Prospect and Challenges of Volatile Organic Compound Breath Testing in Non-Cancer Gastrointestinal Disorders
Abstract
1. Introduction
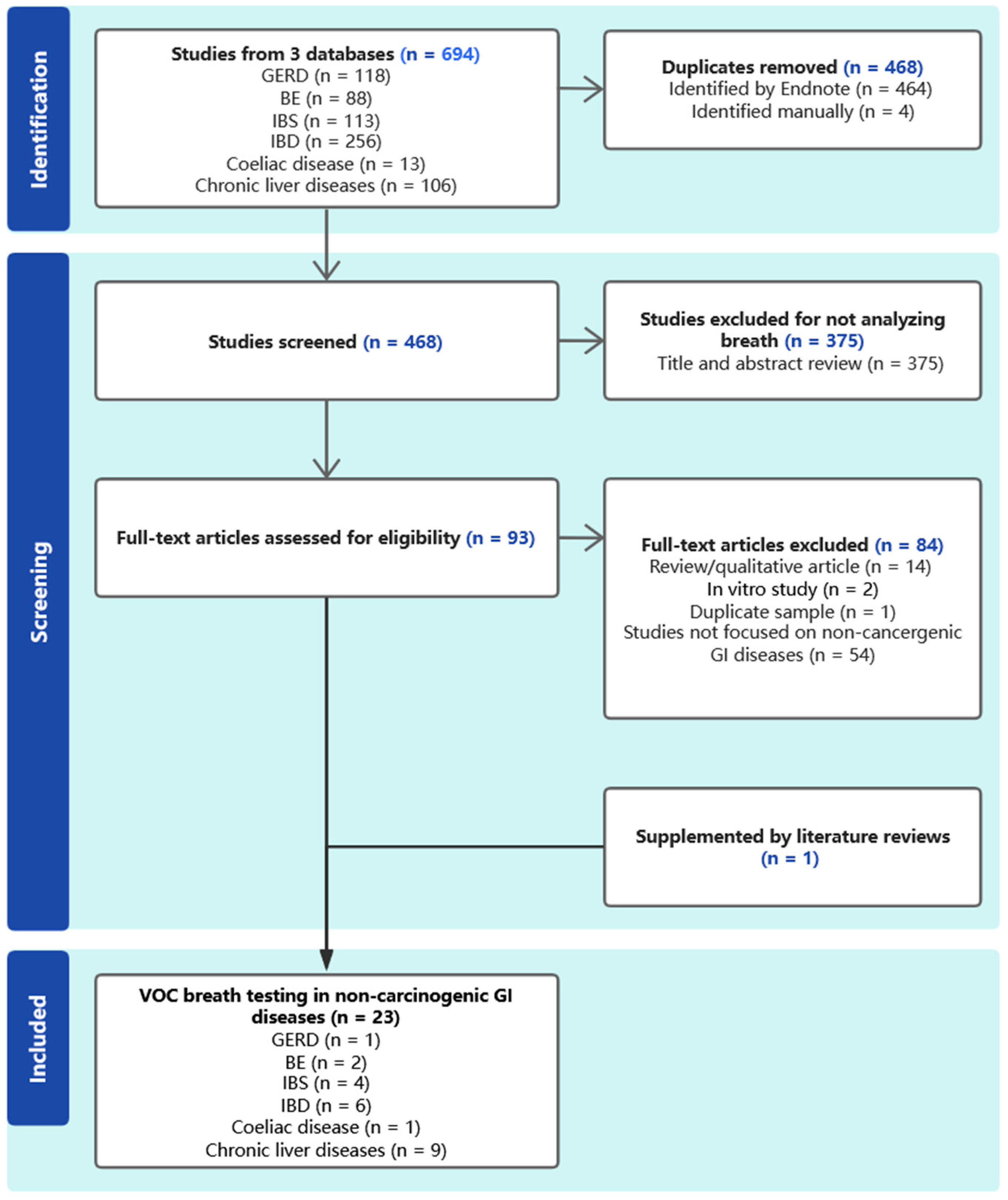
2. Method
Search Strategy
| Reference | Analytical Platform | Reference Test | Disorder Type and Stage, and Group Size | Sensitivity | Specificity | AUC | Breath Biomarkers |
|---|---|---|---|---|---|---|---|
| BE | |||||||
| Peters (2020) [9] | e-nose | Endoscopy | BE (n = 129) and HC (n = 132) | 91% | 74% | - | - |
| Chan (2017) [10] | e-nose (AeonoseTM) | Endoscopy | BE (n = 66) and patients with suspicious history but clarified with endoscopy (n = 56) | 82% | 80% | 0.79 | - |
| GERD | |||||||
| Dryahina (2014) [11] | SIFT-MS | Symptom-based diagnosis | GERD (n = 22) and HC (n = 24) | n.a. | n.a. | n.a. | Acetic acid (+) |
| IBD | |||||||
| Arroyo-Manzanares (2023) [12] | GC-MS | n.a. | IBD (n = 56) and HC (n = 48) | 100% | 100% | n.a. | n.a. |
| Smolinska (2018) [13] | GC-MS | SCCAI | UC (n = 76) and non-IBD (n = 22) | 92% | 77% | 0.94 | Cumene (+), 2,4-dimethylpentane (+), methylcyclopentene (+), C14H30 branched (+) Pentadecane (−), 3-methyl-1-butanol (−), octane (−), acetic acid (−), α-pinene (−), m-cymene (−) |
| Dryahina (2017) [14] | SIFT-MS | HBI, SCCAI | CD (n = 136) and UC (n = 51) and HC (n = 14) | n.a. | n.a. | n.a. | Pentane, Isoprene, Hydrogen sulphide, Carboxylic acids |
