The Contribution of the Brain–Gut Axis to the Human Reward System
Abstract
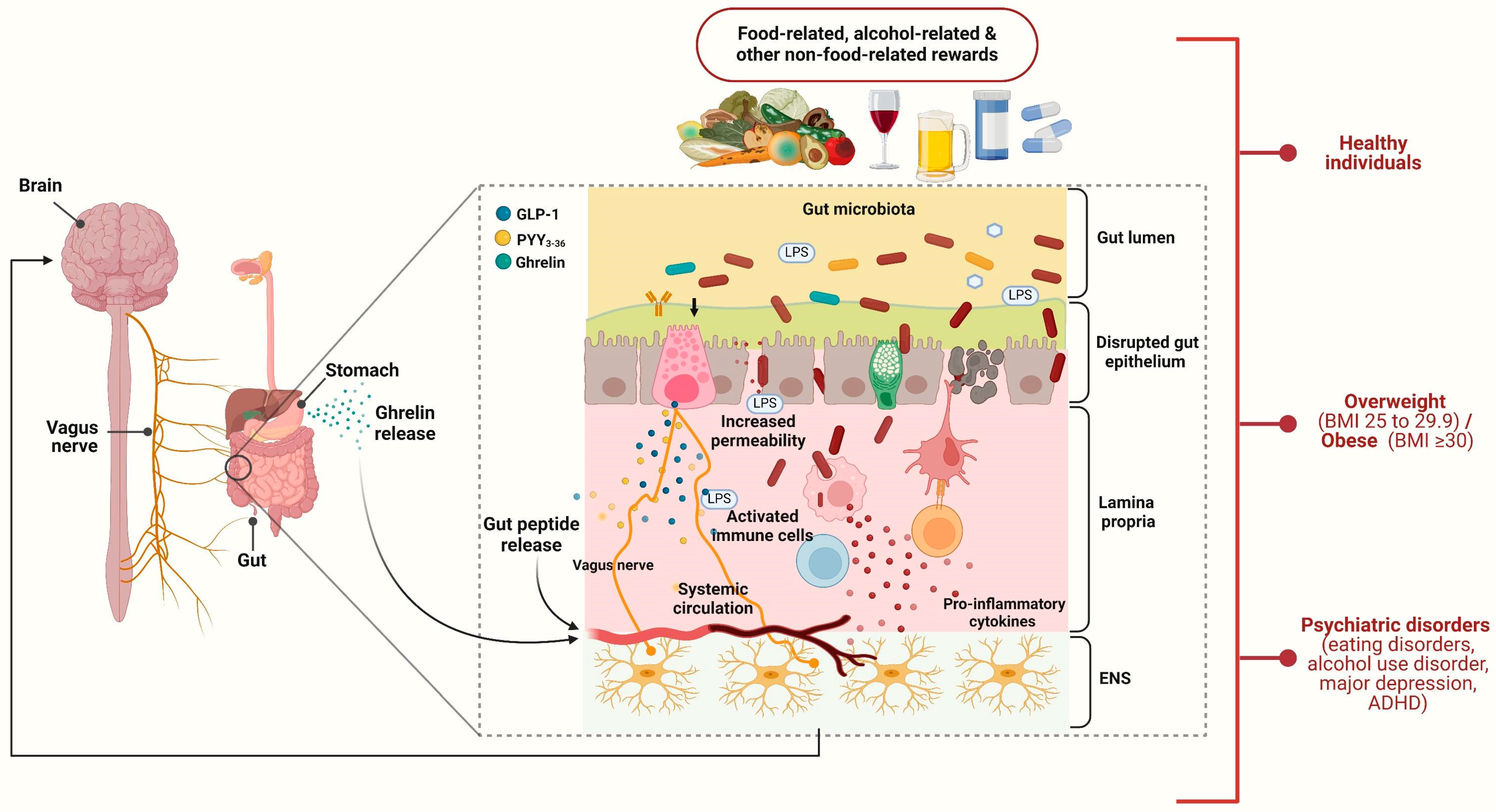
1. Introduction
2. Characteristics of Studies
3. Healthy Subjects
4. Obesity
5. Psychiatric Disorders
5.1. Alcohol-Use Disorder
5.2. Attention Deficit Hyperactivity Disorder and Depression
5.3. Eating Disorders
6. Discussion
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Bayassi-Jakowicka, M.; Lietzau, G. Neuroplasticity and Multilevel System of Connections Determine the Integrative Role of Nucleus Accumbens in the Brain Reward System. Int. J. Mol. Sci. 2021, 22, 9806. [Google Scholar] [CrossRef] [PubMed]
- García-Cabrerizo, R.; Carbia, C.; KJ, O.R. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef]
- Pfabigan, D.M.; Frogner, E.R. Ghrelin is related to lower brain reward activation during touch. Psychophysiology 2024, 61, e14443. [Google Scholar] [CrossRef]
- Sailer, U.; Riva, F.; Lieberz, J.; Campbell-Meiklejohn, D.; Scheele, D.; Pfabigan, D.M. Hungry for compliments? Ghrelin is not associated with neural responses to social rewards or their pleasantness. Front. Psychiatry 2023, 14, 1104305. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Guan, M.; Mayer, E.A.; Stains, J.; Liu, C.; Vora, P.; Jacobs, J.P. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes 2022, 14, 2051999. [Google Scholar] [CrossRef]
- Klausen, M.K.; Jensen, M.E.; Møller, M.; Le Dous, N.; Jensen, A.; Zeeman, V.A.; Johannsen, C.F.; Lee, A.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 2022, 7, e159863. [Google Scholar] [CrossRef]
- Bernardoni, F.; Bernhardt, N.; Pooseh, S.; King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roessner, V.; Smolka, M.N.; et al. Metabolic state and value-based decision-making in acute and recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2020, 45, 253–261. [Google Scholar] [CrossRef]
- Bogdanov, V.B.; Bogdanova, O.V.; Dexpert, S.; Delgado, I.; Beyer, H.; Aubert, A.; Dilharreguy, B.; Beau, C.; Forestier, D.; Ledaguenel, P.; et al. Reward-related brain activity and behavior are associated with peripheral ghrelin levels in obesity. Psychoneuroendocrinology 2020, 112, 104520. [Google Scholar] [CrossRef]
- Dong, T.S.; Mayer, E.A. A Distinct Brain-Gut-Microbiome Profile Exists for Females with Obesity and Food Addiction. Obesity 2020, 28, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Gupta, A.; Jacobs, J.P. Improvement in Uncontrolled Eating Behavior after Laparoscopic Sleeve Gastrectomy Is Associated with Alterations in the Brain-Gut-Microbiome Axis in Obese Women. Nutrients 2020, 12, 2924. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.K.W.; Dong, T.S. Understanding the Heterogeneity of Obesity and the Relationship to the Brain-Gut Axis. Nutrients 2020, 12, 3701. [Google Scholar] [CrossRef]
- Cerit, H.; Christensen, K.; Moondra, P.; Klibanski, A.; Goldstein, J.M.; Holsen, L.M. Divergent associations between ghrelin and neural responsivity to palatable food in hyperphagic and hypophagic depression. J. Affect. Disord. 2019, 242, 29–38. [Google Scholar] [CrossRef]
- Farokhnia, M.; Grodin, E.N.; Lee, M.R.; Oot, E.N.; Blackburn, A.N.; Stangl, B.L.; Schwandt, M.L.; Farinelli, L.A.; Momenan, R.; Ramchandani, V.A.; et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol. Psychiatry 2018, 23, 2029–2038. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e623. [Google Scholar] [CrossRef]
