Dropped Head Syndrome: The Importance of Neurophysiology in Distinguishing Myasthenia Gravis from Parkinson’s Disease
Abstract
1. Introduction
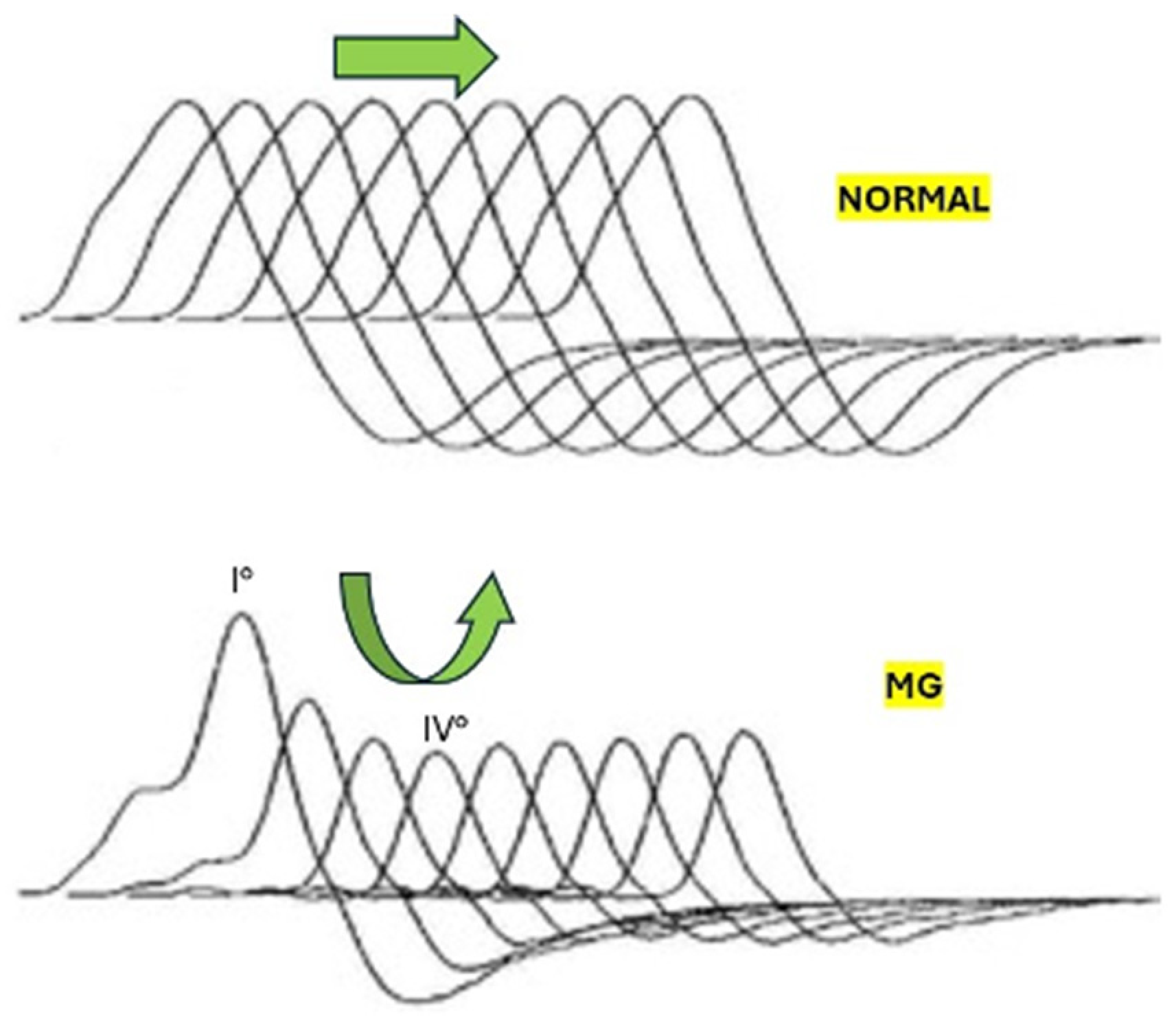
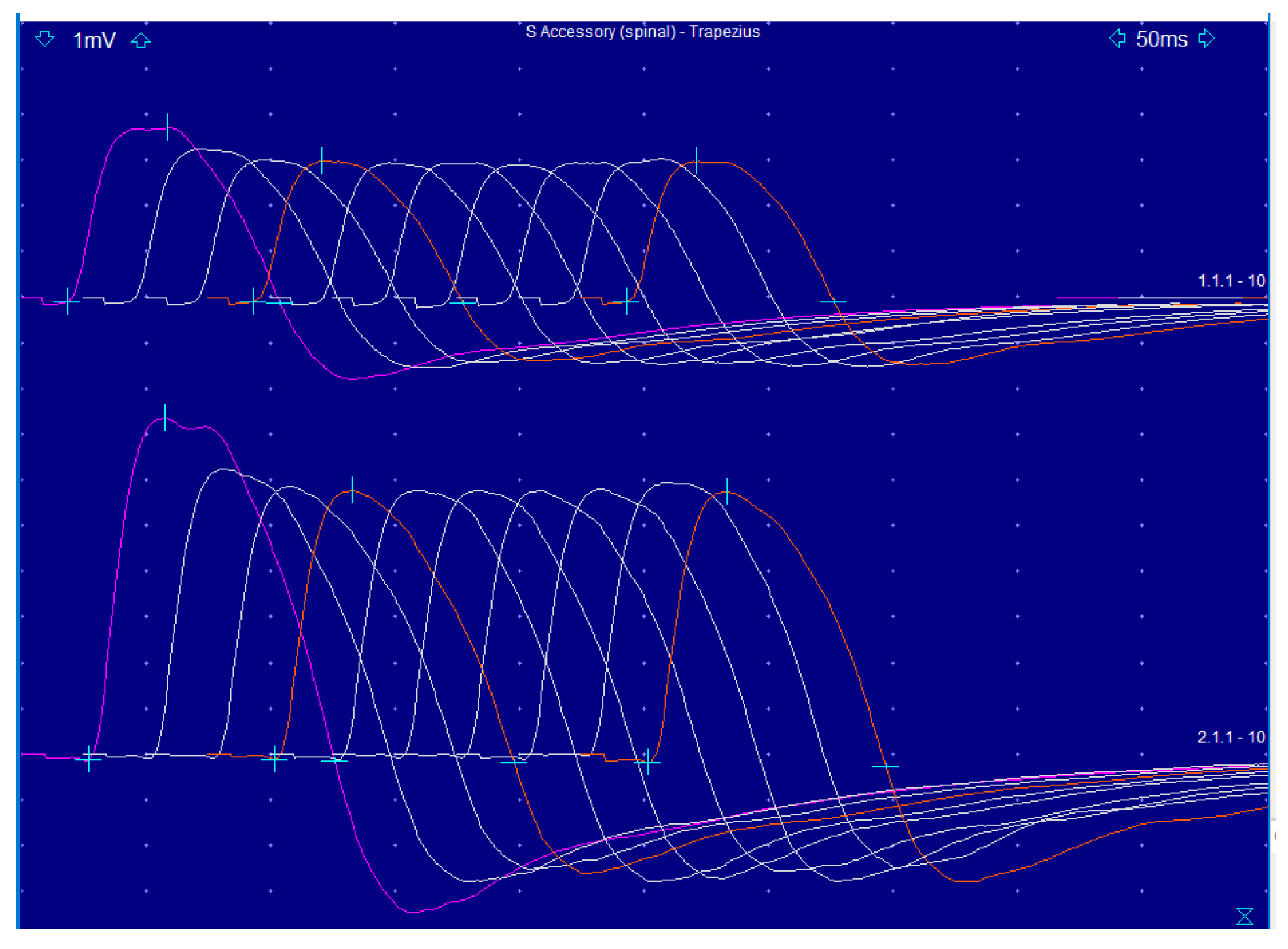
2. Detailed Case Description
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, A.R.; Reddy, R.; Fehlings, M.G. Dropped head syndrome: Diagnosis and management. Evid.-Based Spine-Care J. 2011, 2, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.D. Head drop and camptocormia. J. Neurol. Neurosurg. Psychiatry 2003, 74, 692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doherty, K.M.; van de Warrenburg, B.P.; Peralta, M.C.; Silveira-Moriyama, L.; Azulay, J.P.; Gershanik, O.S.; Bloem, B.R. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011, 10, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Cauchi, M.; Marsh, E. A practical approach to the patient presenting with dropped head. Pract. Neurol. 2016, 16, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Evoli, A.; Piano, C.; Tonali, P.A.; Bentivoglio, A.R. Myasthenia gravis: An unrecognized cause of head drop in Parkinson’s disease. Park. Relat. Disord. 2008, 14, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Bologna, M.; Kojovic, M.; Berardelli, A.; Espay, A.J.; Colosimo, C. Dystonia in atypical parkinsonian disorders. Park. Relat. Disord. 2019, 66, 25–33. [Google Scholar] [CrossRef]
- Datta, N.; Hoke, A. Repetitive Nerve Stimulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Berrih-Aknin, S.; Frenkian-Cuvelier, M.; Eymard, B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J. Autoimmun. 2014, 48–49, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Verschuuren, J.J.; Huijbers, M.G.; Plomp, J.J.; Niks, E.H.; Molenaar, P.C.; Martinez-Martinez, P.; Gomez, A.M.; De Baets, M.H.; Losen, M. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun. Rev. 2013, 12, 918–923. [Google Scholar] [CrossRef]
- Leite Schetino, L.P.; de Castro Fonseca, M.; Magalhães Gomes, M.P.S.; Costa Valadão, P.A.; de Camargo, W.L.; Rodrigues, H.A.; Andrade, J.N.; Arantes-Costa, F.M.; Naves, L.A.; Prado, C.M.; et al. Evaluation of the neuromuscular junction in a middle-aged mouse model of congenital myasthenic syndrome. Muscle Nerve 2019, 60, 790–800. [Google Scholar] [CrossRef]
- Tannemaat, M.R.; Huijbers, M.G.; Verschuuren, J. Myasthenia gravis—Pathophysiology, diagnosis, and treatment. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2024; Volume 200, pp. 283–305. [Google Scholar] [CrossRef]