| Arasaradnam (2016) [15] | FAIMS | HBI, SCCAI | IBD (n = 54) and HC (n = 22) CD (n = 25) and HC (n = 22) UC (n = 29) and HC (n = 22) CD (n = 25) and UC (n = 29) | 74% 69% 61% 67% | 75% 67% 62% 67% | 0.82 0.77 0.70 0.70 | n.a. |
| Rieder (2016) [16] | SIFT-MS | n.a. | IBD (n = 35) and patients with history or suspicious symptoms (n = 6) | n.a. | n.a. | 0.81 | Acetone, acrylonitrile, carbon disulfide, and triethylamine |
| Baranska (2016) [17] | GC-MS | Rome III | IBS (n = 170) and HC (n = 153) | 89.4% | 77.3% | 0.83 | n.a. |
| IBS | |||||||
| Van Malderen (2022) [18] | Multicapillary Column/IMS | Rome III | IBS (n = 72) and HC (n = 24) IBS-D (n = 21) and HC (n = 24) IBS-C (n = 24) and HC (n = 24) | 97% 67% 67% 92% | 21% 75% 92% 50% | 0.62 0.70 0.81 0.68 | n.a. |
| Baranska (2016) [17] | GC-MS | Rome III | IBS (n = 170) and HC (n = 153) | 89.4% | 77.3% | 0.83 | n.a. |
| Cauchi (2014) [19] | GC-MS | Rome III | IBS (n = 28) and HC (n = 20) | 41% | 72% | 0.44 | n.a. |
| Patel (2014) [20] | SIFT-MS | Rome III | n.a. | n.a. | 0.99 | Benzene (+) Dimethyl sulfide (+) 1-octene (+) 1-3-methyhexane (+) | |
| Chronic liver diseases | |||||||
| Ferrandino (2020) [21] | GC-MS | Cirrhotic patients had an established diagnosis according to EASL and AASLD guidelines | LC (n = 32) and HC (n = 40) | n.a. | n.a. | n.a. | limonene |
| Vincentis (2017) [22] | e-nose (BIONOTETM) | Child–Pugh Classification and MELD (A combination of clinical, biochemical, radiological, and endoscopic findings, with confirmatory liver biopsy in doubtful cases) | Child–Pugh A (n = 37), B (n = 33) and C (n = 19) | n.a. | n.a. | n.a. | n.a. |
