Human Serum, Following Absorption of Fish Cartilage Hydrolysate, Promotes Dermal Fibroblast Healing through Anti-Inflammatory and Immunomodulatory Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Product Tested
2.2. Human Study Protocol
2.3. Human Primary Dermal Fibroblast (HDF) Cultures
2.4. In Vitro Scratch Assay
2.4.1. Cell Treatment
2.4.2. Statistics
2.5. DiaPASEF Proteomic Analysis
2.5.1. Cell Treatment
2.5.2. Spectral Library Creation
2.5.3. Sample Preparation and Acquisition
2.5.4. MS Data Processing
2.5.5. Data Analysis—Library Generation
2.5.6. Library Search of DIA Data
2.5.7. Quantification and Statistical Analyses of Proteomics Data
3. Results
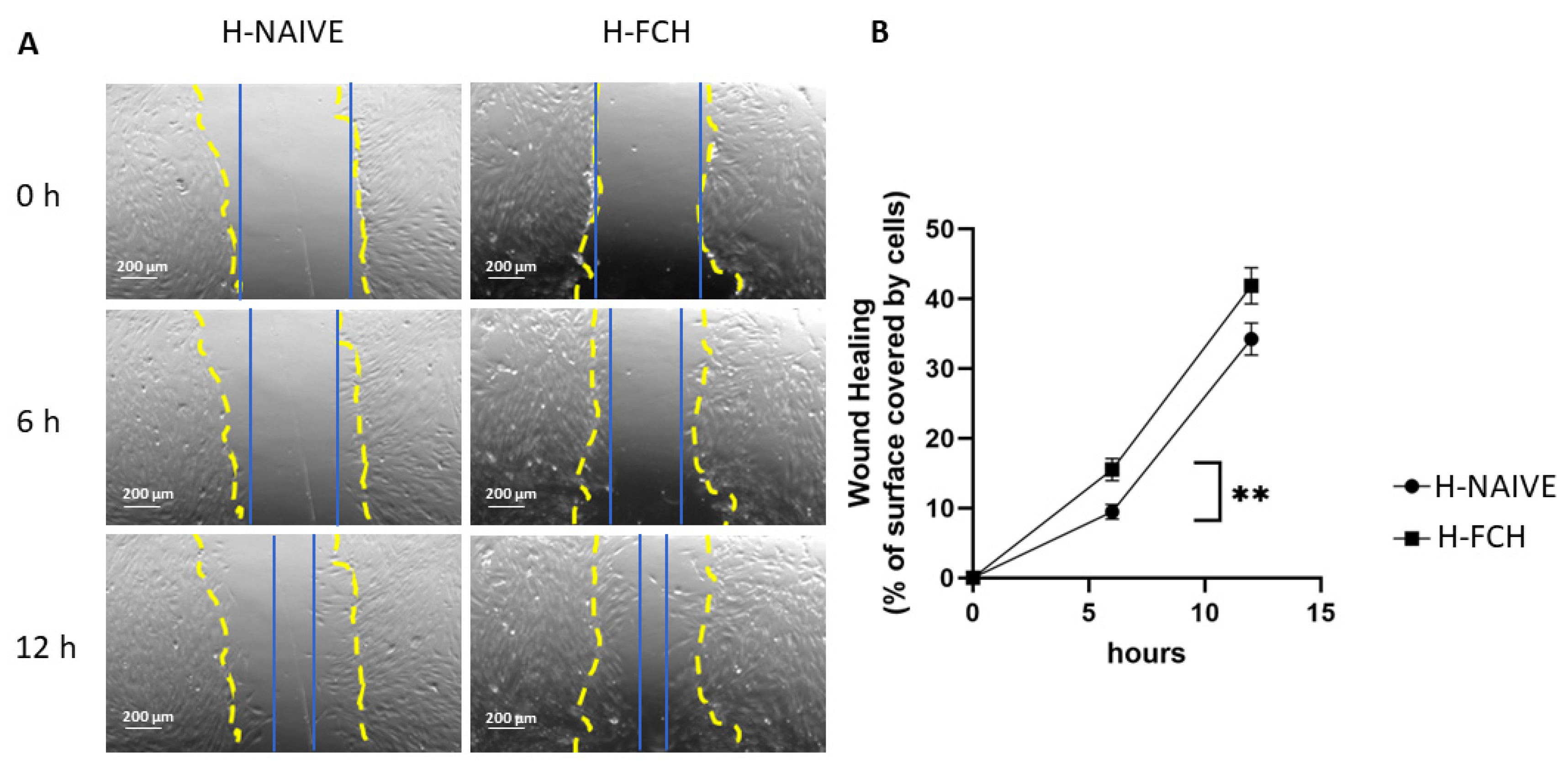
3.1. FCH-Enriched Serum Accelerates Scratch WH of HDFs
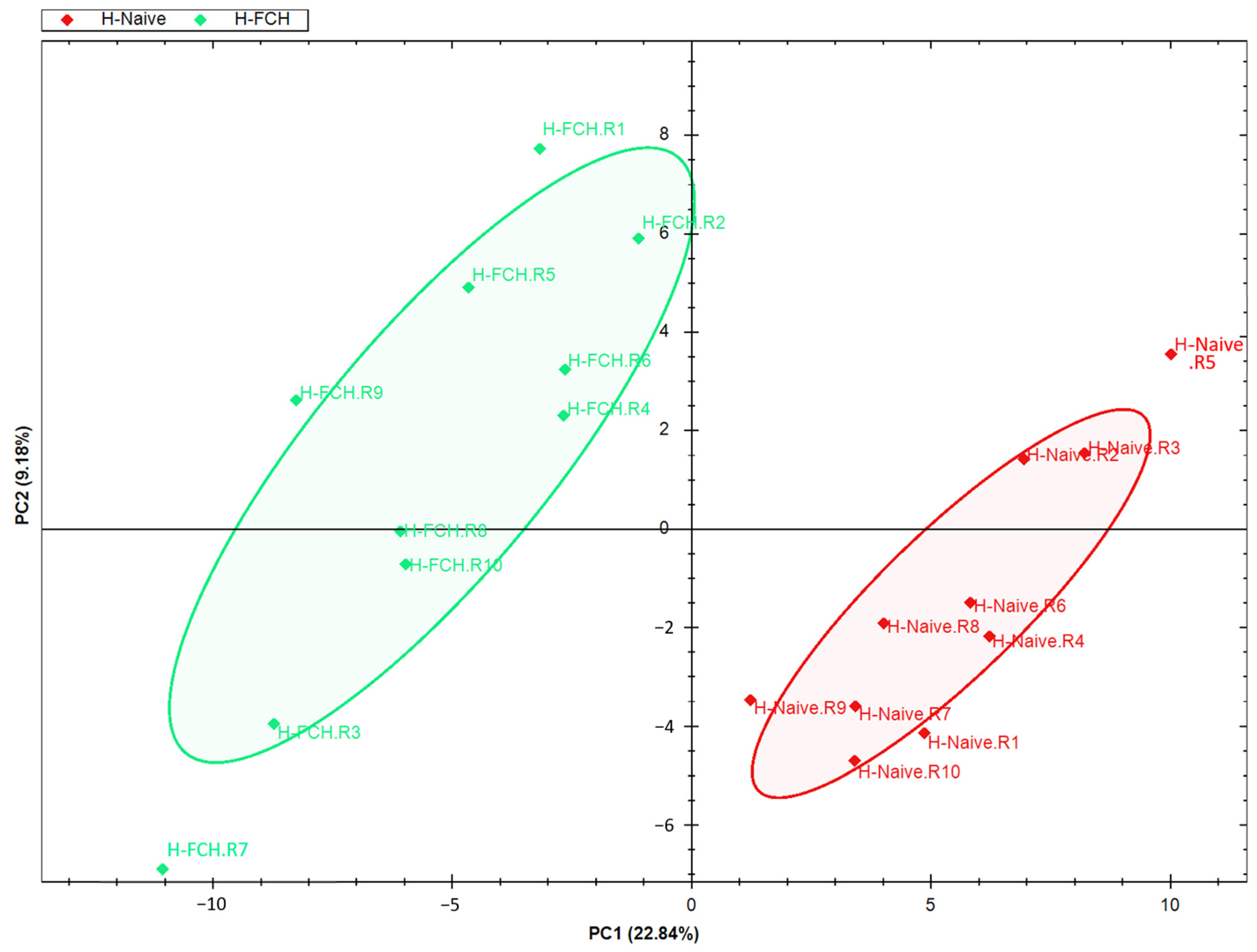
3.2. FCH-Enriched Serum Increases Expression of Proteins Involved in WH
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, P.M. The Skin Barrier as an Innate Immune Element. Semin. Immunopathol. 2007, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin Tissue Regeneration for Burn Injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Quondamatteo, F. Skin and Diabetes Mellitus: What Do We Know? Cell Tissue Res. 2014, 355, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2016, 58, 81–94. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis Niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, K.; Li, B.; Hou, H. Effects of Oral Administration of Peptides with Low Molecular Weight from Alaska Pollock (Theragra Chalcogramma) on Cutaneous Wound Healing. J. Funct. Foods 2018, 48, 682–691. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral Administration of Marine Collagen Peptides Prepared from Chum Salmon (Oncorhynchus Keta) Improves Wound Healing Following Cesarean Section in Rats. Food Nutr. Res. 2015, 59, 26411. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Marine Collagen Peptides from Chum Salmon Skin Enhances Cutaneous Wound Healing and Angiogenesis in Rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Samà, D.; Calatroni, A. Glycosaminoglycans Modulate Inflammation and Apoptosis in LPS-Treated Chondrocytes. J. Cell. Biochem. 2009, 106, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.-K.; Tabata, Y. Chondroitin-6-Sulfate Attenuates Inflammatory Responses in Murine Macrophages via Suppression of NF-κB Nuclear Translocation. Acta Biomater. 2014, 10, 2684–2692. [Google Scholar] [CrossRef]