- Mantegazza, R.; Vanoli, F.; Frangiamore, R.; Cavalcante, P. Complement Inhibition for the Treatment of Myasthenia Gravis. Immunotargets Ther. 2020, 9, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.H.; Lindstrom, J.M.; Lennon, V.A. End-plate potentials in experimental autoimmune myasthenia gravis in rats. Ann. N. Y. Acad. Sci. 1976, 274, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Unal-Cevik, I.; Temucin, C.M. Head drop in an elder Parkinson’s disease after development of myasthenia gravis. Mov. Disord. 2009, 24, 2025–2026. [Google Scholar] [CrossRef] [PubMed]
- Brázdil, M.; Fojtíková, D.; Kost’álová, E.; Bares, M.; Kuba, R.; Pazourková, M.; Rektor, I. Dropped head syndrome in severe intractable epilepsies with mental retardation. Seizure 2005, 14, 282–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillai, K.S.; Maramattom, B.V.; Santhamma, S.G.; Kishore, A. Head Drop and Trunk Flexion as an Early Manifestation of Anti-IgLON5 Disease. Mov. Disord. Clin. Pract. 2023, 10, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Tatu, L.; Pandey, S.; Sławek, J.; Drużdż, A.; Biering-Sørensen, B.; Altmann, C.F.; Kreisler, A. Frequency of different subtypes of cervical dystonia: A prospective multicenter study according to Col-Cap concept. J. Neural Transm. 2020, 127, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Piepgras, J.; Höltje, M.; Michel, K.; Li, Q.; Otto, C.; Drenckhahn, C.; Probst, C.; Schemann, M.; Jarius, S.; Stöcker, W.; et al. Anti-DPPX encephalitis: Pathogenic effects of antibodies on gut and brain neurons. Neurology 2015, 85, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Lang, A.E. Parkinson Diseases in the 2020s and Beyond: Replacing Clinico-Pathologic Convergence with Systems Biology Divergence. J. Park. Dis. 2018, 8, S59–S64. [Google Scholar] [CrossRef]
- Zis, P.; Argiriadou, V.; Temperikidis, P.P.; Zikou, L.; Tzartos, S.J.; Tavernarakis, A. Parkinson’s disease associated with myasthenia gravis and rheumatoid arthritis. Neurol. Sci. 2014, 35, 797–799. [Google Scholar] [CrossRef]
- Marano, M.; Lanzone, J.; di Biase, L.; Pepe, A.; Di Santo, A.; Di Lazzaro, V. A rare cause of axial worsening in Parkinson’s disease: A case of myasthenic pseudo-parkinsonism. Clin. Neurol. Neurosurg. 2019, 179, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Nie, Q.; Xue, Z.; Li, Z. Coexistence of Parkinson’s disease and myasthenia gravis: A case report and literature review. Exp. Ther. Med. 2024, 28, 282. [Google Scholar] [CrossRef] [PubMed]
- Gamez, J.; Carmona, F.; Lorenzo-Bosquet, C.; Cuberas-Borrós, G.; de Fabregues, O.; Gamez, A. Myasthenia gravis concurrent with Parkinson’s disease in a Spanish cohort. Causation or correlation? Neurol. Sci. 2024, 45, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Marsili, L.; Truong, D.D. Parkinsonism in Viral, Paraneoplastic, and Autoimmune Diseases. J. Neurol. Sci. 2021, 433, 120014. [Google Scholar] [CrossRef] [PubMed]