| Vincentis (2016) [23] | e-nose with PLS-DA | Blood test, ultrasound | CLD (n = 104) and HC (n = 56) LC (n = 65) and NC-CLD (n = 39) | 86.2% 87.5% | 98.2% 69.2% | 0.84 n.a. | n.a. |
| Pijls (2016) [24] | GC-MS | Liver biopsy and symptom | Compensated cirrhosis (n = 34) and CLD without cirrhosis (n = 87) | 83% | 87% | 0.90 | 3-methylbutanal, Propanoic acid, Octane, Terpene (C10H16), Terpenoid: α-pinene, 3-carene, Branched C16H34, 1-hexadecanol Branched C16H34, Dimethyl disulfide |
| Alkhouri (2015) [25] | SIFT-MS | Liver biopsy | Advanced fibrosis (F3–4, n = 20) and CLD without advanced fibrosis (n = 41) | 85% | 68% | 0.855 | isoprene |
| Fernández (2015) [26] | PTR–MS | Liver biopsy | LC (n = 31) and HC (n = 30) | 97% | 70% | 0.95 | Methanol, 2-pentanone and limonene |
| Hanouneh (2014) [27] | SIFT-MS | Liver biopsy and etiology | AH (n = 40) and non-AH (n = 40) | 80–97% | 72–86% | 0.92 | TMA + Pentane, trimethylamine |
| Morisco (2013) [28] | PTR-ToF-MS | Liver biopsy | LC (n = 12) and HC (n = 14) | 83% | 86% | 0.8869 | 2-butanone, 2- or 3-pentanone, C8-ketone, C9-ketone, Monoterpene, Terpene related, S-compound, Sulfoxide-compound, N-compound, Hepadienol, Methanol |
| Dadamio (2012) [29] | GC-MS and linear discriminant analysis | Liver biopsy | LC (n = 35) and HC (n = 49) | 82–88% | 96–100% | n.a. | Acetone, Styrene, Branched chain alkane, Dimethyl sulfide, Dimethylselenea, Phenola, Tetradecane, Branched chain alkane, Indolea, Octane, Isoprene, Nonane, gamma-Terpinene, 2-Methyl-1-propene, 2-Butanone, beta-Pinene, Caryophyllene |