- Krichen, F.; Ghlissi, Z.; Abdallah, R.B.; Kallel, R.; Martinez-Alvarez, O.; Carmen Gómez-Guillén, M.; Sila, A.; Boudawara, T.; Sahnoun, Z.; Bougatef, A. Glycosaminoglycans from Grey Triggerfish and Smooth Hound Skins: Rheological, Anti-Inflammatory and Wound Healing Properties. Int. J. Biol. Macromol. 2018, 118, 965–975. [Google Scholar] [CrossRef]
- Henrotin, Y.; Herman, J.; Uebelhoer, M.; Wauquier, F.; Boutin-Wittrant, L.; Donneau, A.-F.; Monseur, J.; Fotso, V.M.; Duquenne, M.; Wagner, M.; et al. Oral Supplementation with Fish Cartilage Hydrolysate in an Adult Population Suffering from Knee Pain and Function Discomfort: Results from an Innovative Approach Combining an Exploratory Clinical Study and an Ex Vivo Clinical Investigation. BMC Musculoskelet. Disord. 2023, 24, 748. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules 2021, 26, 4880. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix Metalloproteinase Interactions with Collagen and Elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin Coordinates Fibroblast Proliferation and Keratinocyte Differentiation in Wound Healing via TGF-β–Slug Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Bali, J.-P.; Cousse, H.; Neuzil, E. Biochemical Basis of the Pharmacologic Action of Chondroitin Sulfates on the Osteoarticular System. Semin. Arthritis Rheum. 2001, 31, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Final Report on the Safety Assessment of Hydrolyzed Collagen. J. Am. Coll. Toxicol. 1985, 4, 199–221. [CrossRef]
- Wauquier, F.; Daneault, A.; Granel, H.; Prawitt, J.; Fabien Soulé, V.; Berger, J.; Pereira, B.; Guicheux, J.; Rochefort, G.Y.; Meunier, N.; et al. Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: From Bedside to Bench and Vice Versa. Nutrients 2019, 11, 1249. [Google Scholar] [CrossRef]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef]
- Kleinnijenhuis, A.J.; van Holthoon, F.L.; Maathuis, A.J.H.; Vanhoecke, B.; Prawitt, J.; Wauquier, F.; Wittrant, Y. Non-Targeted and Targeted Analysis of Collagen Hydrolysates during the Course of Digestion and Absorption. Anal. Bioanal. Chem. 2020, 412, 973–982. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’InsideTM) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [Google Scholar] [CrossRef]
- Yilmaz, O.; Com, E.; Lavigne, R.; Pineau, C.; Bobe, J. Liquid Chromatography and Tandem Mass Spectrometry in Label-Free Protein Quantification of Zebrafish (Danio Rerio) Eggs. In Germline Development in the Zebrafish: Methods and Protocols; Methods in Molecular Biology; Dosch, R., Ed.; Springer: New York, NY, USA, 2021; pp. 277–290. ISBN 978-1-07-160970-5. [Google Scholar]