- Koutsilieri, E.; Lutz, M.B.; Scheller, C. Autoimmunity, dendritic cells and relevance for Parkinson’s disease. J. Neural Transm. 2013, 120, 75–81. [Google Scholar] [CrossRef]
- Xie, H.; Liu, Y.; Schmidt, C.; Chien, J.H.; Wang, C. Therapeutic Effect and Side Effects of Pharmacotherapy in Patients with Parkinson Disease and Myasthenia Gravis: A Systematic Review of Case Reports and Case Series Studies. Clin. Ther. 2024, 46, 275–284. [Google Scholar] [CrossRef]
- Espay, A.J.; Marsili, L.; Mahajan, A.; Sturchio, A.; Pathan, R.; Pilotto, A.; Elango, D.S.; Pezous, N.; Masellis, M.; Gomez-Mancilla, B. Rivastigmine in Parkinson’s Disease Dementia with Orthostatic Hypotension. Ann. Neurol. 2021, 89, 91–98. [Google Scholar] [CrossRef]
| Condition | Diagnosis | Clinical Signs | Notes | References |
|---|---|---|---|---|
| Dropped head syndrome | ||||
| Myasthenia Gravis | EMG, Ach/mUSK antibodies | Dysphagia, dysphonia, weakness, fluctuating fatigue | Responds to pyridostigmine and plasmapheresis | [5,16] |
| Motor Neuron Disease (ALS, PPS) * | EMG | Hyperreflexia, Babinski, weakness, fasciculations | Can have dysphonia and dysphagia | [3] |
| Myopathy (PM, IBM, NM, MM, CD, FSHD, Radiation-induced, AED-induced carnitine deficiency) | Muscle biopsy | Weakness | LGMD, FSHD, IBM, other myositis, amyloid, thyroid myopathy, pay attention to epileptic patients taking valproic acid | [3,17] |
| IGLON-5 Disease | IGLON-5 antibodies | Weakness, bulbar symptoms, sleep disorders | Tauopathy, rarely responds to immune therapy, severe prognosis | [18] |
| Antecollis | ||||
| Parkinson’s Disease | Clinical | Bradykinesia plus at least one between tremor, rigidity, postural instability | Responds to levodopa/carbidopa | [6] |
| Multiple System Atrophy | Clinical | Dysautonomia, atypical Parkinsonism, cerebellar symptoms | More frequently displays antecollis than idiopathic PD | [7] |
| Idiopathic Cervical Dystonia * | Clinical | Sensory trick | Responds to botulinum toxin injections in anterior neck muscle groups and anticholinergic drugs | [19] |
| DPPX-Encephalopathy | DPPX antibodies | Weight loss, dysautonomia, GI symptoms | Responds to immune therapies | [20] |
| Condition | Treatment | Notes |
|---|---|---|
| Dropped head syndrome | ||
| Myasthenia Gravis | IVIG, PLEX, Steroids | May improve/resolve |
| Motor Neuron Disease (ALS, PPS) | - | Consider neck brace, treat dysphagia if associated |
| Myopathy (PM, IBM, NM, MM, CD, FSHD, Radiation-induced, and AED-induced carnitine deficiency) | Consider steroids in responsive forms; discontinue valproic acid in epileptic patients | Consider neck brace, physical therapy |
| IGLON-5 Disease | IVIG, PLEX, Steroids | Weak response |