- Osadchiy, V.; Labus, J.S.; Gupta, A.; Jacobs, J.; Ashe-McNalley, C.; Hsiao, E.Y.; Mayer, E.A. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 2018, 13, e0201772. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Ly, H.G.; Dupont, P.; Van Laere, K.; Depoortere, I.; Tack, J.; Van Oudenhove, L. Differential brain responses to gradual intragastric nutrient infusion and gastric balloon distension: A role for gut peptides? Neuroimage 2017, 144, 101–112. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef]
- Maria Monteleone, A.; Monteleone, P.; Dalle Grave, R.; Nigro, M.; El Ghoch, M.; Calugi, S.; Cimino, M.; Maj, M. Ghrelin response to hedonic eating in underweight and short-term weight restored patients with anorexia nervosa. Psychiatry Res. 2016, 235, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Suchankova, P.; Yan, J.; Schwandt, M.L.; Stangl, B.L.; Caparelli, E.C.; Momenan, R.; Jerlhag, E.; Engel, J.A.; Hodgkinson, C.A.; Egli, M.; et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: Evidence from human genetic association studies and a mouse model of alcohol dependence. Transl. Psychiatry 2015, 5, e583. [Google Scholar] [CrossRef]
- Leggio, L.; Zywiak, W.H.; Fricchione, S.R.; Edwards, S.M.; de la Monte, S.M.; Swift, R.M.; Kenna, G.A. Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: A preliminary investigation. Biol. Psychiatry 2014, 76, 734–741. [Google Scholar] [CrossRef]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef]
- Sun, X.; Veldhuizen, M.G.; Wray, A.E.; de Araujo, I.E.; Sherwin, R.S.; Sinha, R.; Small, D.M. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol. Behav. 2014, 136, 63–73. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, L.; RG, I.J.; Ten Kulve, J.S.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Puzziferri, N.; Zigman, J.M.; Thomas, B.P.; Mihalakos, P.; Gallagher, R.; Lutter, M.; Carmody, T.; Lu, H.; Tamminga, C.A. Brain imaging demonstrates a reduced neural impact of eating in obesity. Obesity (Silver Spring) 2016, 24, 829–836. [Google Scholar] [CrossRef]
- Cassioli, E.; Rossi, E.; Squecco, R.; Baccari, M.C.; Maggi, M.; Vignozzi, L.; Comeglio, P.; Gironi, V.; Lelli, L.; Rotella, F.; et al. Reward and psychopathological correlates of eating disorders: The explanatory role of leptin. Psychiatry Res. 2020, 290, 113071. [Google Scholar] [CrossRef]
- Zald, D.H.; Treadway, M.T. Reward Processing, Neuroeconomics, and Psychopathology. Annu. Rev. Clin. Psychol. 2017, 13, 471–495. [Google Scholar] [CrossRef]
- Jerlhag, E. The therapeutic potential of glucagon-like peptide-1 for persons with addictions based on findings from preclinical and clinical studies. Front. Pharmacol. 2023, 14, 1063033. [Google Scholar] [CrossRef]
- Tufvesson-Alm, M.; Shevchouk, O.T.; Jerlhag, E. Insight into the role of the gut-brain axis in alcohol-related responses: Emphasis on GLP-1, amylin, and ghrelin. Front. Psychiatry 2022, 13, 1092828. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Sternat, T.; Katzman, M.A. Neurobiology of hedonic tone: The relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatr. Dis. Treat. 2016, 12, 2149–2164. [Google Scholar] [CrossRef]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.A.; Brown, T.A.; Lavender, J.M.; Lopez, E.; Wierenga, C.E.; Kaye, W.H. Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: Beyond leptin and ghrelin. Mol. Cell Endocrinol. 2019, 497, 110320. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Berner, L.A.; Attia, E. Cognitive Neuroscience of Eating Disorders. Psychiatr. Clin. N. Am. 2019, 42, 75–91. [Google Scholar] [CrossRef]