- Onfray, C.; Chevolleau, S.; Moinard, E.; Girard, O.; Mahadik, K.; Allsop, R.; Georgolopoulos, G.; Lavigne, R.; Renoult, O.; Aksoy, I.; et al. Unraveling Hallmark Suitability for Staging Pre- and Post-Implantation Stem Cell Models. Cell Rep. 2024, 43, 114232. [Google Scholar] [CrossRef]
- Callister, S.J.; Barry, R.C.; Adkins, J.N.; Johnson, E.T.; Qian, W.-J.; Webb-Robertson, B.-J.M.; Smith, R.D.; Lipton, M.S. Normalization Approaches for Removing Systematic Biases Associated with Mass Spectrometry and Label-Free Proteomics. J. Proteome Res. 2006, 5, 277–286. [Google Scholar] [CrossRef]
- Achcar, F.; Camadro, J.-M.; Mestivier, D. AutoClass@IJM: A Powerful Tool for Bayesian Classification of Heterogeneous Data in Biology. Nucleic Acids Res. 2009, 37, W63–W67. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape Plugin to Assess Overrepresentation of Gene Ontology Categories in Biological Networks. Bioinform. Oxf. Engl. 2005, 21, 3448–3449. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, T.; Gillard, M.; Nagel, D. A Simple Protocol for Informative Visualization of Enriched Gene Ontology Terms. Bio-Protoc. 2019, 9, e3429. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Demichev, V.; Szyrwiel, L.; Yu, F.; Teo, G.C.; Rosenberger, G.; Niewienda, A.; Ludwig, D.; Decker, J.; Kaspar-Schoenefeld, S.; Lilley, K.S.; et al. Dia-PASEF Data Analysis Using FragPipe and DIA-NN for Deep Proteomics of Low Sample Amounts. Nat. Commun. 2022, 13, 3944. [Google Scholar] [CrossRef]
- Guergues, J.; Wohlfahrt, J.; Stevens, S.M., Jr. Enhancement of Proteome Coverage by Ion Mobility Fractionation Coupled to PASEF on a TIMS–QTOF Instrument. J. Proteome Res. 2022, 21, 2036–2044. [Google Scholar] [CrossRef]
- Seaman, L.; Meixner, W.; Snyder, J.; Rajapakse, I. Periodicity of Nuclear Morphology in Human Fibroblasts. Nucleus 2015, 6, 408–416. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2021, 11, 29. [Google Scholar] [CrossRef]
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An Overview of the Serpin Superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef][Green Version]
- Park, D.J.; Duggan, E.; Ho, K.; Dorschner, R.A.; Dobke, M.; Nolan, J.P.; Eliceiri, B.P. Serpin-Loaded Extracellular Vesicles Promote Tissue Repair in a Mouse Model of Impaired Wound Healing. J. Nanobiotechnol. 2022, 20, 474. [Google Scholar] [CrossRef]
- Grimstein, C.; Choi, Y.-K.; Satoh, M.; Lu, Y.; Wang, X.; Campbell-Thompson, M.; Song, S. Combination of Alpha-1 Antitrypsin and Doxycycline Suppresses Collagen-Induced Arthritis. J. Gene Med. 2010, 12, 35–44. [Google Scholar] [CrossRef]