| Antecollis | ||
| Parkinson’s Disease | Discontinue dopamine-agonists or other recently added treatments; consider levodopa increase and muscle relaxants | Physical therapy, consider BoNT injections in anterior neck muscles |
| Multiple System Atrophy | Same as Parkinson’s disease | - |
| Idiopathic Cervical Dystonia | Consider BoNT injections in anterior neck muscles and anticholinergics | Effective, but increased risk of dysphagia |
| DPPX-Encephalopathy | IVIG, PLEX, Steroids | May improve |
| Treatment | Description | Mechanism of Action | Notes |
|---|---|---|---|
| Acetylcholinesterase inhibitors | Medications that improve communication between nerves and muscles | Inhibit breakdown of acetylcholine, increasing its availability at neuromuscular junctions | First-line treatment; includes pyridostigmine. May cause gastrointestinal side effects |
| Corticosteroids | Anti-inflammatory drugs used to suppress immune response | Reduce autoantibody production and inflammation | Effective for moderate to severe MG; long-term use can cause significant side effects like osteoporosis and diabetes |
| Immunosuppressants | Drugs that suppress the immune system to reduce autoantibody production | Target immune cells to decrease autoantibody levels | Include azathioprine, mycophenolate mofetil, and cyclosporine. Regular monitoring required due to risk of infection and other side effects |
| Plasma exchange | Procedure aiming to remove antibodies from the blood. | Direct removal of circulating autoantibodies | Used in acute exacerbations or crisis situations. Short-term relief; risk of complications like infection or blood clots |
| Intravenous immunoglobulins (IVIG) | Infusion of antibodies from donated blood to modulate the immune system | Provides normal antibodies to alter immune response and neutralize autoantibodies | Used in acute exacerbations or as maintenance therapy. High cost and risk of allergic reactions |
| Thymectomy | Surgical removal of the thymus | Potentially reduces the production of autoantibodies by removing a source of abnormal immune response | May lead to remission or reduced medication requirement; most effective in patients with thymoma or early-onset MG |
| Monoclonal antibodies | Targeted biologic therapy that specifically inhibits components of the immune system | Block specific immune pathways, such as complement activation or B-cell function | Include rituximab and eculizumab. Used in refractory cases; expensive and require careful monitoring |
| Novel/experimental therapies | |||
| - Efgartigimod | Neonatal Fc receptor (FcRn) antagonist | Reduces levels of pathogenic IgG antibodies | Demonstrates efficacy in clinical trials; may cause headache, upper respiratory infections |