| Brain–gut axis synonyms and related terms | Reward system synonyms and related terms |
| Brain–gut axis, intestinal microbiota, microbiota metabolites, gut-derived hormones, ghrelin, glucagon-like peptide 1, PYY3–36 | Reward system, reward processing, reward network, pleasure-seeking behaviors |
| Author | Ref. | Country | Publication Year | Demographic and Clinical Characteristics of Participants | Design | Methodology |
|---|---|---|---|---|---|---|
| Pfabigan et al. | [5] | Norway and Sweden | 2024 | A total of 68 healthy volunteers (47 males); race: 66 of European descent and 2 of Asian descent; age: 18–55 yrs | CS | ELISA and brain fMRI during a CT-targeted touch task |
| Sailer et al. | [6] | Norway, Germany, and the UK | 2023 | A total of 68 healthy volunteers (47 males); race: 66 of European descent and 2 of Asian descent; age: 18–55 yrs | CS | ELISA and brain fMRI during a social-recognition-by-experts task and a social affirmation task |
| Dong et al. | [7] | The USA | 2022 | A total of 81 obese and 216 normal or overweight individuals; gender: 99 males; race: 110 Non-Hispanic White, 92 Hispanic, 65 Asian, 24 Black, and 6 Native American; age: 21–41.5 yrs | CS | 16s RNA gene sequencing, metabolite analysis, and brain structural MRI and fMRI |
| Klausen et al. | [8] | Denmark, the USA, and Germany | 2022 | A total of 127 patients with AUD (76 males), with 62 randomized to exenatide and 65 randomized to a placebo; race: all White; mean age: 52 yrs | RCT | Symptom questionnaires, brain fMRI, and SPECT DAT scan |
| Bernardoni et al. | [9] | Germany | 2020 | A total of 94 acutely underweight female AN patients, 37 recovered female AN patients, and 119 female healthy controls; mean age: 16.1–22.2 yrs | CS | ELISA, brain MRI, delay discounting task, risk aversion for probabilistic gains (PDGs), and probabilistic losses tests |
| Bogdanov et al. | [10] | France, the USA, and Italy | 2020 | A total of 15 severely obese subjects (3 males) and 15 non-obese healthy controls (2 males); mean age: 37–38.7 yrs | CS | ELISA and brain fMRI during a guessing task |
| Dong et al. | [11] | The USA | 2020 | A total of 86 obese females with FA and 19 obese females without FA; race: 41 Hispanic, 28 Caucasian, 13 African American, 21 Asian, and 2 Other; age: 18–50 yrs | CS | 16s ribosomal RNA gene sequencing, metabolomics, and brain MRI |
| Dong et al. | [12] | The USA | 2020 | A total of 18 female obese patients who underwent LSG; race: 8 Non-Hispanic White, 2 African American, 2 Asian, and 6 Hispanic; age: 18–55 yrs | Prospective trial | 16s ribosomal RNA gene sequencing, mass spectrometry, and brain structural and rs-fMRI |
| Hung et al. | [13] | The USA | 2020 | A total of 130 overweight or obese individuals (43 males); race: 52 Hispanic and 78 Non-Hispanic; age: 18–60 yrs | CS | 16s ribosomal RNA sequencing, mass spectroscopy, and brain structural MRI |
| Cerit et al. | [14] | The USA | 2019 | A total of 10 female hyperphagic MDD patients in remission, 18 female hypophagic MDD patients in remission, and 18 healthy controls; age: 22–42 yrs | CS | Brain fMRI during exposure to food pictures and radioimmunoassay |
| Farokhnia et al. | [15] | The USA | 2018 | A total of 11 heavy drinkers (8 males); race: 9 African American; mean age: 40 yrs | Double-blind RCT | Brain fMRI |