- Janciauskiene, S.M.; Nita, I.M.; Stevens, T. A1-Antitrypsin, Old Dog, New Tricks: A1-Antitrypsin Exerts in Vitro Anti-Inflammatory Activity in Human Monocytes by Elevating cAMP. J. Biol. Chem. 2007, 282, 8573–8582. [Google Scholar] [CrossRef]
- Congote, L.F.; Temmel, N.; Sadvakassova, G.; Dobocan, M.C. Comparison of the Effects of Serpin A1, a Recombinant Serpin A1-IGF Chimera and Serpin A1 C-Terminal Peptide on Wound Healing. Peptides 2008, 29, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.C.; Textoris, C.; Oehme, F.; Klaassen, T.; Goppelt, A.; Römer, A.; Fugmann, B.; Davidson, J.M.; Werner, S.; Krieg, T.; et al. Pivotal Role for A1-Antichymotrypsin in Skin Repair*. J. Biol. Chem. 2011, 286, 28889–28901. [Google Scholar] [CrossRef] [PubMed]
- Kirschfink, M.; Nürnberger, W. C1 Inhibitor in Anti-Inflammatory Therapy: From Animal Experiment to Clinical Application. Mol. Immunol. 1999, 36, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Begieneman, M.P.V.; Kubat, B.; Ulrich, M.M.W.; Hahn, N.E.; Stumpf-Stolker, Y.; Tempelaars, M.; Middelkoop, E.; Zeerleder, S.; Wouters, D.; van Ham, M.S.; et al. Prolonged C1 Inhibitor Administration Improves Local Healing of Burn Wounds and Reduces Myocardial Inflammation in a Rat Burn Wound Model. J. Burn Care Res. 2012, 33, 544–551. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Khaldoyanidi, S.K.; DiScipio, R.G. Complement Activation in the Context of Stem Cells and Tissue Repair. World J. Stem Cells 2015, 7, 1090–1108. [Google Scholar] [CrossRef]
- Sinno, H.; Malholtra, M.; Lutfy, J.; Jardin, B.; Winocour, S.; Brimo, F.; Beckman, L.; Watters, K.; Philip, A.; Williams, B.; et al. Topical Application of Complement C3 in Collagen Formulation Increases Early Wound Healing. J. Dermatol. Treat. 2013, 24, 141–147. [Google Scholar] [CrossRef]
- Sinno, H.; Malhotra, M.; Lutfy, J.; Jardin, B.; Winocour, S.; Brimo, F.; Beckman, L.; Watters, K.; Philip, A.; Williams, B.; et al. Accelerated Wound Healing with Topical Application of Complement C5. Plast. Reconstr. Surg. 2012, 130, 523. [Google Scholar] [CrossRef]
- Man, R.C.; Idrus, R.B.H.; Ibrahim, W.I.W.; Saim, A.B.; Lokanathan, Y. Secretome Analysis of Human Nasal Fibroblast Identifies Proteins That Promote Wound Healing. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–18. [Google Scholar]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. A1-Acid Glycoprotein: An Acute Phase Protein with Inflammatory and Immunomodulating Properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Ito, S.; Suzuki, H.; Isobe, K.-I. Antibodies to Wounded Tissue Enhance Cutaneous Wound Healing. Immunology 2009, 128, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Leung, D.Y.M. Atopic Dermatitis: A Disease of Altered Skin Barrier and Immune Dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef]
