Prognostic Values of Ferroptosis-Related Proteins ACSL4, SLC7A11, and CHAC1 in Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Patient Clinicopathological and Laboratory Data
2.3. Immunohistochemistry of the Tissue Microarray
2.4. Indirect ELISA of the CCA Sera
2.5. Statistical Analyses
3. Results
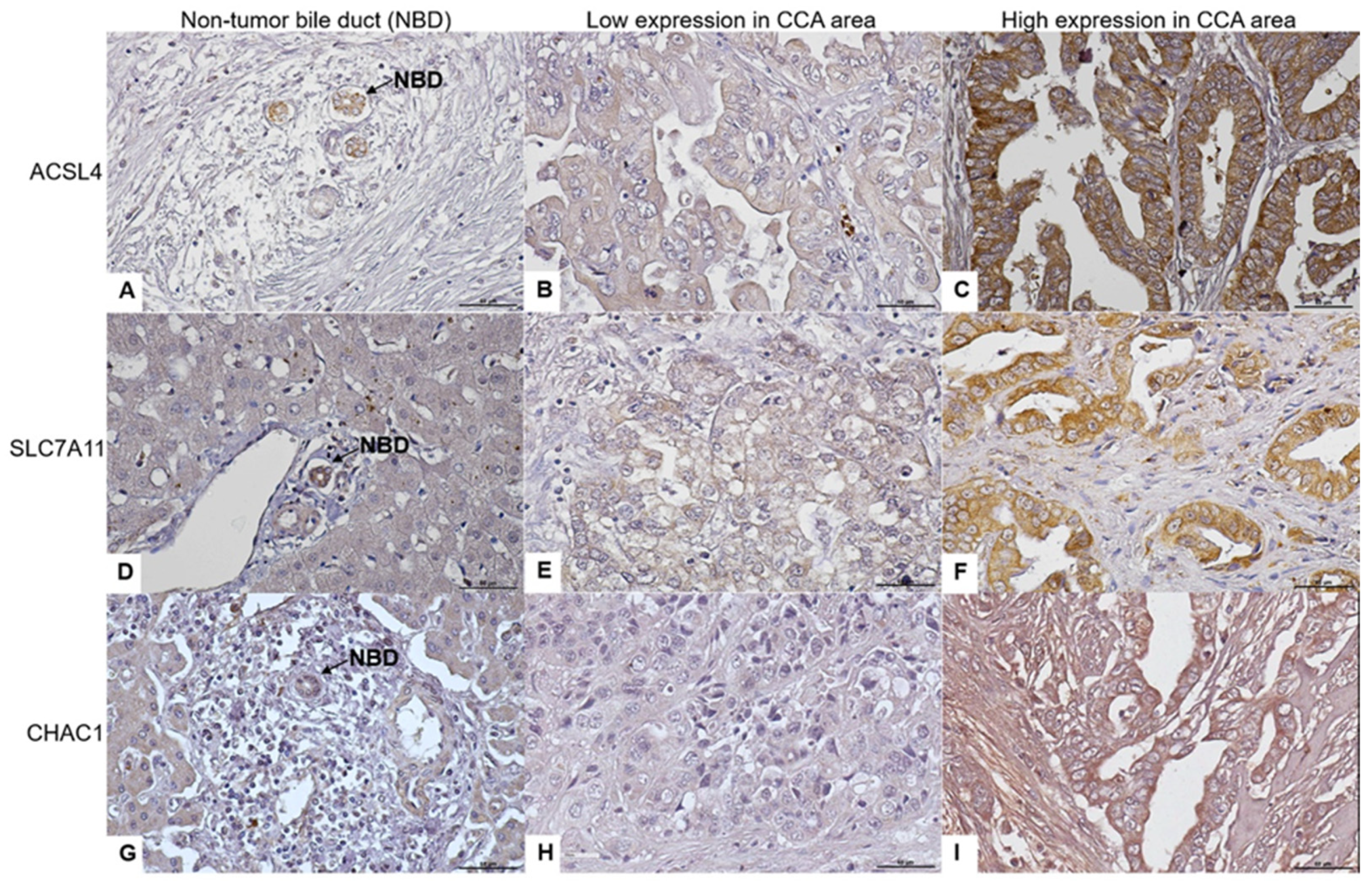
3.1. ACLS4, SLC7A11, and CHAC1 Protein Expression Levels in CCA Tissues
3.2. Association of ACLS4, SLC7A11, and CHAC1 Protein Levels with Clinicopathological Data and Laboratory Results
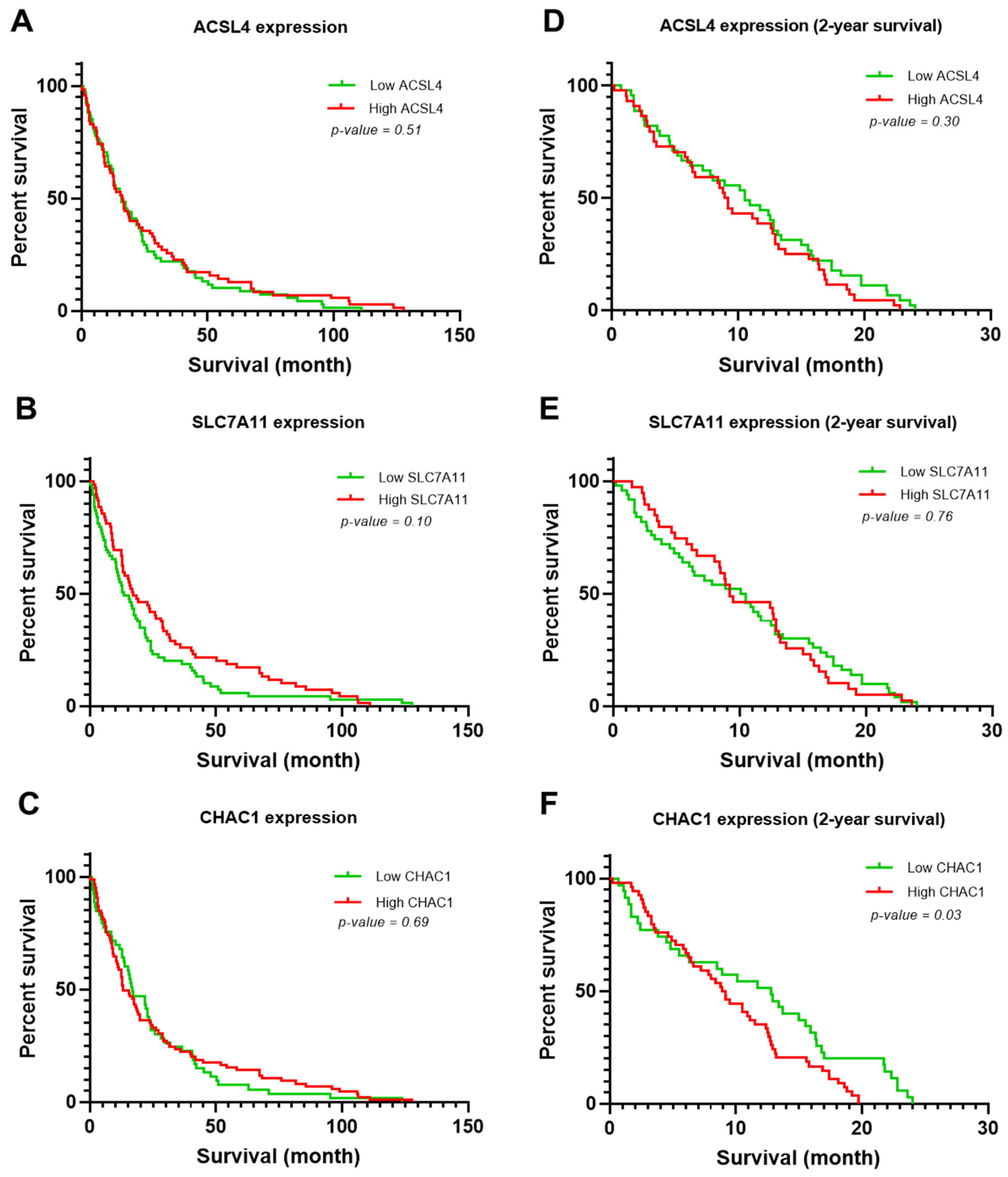
3.3. Clinicopathological Variables Potentially Associated with the Survival Rate of Patients with CCA
3.4. ACLS4 and SLC7A11 Levels in CCA Sera
3.5. Association of Clinicopathological Variables with ACSL4 and SLC7A11 Levels in CCA Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moriconi, F.; Ramadori, P.; Schultze, F.C.; Blaschke, M.; Amanzada, A.; Khan, S.; Ramadori, G. Characterization of the erythropoietin/erythropoietin receptor axis in a rat model of liver damage and cholangiocarcinoma development. Histochem. Cell Biol. 2012, 139, 473–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Luvira, V.; Nilprapha, K.; Bhudhisawasdi, V.; Pugkhem, A.; Chamadol, N.; Kamsa-Ard, S. Cholangiocarcinoma Patient Outcome in Northeastern Thailand: Single-Center Prospective Study. Asian Pac. J. Cancer Prev. 2016, 17, 401–406. [Google Scholar] [CrossRef]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2016, 21, 648–657. [Google Scholar] [CrossRef]
- Kimawaha, P.; Jusakul, A.; Junsawang, P.; Thanan, R.; Titapun, A.; Khuntikeo, N.; Techasen, A. Establishment of a Potential Serum Biomarker Panel for the Diagnosis and Prognosis of Cholangiocarcinoma Using Decision Tree Algorithms. Diagnostics 2021, 11, 589. [Google Scholar] [CrossRef]
- Kimawaha, P.; Thanan, R.; Jusakul, A.; Jamnongkan, W.; Silsirivanit, A.; Sa-Ngaimwibool, P.; Titapun, A.; Khuntikeo, N.; Sithithaworn, P.; Worasith, C.; et al. Serum α2,6-sialylated glycoform of serotransferrin as a glycobiomarker for diagnosis and prediction of clinical severity in cholangiocarcinoma. Clin. Chim. Acta 2022, 536, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Sirithawat, P.; Jusakul, A.; Kongpetch, S.; Thanee, M.; Srichanchara, P.; Panjaroensak, S.; Kimawaha, P.; Janthamala, S.; Aphivatanasiri, C.; Techasen, A. Alteration of STK11 Expression Associated With Cholangiocarcinoma Progression. In Vivo 2023, 37, 1638–1648. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Fujihara, K.M.; Zhang, B.Z.; Clemons, N.J. Opportunities for Ferroptosis in Cancer Therapy. Antioxidants 2021, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-F.; Zou, T.; Tuo, Q.-Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, P.-Z.; Zhu, G.-Z.; Hooi, S.C.; Wu, Y.; Huang, X.-W.; Dai, H.-Q.; Chen, P.-H.; Li, Z.-J.; Su, W.-J.; et al. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol. Sin. 2020, 42, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, H.; Lian, M.; Yue, C.; Dong, G.; Jin, Y.; Li, R.; Wan, H.; Wang, R.; Wang, Y.; et al. SLC7A11, a component of cysteine/glutamate transporter, is a novel biomarker for the diagnosis and prognosis in laryngeal squamous cell carcinoma. Oncol. Rep. 2017, 38, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2020, 12, 599–620. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Pagnon, J.; Kohannim, O.; Gargalovic, P.S.; Lusis, A.J. CHAC1/MGC4504 Is a Novel Proapoptotic Component of the Unfolded Protein Response, Downstream of the ATF4-ATF3-CHOP Cascade. J. Immunol. 2009, 182, 466–476. [Google Scholar] [CrossRef]
- Mamoor, S. CHAC1 is differentially expressed in central nervous system metastasis in human breast cancer. OSF Prepr. 2023. [Google Scholar] [CrossRef]
- He, S.; Zhang, M.; Ye, Y.; Zhuang, J.; Ma, X.; Song, Y.; Xia, W. ChaC glutathione specific γ-glutamylcyclotransferase 1 inhibits cell viability and increases the sensitivity of prostate cancer cells to docetaxel by inducing endoplasmic reticulum stress and ferroptosis. Exp. Ther. Med. 2021, 22, 1–9. [Google Scholar] [CrossRef]
- Yu, W.B.; Wang, L.M.; Liu, S.; Liu, Y.M.; Wang, S.B.; Sun, X.B. Combination of serum ACSL4 levels and low-dose 256-slice spiral CT exhibits the potential in the early screening of lung cancer. Medicine 2023, 102, e32733. [Google Scholar] [CrossRef]
