 | 34.00 | 51.00 | 112.00 | |||||
| Test Statistics a,d | ||||||
|---|---|---|---|---|---|---|
| NPRS2—NPRS1 | P2—P1 | S2—S1 | PF2—PF1 | W2—W1 | Q2—Q1 | |
| Z | −0.736 b | −1.442 b | −2.032 b | −1.014 b | −1.120 b | −1.521 c |
| Asymp. Sig. (2-tailed) | 0.461 | 0.149 | 0.042 | 0.310 | 0.263 | 0.128 |
| r | ||||||
| FNPRS—NPRS2 | FP—P2 | FS—S2 | FPF—PF2 | FW—W2 | FQ—Q2 | |
| Z | −1.761 b | −2.154 b | −1.200 b | −2.524 b | −2.492 b | −0.140 c |
| Asymp. Sig. (2-tailed) | 0.078 | 0.031 | 0.230 | 0.012 | 0.013 | 0.889 |
| r | 0.42 | 0.42 | ||||
| FNPRS—NPRS1 | FP—P1 | FS—S1 | FPF—PF1 | FW—W1 | FQ—Q1 | |
| Z | −2.124 b | −2.680 b | −2.536 b | −2.547 b | −2.668 b | −1.016 c |
| Asymp. Sig. (2-tailed) | 0.034 | 0.007 | 0.011 | 0.011 | 0.008 | 0.310 |
| r | 0.60 | 0.63 | 0.63 | 0.60 | ||
| Bootstrap for Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Model | B | Bootstrap a | |||||
| Bias | Std. Error | Sig. (2-Tailed) | BCa 95% Confidence Interval | ||||
| Lower | Upper | ||||||
| 1 | (Constant) | −29.100 | 0.085 b | 23.433 b | 0.389 b | −99.375 b | 5.550 b |
| Gender | 6.850 | 0.000 b | 12.456 b | 0.624 b | −11.333 b | 30.333 b | |
| Bootstrap for Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Model | B | Bootstrap a | |||||
| Bias | Std. Error | Sig. (2-Tailed) | BCa 95% Confidence Interval | ||||
| Lower | Upper | ||||||
| 1 | (Constant) | 53.234 | 1.033 | 18.897 | 0.039 | −2.540 | 120.909 |
| Age | −0.916 | −0.010 | 0.259 | 0.028 | −1.280 | −0.601 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baravarian, B.; Kwon, G.; Tamea, C.; Shou, J.; Lambert, N.; Lee, A.; Castle, E.; Barrett, T. Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series. Biomedicines 2025, 13, 2328. https://doi.org/10.3390/biomedicines13102328
Baravarian B, Kwon G, Tamea C, Shou J, Lambert N, Lee A, Castle E, Barrett T. Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series. Biomedicines. 2025; 13(10):2328. https://doi.org/10.3390/biomedicines13102328
Chicago/Turabian StyleBaravarian, Babak, Gi Kwon, Conrad Tamea, John Shou, Naomi Lambert, Alexis Lee, Eva Castle, and Tyler Barrett. 2025. "Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series" Biomedicines 13, no. 10: 2328. https://doi.org/10.3390/biomedicines13102328
APA StyleBaravarian, B., Kwon, G., Tamea, C., Shou, J., Lambert, N., Lee, A., Castle, E., & Barrett, T. (2025). Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series. Biomedicines, 13(10), 2328. https://doi.org/10.3390/biomedicines13102328