- Elder, J.T.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Stuart, P.E.; Tejasvi, T.; Voorhees, J.J.; Abecasis, G.R.; Nair, R.P. Molecular Dissection of Psoriasis: Integrating Genetics and Biology. J. Investig. Dermatol. 2010, 130, 1213–1226. [Google Scholar] [CrossRef]
- Noh, J.Y.; Shin, J.U.; Kim, J.H.; Kim, S.H.; Kim, B.-M.; Kim, Y.H.; Park, S.; Kim, T.-G.; Shin, K.-O.; Park, K.; et al. ZAG Regulates the Skin Barrier and Immunity in Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 1648–1657. [Google Scholar] [CrossRef]
- Haque, M.; Siegel, R.J.; Fox, D.A.; Ahmed, S. Interferon-Stimulated GTPases in Autoimmune and Inflammatory Diseases: Promising Role for the Guanylate-Binding Protein (GBP) Family. Rheumatology 2021, 60, 494–506. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Shannon, W.; Du, F.; Duncan, J.; Cao, K.; Aftergut, K.; Catier, J.; Fernandez-Vina, M.A.; Menter, A. Insights into Psoriasis and Other Inflammatory Diseases from Large-Scale Gene Expression Studies. Hum. Mol. Genet. 2001, 10, 1793–1805. [Google Scholar] [CrossRef]
- Park, S.Y.; Gupta, D.; Hurwich, R.; Kim, C.H.; Dziarski, R. Peptidoglycan Recognition Protein Pglyrp2 Protects Mice from Psoriasis-Like Skin Inflammation by Promoting Treg and Limiting Th17 Responses. J. Immunol. 2011, 187, 5813–5823. [Google Scholar] [CrossRef]
- Chen, C.-C.; Mo, F.-E.; Lau, L.F. The Angiogenic Factor Cyr61 Activates a Genetic Program for Wound Healing in Human Skin Fibroblasts *. J. Biol. Chem. 2001, 276, 47329–47337. [Google Scholar] [CrossRef]
- Bost, F.; Diarra-Mehrpour, M.; Martin, J.-P. Inter-α-Trypsin Inhibitor Proteoglycan Family. Eur. J. Biochem. 1998, 252, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Asano, Y.; Saigusa, R.; Taniguchi, T.; Nakamura, K.; Miura, S.; Toyama, T.; Takahashi, T.; Ichimura, Y.; Hirabayashi, M.; et al. Increased Expression of Aquaporin-1 in Dermal Fibroblasts and Dermal Microvascular Endothelial Cells Possibly Contributes to Skin Fibrosis and Edema in Patients with Systemic Sclerosis. J. Dermatol. Sci. 2019, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. Pulp Fibroblasts Synthesize Functional Complement Proteins Involved in Initiating Dentin-Pulp Regeneration. Am. J. Pathol. 2014, 184, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Kulics, J.; Circolo, A.; Strunk, R.C.; Colten, H.R. Regulation of Synthesis of Complement Protein C4 in Human Fibroblasts: Cell- and Gene-Specific Effects of Cytokines and Lipopolysaccharide. Immunology 1994, 82, 509–515. [Google Scholar]