- Zhan, Y.-F.; Zhang, J.; Wang, P. Changes and clinical significance of serum SLC7A11 in patients with hepatocellular carcinoma. Chin. Hepatol. 2022, 27, 1288–1291. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sa-Ngiamwibool, P.; Aphivatanasiri, C.; Sangkhamanon, S.; Intarawichian, P.; Kunprom, W.; Thanee, M.; Prajumwongs, P.; Loilome, W.; Khuntikeo, N.; Titapun, A.; et al. Modification of the AJCC/UICC 8th edition staging system for intrahepatic cholangiocarcinoma: Proposal for an alternative staging system from cholangiocarcinoma-prevalent Northeast Thailand. HPB 2022, 24, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Lu, Y.; Marchbanks, P.A.; Folger, S.G.; Strom, B.L.; McDonald, J.A.; Simon, M.S.; Weiss, L.K.; Malone, K.E.; Burkman, R.T.; et al. Quantitative measures of estrogen receptor expression in relation to breast cancer-specific mortality risk among white women and black women. Breast Cancer Res. 2013, 15, R90. [Google Scholar] [CrossRef]
- Zhou, R.-P.; Chen, Y.; Wei, X.; Yu, B.; Xiong, Z.-G.; Lu, C.; Hu, W. Novel insights into ferroptosis: Implications for age-related diseases. Theranostics 2020, 10, 11976–11997. [Google Scholar] [CrossRef]
- Chornyi, S.; Koster, J.; Ijlst, L.; Waterham, H.R. Studying the topology of peroxisomal acyl-CoA synthetases using self-assembling split sfGFP. Histochem. Cell Biol. 2024, 161, 133–144. [Google Scholar] [CrossRef]
- Liu, S.; Fan, S.; Wang, Y.; Chen, R.; Wang, Z.; Zhang, Y.; Jiang, W.; Chen, Y.; Xu, X.; Yu, Y.; et al. ACSL4 serves as a novel prognostic biomarker correlated with immune infiltration in Cholangiocarcinoma. BMC Cancer 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Dinarvand, N.; Khanahmad, H.; Hakimian, S.M.; Sheikhi, A.; Rashidi, B. Evaluation of long-chain acyl-coenzyme A synthetase 4 (ACSL4) expression in human breast cancer. Res. Pharm. Sci. 2020, 15, 48–56. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, C.-Y.; Hung, Y.-H.; Weng, T.-Y.; Yen, M.-C.; Lai, M.-D. Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer. PLoS ONE 2016, 11, e0155660. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-J.; Xu, G.-L. Overexpression of Acyl-CoA Ligase 4 (ACSL4) in Patients with Hepatocellular Carcinoma and its Prognosis. Med. Sci. Monit. 2017, 23, 4343–4350. [Google Scholar] [CrossRef]
- Toshida, K.; Itoh, S.; Iseda, N.; Tomiyama, T.; Yoshiya, S.; Toshima, T.; Liu, Y.; Iwasaki, T.; Okuzaki, D.; Taniguchi, K.; et al. Impact of ACSL4 on the prognosis of hepatocellular carcinoma: Association with cancer-associated fibroblasts and the tumour immune microenvironment. Liver Int. 2024, 44, 1011–1023. [Google Scholar] [CrossRef]