| - Rozanolixizumab | Humanized monoclonal antibody targeting the neonatal FcRn | Lowers circulating IgG autoantibodies by blocking FcRn | Under investigation; shown promising results in reducing symptoms. Infusion-related reactions possible |
| Condition | RNS Findings | Clinical Features | Diagnostic Tests | Notes |
|---|---|---|---|---|
| Myasthenia Gravis (MG) | Decremental response in CMAP amplitude, often U-shaped | Fluctuating muscle weakness, fatigability, dysphagia, dysphonia | Acetylcholine receptor antibodies, SFEMG | High specificity with RNS; SFEMG for inconclusive cases |
| Lambert-Eaton Myasthenic Syndrome (LEMS) | Incremental response in CMAP amplitude upon high-frequency stimulation | Proximal muscle weakness, autonomic dysfunction | Voltage-gated calcium channel antibodies | CMAP amplitude increases after exercise or high-frequency stimulation |
| Amyotrophic Lateral Sclerosis (ALS) | Reduced CMAP amplitude, no decremental pattern | Progressive muscle weakness, atrophy, fasciculations | EMG, clinical examination | ALS affects both upper and lower motor neurons |
| Botulism | Decremental response in CMAP amplitude, improvement with rapid RNS | Acute onset of muscle weakness, cranial nerve involvement | Toxin detection in serum, stool, or food | Requires prompt diagnosis and treatment |
| Congenital Myasthenic Syndromes (CMS) | Decremental response in CMAP amplitude | Early onset muscle weakness, often with ocular involvement | Genetic testing, SFEMG | Genetic forms of neuromuscular junction disorders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiardi, M.; Magliozzi, A.; Colosimo, C.; Marsili, L. Dropped Head Syndrome: The Importance of Neurophysiology in Distinguishing Myasthenia Gravis from Parkinson’s Disease. Biomedicines 2024, 12, 1833. https://doi.org/10.3390/biomedicines12081833
Mangiardi M, Magliozzi A, Colosimo C, Marsili L. Dropped Head Syndrome: The Importance of Neurophysiology in Distinguishing Myasthenia Gravis from Parkinson’s Disease. Biomedicines. 2024; 12(8):1833. https://doi.org/10.3390/biomedicines12081833
Chicago/Turabian StyleMangiardi, Marilena, Alessandro Magliozzi, Carlo Colosimo, and Luca Marsili. 2024. "Dropped Head Syndrome: The Importance of Neurophysiology in Distinguishing Myasthenia Gravis from Parkinson’s Disease" Biomedicines 12, no. 8: 1833. https://doi.org/10.3390/biomedicines12081833
APA StyleMangiardi, M., Magliozzi, A., Colosimo, C., & Marsili, L. (2024). Dropped Head Syndrome: The Importance of Neurophysiology in Distinguishing Myasthenia Gravis from Parkinson’s Disease. Biomedicines, 12(8), 1833. https://doi.org/10.3390/biomedicines12081833