- Katz, Y.; Strunk, R.C. Synovial Fibroblast-like Cells Synthesize Seven Proteins of the Complement System. Arthritis Rheum. 1988, 31, 1365–1370. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]


| Pathway | Protein IDs | Gene Name | Protein Name |
|---|---|---|---|
| Response to wounding | P01023 | A2M | Alpha-2-macroglobulin |
| P02743 | APCS | Serum amyloid P-component | |
| P02652 | APOA2 | Apolipoprotein A-II | |
| P02749 | APOH | Beta-2-glycoprotein 1 | |
| P02746 | C1QB | Complement C1q subcomponent subunit B | |
| P02747 | C1QC | Complement C1q subcomponent subunit C | |
| P09871 | C1S | Complement C1s subcomponent | |
| P06681 | C2 | Complement C2 | |
| P01024 | C3 | Complement C3 | |
| P01031 | C5 | Complement C5 | |
| P02748 | C9 | Complement component C9 | |
| P21926 | CD9 | CD9 antigen | |
| P00751 | CFB | Complement factor B | |
| P08603 | CFH | Complement factor H | |
| P00742 | F10 | Coagulation factor X | |
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 | |
| P19652 | ORM2 | Alpha-1-acid glycoprotein 2 | |
| P35542 | SAA4 | Serum amyloid A-4 protein | |
| P01009 | SERPINA1 | Alpha-1-antitrypsin | |
| P01011 | SERPINA3 | Alpha-1-antichymotrypsin | |
| P01008 | SERPINC1 | Antithrombin-III | |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | |
| P02787 | TF | Serotransferrin | |
| Immune response | P25311 | AZGP1 | Zinc-alpha-2-glycoprotein |
| P02746 | C1QB | Complement C1q subcomponent subunit B | |
| P02747 | C1QC | Complement C1q subcomponent subunit C | |
| P09871 | C1S | Complement C1s subcomponent | |
| P06681 | C2 | Complement C2 | |
| P01024 | C3 | Complement C3 | |
| P01031 | C5 | Complement C5 | |
| P02748 | C9 | Complement component C9 | |
| P00751 | CFB | Complement factor B | |
| P08603 | CFH | Complement factor H | |
| Q92989 | CLP1 | Polyribonucleotide 5’-hydroxyl-kinase Clp1 | |
| P32456 | GBP2 | Guanylate-binding protein 2 | |
| P01876 | IGHA1 | Immunoglobulin heavy constant alpha 1 | |
| P01877 | IGHA2 | Immunoglobulin heavy constant alpha 2 | |
| P01857 | IGHG1 | Immunoglobulin heavy constant gamma 1 | |
| P01859 | IGHG2 | Immunoglobulin heavy constant gamma 2 | |
| P01860 | IGHG3 | Immunoglobulin heavy constant gamma 3 | |