- Wu, X.; Deng, F.; Li, Y.; Daniels, G.; Du, X.; Ren, Q.; Wang, J.; Wang, L.H.; Yang, Y.; Zhang, V.; et al. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget 2015, 6, 44849–44863. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wang, Z.; Duan, R.; Yang, C.; Zhao, R.; Feng, Q.; Qin, Y.; Jiang, J.; Gu, S.; Lv, K.; et al. Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology 2021, 75, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Ling, J.; Zhuo, W.; Yu, Z.; Luo, Y.; Zhu, Y. Overexpression of SLC7A11: A novel oncogene and an indicator of unfavorable prognosis for liver carcinoma. Future Oncol. 2018, 14, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Oh, S.H.; Dutta, R.K.; Sun, T.; Yang, W.; Chi, J.A.; Diehl, A.M. Inhibiting xCT/SLC7A11 induces ferroptosis of myofibroblastic hepatic stellate cells but exacerbates chronic liver injury. Liver Int. 2021, 41, 2214–2227. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Wei, C.; Zheng, D.; Lu, X.; Yang, Y.; Luo, A.; Zhang, K.; Duan, X.; Wang, Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur. J. Pharm. Sci. 2020, 152, 105450. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, K.; Sun, L.; Yin, X.; Zhang, J.; Liu, C.; Li, B. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J. Transl. Med. 2021, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Maeda, K.; Ohtani, H.; Nagahara, H.; Shibutani, M.; Hirakawa, K. Expression of xCT as a predictor of disease recurrence in patients with colorectal cancer. Anticancer Res. 2015, 35, 677–682. [Google Scholar]
- Xu, J.; Zhao, L.; Zhang, X.; Ying, K.; Zhou, R.; Cai, W.; Wu, X.; Jiang, H.; Xu, Q.; Miao, D.; et al. Salidroside ameliorates acetaminophen-induced acute liver injury through the inhibition of endoplasmic reticulum stress-mediated ferroptosis by activating the AMPK/SIRT1 pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115331. [Google Scholar] [CrossRef]
- Mehta, V.; Meena, J.; Kasana, H.; Munshi, A.; Chander, H. Prognostic significance of CHAC1 expression in breast cancer. Mol. Biol. Rep. 2022, 49, 8517–8526. [Google Scholar] [CrossRef]