| Han et al. | [16] | Canada | 2018 | A total of 38 healthy subjects (21 males), who were administered either IV ghrelin or saline; mean age: 22.5 yrs | Single-blind, counterbalanced prospective trial | Brain fMRI during a food odor-conditioning task |
| Osadchiy et al. | [17] | The USA | 2018 | A total of 63 healthy individuals (29 males); age: 18–60 yrs | CS | Mass spectrometry, brain structural MRI, brain functional MRI, and diffusion-weighted MRI |
| Aarts et al. | [18] | The Netherlands | 2017 | A total of 19 ADHD patients (13 males) and 77 controls (41 males); mean age: 19.1–27.5 yrs | CS | 16s ribosomal RNA gene sequencing fMRI |
| Ly et al. | [19] | Belgium | 2017 | A total of 15 healthy volunteers (8 males); mean age: 27.3 yrs | Prospective counterbalanced trial | RIA and H215O-PET after a balloon- and nutrient-induced distension |
| Byrne et al. | [20] | The UK | 2016 | A total of 20 healthy non-obese men; race: 18 European Caucasian; age: 18–65 yrs | RCT | fMRI |
| Monteleone et al. | [21] | Italy | 2016 | A total of 7 underweight AN patients (1 male), 7 weight-restored AN patients (2 males), and 7 healthy controls (2 males); age: 18–35 yrs | Prospective trial | Enzyme immunoassay |
| Suchankova et al. | [22] | Sweden and the USA | 2015 | A total of 84 nondependent drinkers (37 males); race: 72 Caucasian and 12 African American; age: 21–44 yrs | CS | Exposure to a breath alcohol concentration test and brain fMRI during the a Monetary Incentive Delay task |
| Leggio et al. | [23] | The USA | 2014 | A total of 45 heavy alcohol drinkers (9 males) randomized to ghrelin or a placebo; race: 14 Black, 24 White, 2 Latino, and 5 Other; age: 25–62 yrs | RCT | Cue-reactivity procedure and AVAS |
| Scholtz et al. | [24] | Ireland and the UK | 2014 | A total of 21 RYGB (4 males), 20 BAND (1 male), and 20 BMI-matched unoperated controls (3 males); race: 41 European Caucasian; age: 20–59 yrs | CS | Brain structural MRI and fMRI during exposure to food pictures, radioimmunoassays, ELISA, and mass spectroscopy |
| Sun et al. | [25] | The USA and Germany | 2014 | A total of 32 healthy subjects (14 males); age: 18–39 yrs | CS | Brain fMRI after food delivery and radioimmunoassays |
| van Bloemendaal et al. | [26] | The Netherlands | 2014 | A total of 16 obese T2DM subjects (8 males), 16 normoglycemic obese subjects (8 males), and 16 healthy lean individuals (8 males); race: all Caucasian; age: 40–70 yrs | RCT | fMRI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaivazoglou, K.; Aggeletopoulou, I.; Triantos, C. The Contribution of the Brain–Gut Axis to the Human Reward System. Biomedicines 2024, 12, 1861. https://doi.org/10.3390/biomedicines12081861
Karaivazoglou K, Aggeletopoulou I, Triantos C. The Contribution of the Brain–Gut Axis to the Human Reward System. Biomedicines. 2024; 12(8):1861. https://doi.org/10.3390/biomedicines12081861
Chicago/Turabian StyleKaraivazoglou, Katerina, Ioanna Aggeletopoulou, and Christos Triantos. 2024. "The Contribution of the Brain–Gut Axis to the Human Reward System" Biomedicines 12, no. 8: 1861. https://doi.org/10.3390/biomedicines12081861
APA StyleKaraivazoglou, K., Aggeletopoulou, I., & Triantos, C. (2024). The Contribution of the Brain–Gut Axis to the Human Reward System. Biomedicines, 12(8), 1861. https://doi.org/10.3390/biomedicines12081861