| P01861 | IGHG4 | Immunoglobulin heavy constant gamma 4 | |
| P01871 | IGHM | Immunoglobulin heavy constant mu | |
| P01834 | IGKC | Immunoglobulin kappa constant | |
| P01602 | IGKV1-5 | Immunoglobulin kappa variable 1-5 | |
| P04433 | IGKV3D-11 | Immunoglobulin kappa variable 3-11 | |
| A0A0C4DH25 | IGKV3D-20 | Immunoglobulin kappa variable 3D-20 | |
| P06312 | IGKV4-1 | Immunoglobulin kappa variable 4-1 | |
| Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | |
| Q9Y535 | POLR3H | DNA-directed RNA polymerase III subunit RPC8 | |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | |
| Glycosaminoglycan binding | Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase |
| P01008 | SERPINC1 | Antithrombin-III | |
| P02749 | APOH | Beta-2-glycoprotein 1 | |
| O00622 | CCN1 | CCN family member 1 | |
| P47914 | RPL29 | 60S ribosomal protein L29 | |
| Glycosaminoglycan metabolic process | Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase |
| P19823 | ITIH2 | Inter-alpha-trypsin inhibitor heavy chain H2 | |
| P19827 | ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Faouder, J.; Guého, A.; Lavigne, R.; Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Com, E.; Wittrant, Y.; Pineau, C. Human Serum, Following Absorption of Fish Cartilage Hydrolysate, Promotes Dermal Fibroblast Healing through Anti-Inflammatory and Immunomodulatory Proteins. Biomedicines 2024, 12, 2132. https://doi.org/10.3390/biomedicines12092132
Le Faouder J, Guého A, Lavigne R, Wauquier F, Boutin-Wittrant L, Bouvret E, Com E, Wittrant Y, Pineau C. Human Serum, Following Absorption of Fish Cartilage Hydrolysate, Promotes Dermal Fibroblast Healing through Anti-Inflammatory and Immunomodulatory Proteins. Biomedicines. 2024; 12(9):2132. https://doi.org/10.3390/biomedicines12092132
Chicago/Turabian StyleLe Faouder, Julie, Aurélie Guého, Régis Lavigne, Fabien Wauquier, Line Boutin-Wittrant, Elodie Bouvret, Emmanuelle Com, Yohann Wittrant, and Charles Pineau. 2024. "Human Serum, Following Absorption of Fish Cartilage Hydrolysate, Promotes Dermal Fibroblast Healing through Anti-Inflammatory and Immunomodulatory Proteins" Biomedicines 12, no. 9: 2132. https://doi.org/10.3390/biomedicines12092132
APA StyleLe Faouder, J., Guého, A., Lavigne, R., Wauquier, F., Boutin-Wittrant, L., Bouvret, E., Com, E., Wittrant, Y., & Pineau, C. (2024). Human Serum, Following Absorption of Fish Cartilage Hydrolysate, Promotes Dermal Fibroblast Healing through Anti-Inflammatory and Immunomodulatory Proteins. Biomedicines, 12(9), 2132. https://doi.org/10.3390/biomedicines12092132