- Mehta, V.; Suman, P.; Chander, H. High levels of unfolded protein response component CHAC1 associates with cancer progression signatures in malignant breast cancer tissues. Clin. Transl. Oncol. 2022, 24, 2351–2365. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Xu, J.; Chen, L.; Xu, C.; Chen, F.; Xu, Z.; Zhang, Y.; Xia, S.; Shao, Y.; et al. Ferroptosis-related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J. Cell. Mol. Med. 2021, 25, 3610–3621. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Q.; Wang, Y. Serum ACSL4 levels in patients with ST-segment elevation myocardial infarction (STEMI) and its association with one-year major adverse cardiovascular events (MACE): A prospective cohort study. Medicine 2024, 103, e36870. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | ACSL4 Expression | SLC7A11 Expression | CHAC1 Expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low n, (%) | High n, (%) | p-Value | Low n, (%) | High n, (%) | p-Value | Low n, (%) | High n, (%) | p-Value | |
| Gender | 0.45 | 0.27 | 0.66 | ||||||
| Female | 15 (45.5%) | 18 (54.5%) | 21 (63.6%) | 12 (36.4%) | 12 (36.4%) | 21 (63.6%) | |||
| Male | 30 (53.6%) | 26 (46.4%) | 29 (51.8%) | 27 (48.2%) | 23 (41.1%) | 33 (58.9%) | |||
| Age (median, years) | 0.75 | 0.62 | 0.96 | ||||||
| <60 | 21 (48.8%) | 22 (51.2%) | 23 (53.5%) | 20 (46.5%) | 17 (39.5%) | 26 (60.5%) | |||
| ≥60 | 24 (52.2%) | 22 (47.8%) | 27 (58.7%) | 19 (41.3%) | 18 (39.1%) | 28 (60.9%) | |||
| Location of tumor | 0.04 | 0.24 | 0.40 | ||||||
| Intrahepatic | 38 (56.7%) | 29 (43.3%) | 40 (59.7%) | 27 (40.3%) | 28 (41.8%) | 39 (58.2%) | |||
| Extrahepatic | 7 (31.8%) | 15 (68.2%) | 10 (45.5%) | 12 (54.5%) | 7 (31.8%) | 15 (68.2%) | |||
| Tumor growth type | 0.03 | 0.12 | 0.38 | ||||||
| Intraductal type | 11 (73.3%) | 4 (26.7%) | 9 (60.0%) | 6 (40.0%) | 6 (40.0%) | 9 (60.0%) | |||
| Mass-forming type | 15 (57.7%) | 11 (42.3%) | 19 (73.1%) | 7 (26.9%) | 14 (53.8%) | 12 (46.2%) | |||
| Mixed type | 14 (36.8%) | 24 (63.2%) | 18 (47.4%) | 20 (52.6%) | 14 (36.8%) | 24 (63.2%) | |||
| Cell type | 0.45 | 0.30 | 0.51 | ||||||
| Papillary | 23 (54.8%) | 19 (45.2%) | 26 (61.9%) | 16 (38.1%) | 15 (35.7%) | 27 (64.3%) | |||
| Non-papillary | 22 (46.8%) | 25 (53.2%) | 24 (51.1%) | 23 (48.9%) | 20 (42.6%) | 27 (57.4%) | |||
| Lymph node metastasis | 0.93 | 0.17 | 0.85 | ||||||
| Yes | 29 (50.9% | 28 (49.1%) | 29 (50.9% | 28 (49.1%) | 22 (38.6% | 35 (61.4%) | |||
| No | 16 (50.0%) | 16 (50.0%) | 29 (50.9% | 28 (49.1%) | 13 (40.6%) | 19 (59.4%) | |||
| Distant metastasis | 0.52 | 0.50 | 0.15 | ||||||
| Yes | 4 (40.0%) | 6 (60.0%) | 7 (70.0%) | 3 (30.0%) | 6 (60.0%) | 4 (40.0%) | |||
| No | 41 (51.9%) | 38 (48.1%) | 43 (54.4%) | 36 (45.6%) | 29 (36.7%) | 50 (63.3%) | |||
| TMN stage | 0.95 | 0.63 | 0.61 | ||||||
| I–II | 9 (50.0%) | 9 (50.0%) | 11 (61.1%) | 7 (38.9%) | 8 (44.4%) | 10 (55.6%) | |||
| III–IV | 36 (50.7%) | 35 (49.3%) | 39 (54.9%) | 32 (45.1%) | 27 (38.0%) | 44 (62.0%) | |||
| OV infection | 0.65 | 0.52 | 0.33 | ||||||
| Yes | 34 (49.3%) | 35 (50.7%) | 40 (58%) | 29 (42%) | 29 (42.0%) | 40 (58.0%) | |||
| No | 34 (49.3%) | 35 (50.7%) | 10 (50%) | 10 (50%) | 6 (30.0%) | 14 (70.0%) | |||
| Total protein (g/dL) | 0.26 | 0.11 | 0.90 | ||||||
| <8.7 | 35 (47.3%) | 39 (52.7%) | 38 (51.4%) | 36 (48.6%) | 30 (40.5%) | 44 (59.5%) | |||
| ≥8.7 | 5 (71.4%) | 2 (28.6%) | 6 (85.7%) | 1 (14.3%) | 3 (42.9%) | 4 (57.1%) | |||
| Globulin (g/dL) | 0.82 | 0.42 | 0.40 | ||||||
| <3.4 | 16 (48.5%) | 17 (51.5%) | 16 (48.5%) | 17 (51.5%) | 15 (45.5%) | 18 (54.5%) | |||
| ≥3.4 | 24 (51.1%) | 23 (48.9%) | 27 (57.4%) | 20 (42.6%) | 17 (36.2%) | 30 (63.8%) | |||
| Total bilirubin (mg/dL) | 0.48 | 0.41 | 0.18 | ||||||
| <1.2 | 31 (51.7%) | 29 (48.3%) | 31 (51.7%) | 29 (48.3%) | 27 (45.0%) | 33 (55.0%) | |||
| ≥1.2 | 9 (42.9%) | 12 (57.1%) | 13 (61.9%) | 8 (38.1%) | 6 (28.6%) | 15 (71.4%) | |||
| Direct bilirubin (mg/dL) | 0.23 | 0.92 | 0.07 | ||||||
| <0.5 | 26 (54.2%) | 22 (45.8%) | 26 (54.2%) | 22 (45.8%) | 23 (47.9%) | 25 (52.1%) | |||
| ≥0.5 | 13 (40.6%) | 19 (59.4%) | 17 (53.1%) | 15 (46.9%) | 9 (28.1%) | 23 (71.9%) | |||
| ALT (U/L) | 0.05 | 0.46 | 0.06 | ||||||
| <33 | 20 (62.5%) | 12 (37.5%) | 19 (59.4%) | 13 (40.6%) | 17 (53.1%) | 15 (46.9%) | |||
| ≥33 | 20 (40.8%) | 29 (59.2%) | 25 (51.0%) | 24 (49.0%) | 16 (32.7%) | 33 (67.3%) | |||
| AST (U/L) | 0.22 | 0.97 | 0.10 | ||||||
| <40 | 19 (57.6%) | 14 (42.4%) | 18 (54.5%) | 15 (45.5%) | 17 (51.5%) | 16 (48.5%) | |||
| ≥40 | 21 (43.8%) | 27 (56.3%) | 26 (54.2%) | 22 (45.8%) | 16 (33.3%) | 32 (66.7%) | |||
| ALP (U/L) | 0.77 | 0.97 | 0.88 | ||||||
| <129 | 16 (53.3%) | 14 (46.7%) | 17 (56.7%) | 13 (43.3%) | 13 (43.3%) | 17 (56.7%) | |||
| ≥129 | 24 (50.0%) | 24 (50.0%) | 27 (56.3%) | 21 (43.8%) | 20 (41.7%) | 28 (58.3%) | |||
| Variable | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Age (≥60) | 0.81 | 0.53 | 1.23 | 0.33 | ||||
| Gender (Male) | 0.76 | 0.49 | 1.18 | 0.22 | ||||
| Location of tumor (Intrahepatic) | 0.96 | 0.59 | 1.57 | 0.90 | ||||
| Cell types (Papillary) | 0.52 | 0.34 | 0.82 | <0.01 | 0.57 | 0.36 | 0.90 | 0.01 |
| Tumor growth type (Mixed type) | 1.27 | 0.68 | 2.34 | 0.44 | ||||
| Lymph node metastasis (Yes) | 1.42 | 0.91 | 2.22 | 0.11 | ||||
| Distant metastasis (Yes) | 2.11 | 1.07 | 4.15 | 0.03 | 2.26 | 1.10 | 4.64 | 0.02 |
| TMN stage (III–IV) | 1.81 | 1.06 | 3.09 | 0.03 | 1.57 | 0.91 | 2.73 | 0.10 |
| OV infection (Positive) | 0.83 | 0.50 | 1.38 | 0.48 | ||||
| ACSL4 (High) | 1.24 | 0.81 | 1.90 | 0.30 | ||||
| SLC7A11 (High) | 1.06 | 0.69 | 1.62 | 0.77 | ||||
| CHAC1 (High) | 1.66 | 1.04 | 2.65 | 0.03 | 1.98 | 1.21 | 3.24 | <0.01 |
| Group Comparison | Protein | AUC (95% CI) | Cut-Off (OD) | Youden Index | Sensitivity (%) | Specificity (%) | LR | p-Value |
|---|---|---|---|---|---|---|---|---|
| Early CCA vs. Advanced CCA | ACSL4 | 0.80 (0.69–0.91) | 0.74 | 0.50 | 96.7 | 53.3 | 2.07 | <0.01 |
| SLC7A11 | 0.70 (0.67–0.90) | 0.90 | 0.54 | 67.7 | 86.6 | 5.08 | <0.01 |
| Characteristics | ACSL4 Expression | SLC7A11 Expression | ||||
|---|---|---|---|---|---|---|
| Low n, (%) | High n, (%) | p-Value | Low n, (%) | High n, (%) | p-Value | |
| Gender | 0.38 | 0.12 | ||||
| Female | 6 (22.2%) | 21 (77.8%) | 13 (48.1%) | 14 (51.9%) | ||
| Male | 11 (32.4%) | 23 (67.6%) | 23 (67.6%) | 11 (32.4%) | ||
| Age (median, years) | 0.57 | 0.34 | ||||
| <60 | 8 (47.1%) | 9 (52.9%) | 13 (76.5%) | 4 (23.5%) | ||
| ≥60 | 8 (57.1%) | 6 (42.9%) | 13 (92.9%) | 1 (7.1%) | ||
| TMN stage | <0.01 | <0.01 | ||||
| I–II | 16 (53.3%) | 14 (46.7%) | 26 (86.7%) | 4 (13.3%) | ||
| III–IV | 1 (3.2%) | 30 (96.8%) | 10 (32.3%) | 21 (67.7%) | ||
| Albumin (g/dL) | 0.11 | <0.01 | ||||
| <3.5 | 4 (16.0%) | 21 (84.0%) | 8 (32.0%) | 17 (68.0%) | ||
| ≥3.5 | 11 (34.4%) | 21 (65.6%) | 24 (75.0%) | 8 (25.0%) | ||
| Total bilirubin (mg/dL) | <0.01 | <0.01 | ||||
| <1.2 | 15 (57.7%) | 11 (42.3%) | 22 (84.6%) | 4 (15.4%) | ||
| ≥1.2 | 1 (3.1%) | 31 (96.9%) | 11 (34.4%) | 21 (65.6%) | ||
| Direct bilirubin (mg/dL) | <0.01 | <0.01 | ||||
| <0.5 | 14 (60.9%) | 9 (39.1%) | 19 (82.6%) | 4 (17.4%) | ||
| ≥0.5 | 2 (5.7%) | 33 (94.3%) | 14 (40.0%) | 21 (60.0%) | ||
| ALT (U/L) | 0.53 | 0.66 | ||||
| <33 | 6 (33.3%) | 12 (66.7%) | 11 (61.1%) | 7 (38.9%) | ||
| ≥33 | 10 (25.0%) | 30 (75.0%) | 22 (55.0%) | 18 (45.0%) | ||
| AST (U/L) | 0.05 | 0.09 | ||||
| <40 | 9 (42.9%) | 12 (57.1%) | 15 (71.4%) | 6 (28.6%) | ||
| ≥40 | 7 (18.9%) | 30 (81.1%) | 18 (48.6%) | 19 (515.4%) | ||
| ALP (U/L) | 0.05 | 0.17 | ||||
| <129 | 8 (47.1%) | 9 (52.9%) | 12 (70.6%) | 5 (29.4%) | ||
| ≥129 | 8 (19.5%) | 33 (80.5%) | 21 (51.2%) | 20 (48.8%) | ||
| CA19-9 (U/mL) | 0.02 | 0.13 | ||||
| <37 | 10 (58.8%) | 7 (41.2%) | 13 (76.5%) | 4 (23.5%) | ||
| ≥37 | 4 (21.1%) | 15 (78.9%) | 10 (52.6%) | 9 (47.4%) | ||
| CEA (ng/mL) | 0.93 | 0.46 | ||||
| <2.5 | 5 (38.5%) | 8 (61.5%) | 8 (61.5%) | 5 (38.5%) | ||
| ≥2.5 | 8 (40.0%) | 12 (60.0%) | 15 (75.0%) | 5 (25.0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amontailak, S.; Titapun, A.; Jusakul, A.; Thanan, R.; Kimawaha, P.; Jamnongkan, W.; Thanee, M.; Sirithawat, P.; Techasen, A. Prognostic Values of Ferroptosis-Related Proteins ACSL4, SLC7A11, and CHAC1 in Cholangiocarcinoma. Biomedicines 2024, 12, 2091. https://doi.org/10.3390/biomedicines12092091
Amontailak S, Titapun A, Jusakul A, Thanan R, Kimawaha P, Jamnongkan W, Thanee M, Sirithawat P, Techasen A. Prognostic Values of Ferroptosis-Related Proteins ACSL4, SLC7A11, and CHAC1 in Cholangiocarcinoma. Biomedicines. 2024; 12(9):2091. https://doi.org/10.3390/biomedicines12092091
Chicago/Turabian StyleAmontailak, Supakan, Attapol Titapun, Apinya Jusakul, Raynoo Thanan, Phongsaran Kimawaha, Wassana Jamnongkan, Malinee Thanee, Papitchaya Sirithawat, and Anchalee Techasen. 2024. "Prognostic Values of Ferroptosis-Related Proteins ACSL4, SLC7A11, and CHAC1 in Cholangiocarcinoma" Biomedicines 12, no. 9: 2091. https://doi.org/10.3390/biomedicines12092091
APA StyleAmontailak, S., Titapun, A., Jusakul, A., Thanan, R., Kimawaha, P., Jamnongkan, W., Thanee, M., Sirithawat, P., & Techasen, A. (2024). Prognostic Values of Ferroptosis-Related Proteins ACSL4, SLC7A11, and CHAC1 in Cholangiocarcinoma. Biomedicines, 12(9), 2091. https://doi.org/10.3390/biomedicines12092091