3. Results
3.1. Quality Assessment of Studies
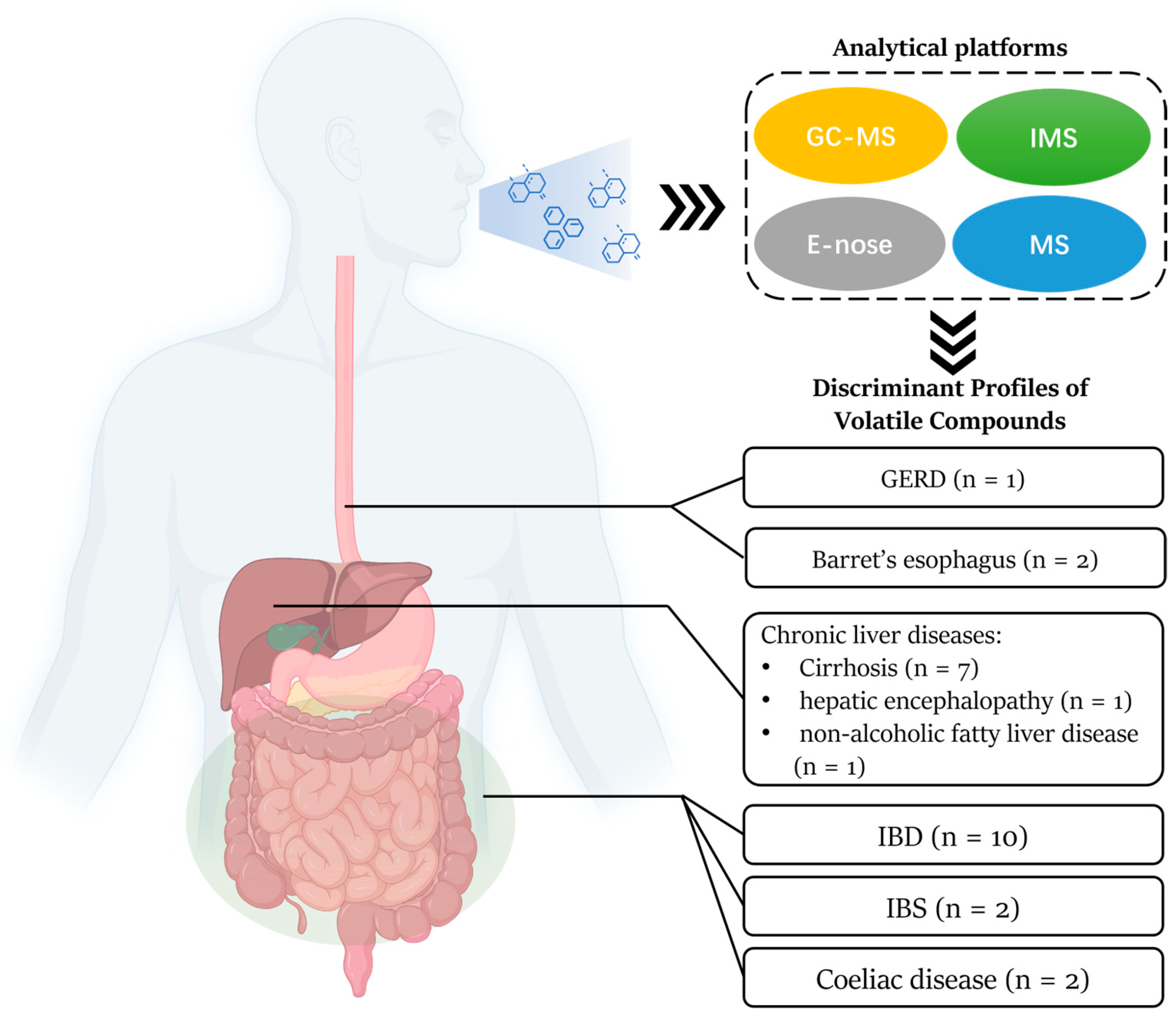
3.2. Current Analytical Platforms of VOCs
3.3. Gastro-Esophageal Reflux Disease and Barrett’s Esophagus
3.4. Coeliac Disease
3.5. Inflammatory Bowel Disease and Irritable Bowel Syndrome
3.6. Chronic Liver Disease
3.6.1. Cirrhosis
3.6.2. Hepatic Encephalopathy
3.6.3. Non-Alcoholic Fatty Liver Disease
3.7. Progressing VOC Breath Tests toward Clinical Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.S. Chapter 17—13C breath tests. In Breathborne Biomarkers and the Human Volatilome, 2nd ed.; Beauchamp, J., Davis, C., Pleil, J., Eds.; Elsevier: Boston, MA, USA, 2020; pp. 273–287. [Google Scholar]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath. Res. 2021, 15, 034001. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.P. Urea breath tests in the management of Helicobacter pylori infection. Gut 1998, 43 (Suppl. 1), S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Lubes, G.; Goodarzi, M. Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics. Chem. Rev. 2017, 117, 6399–6422. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.S. Why have only a handful of breath tests made the transition from R&D to clinical practice? J. Breath Res. 2024, 18, 012001. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Peters, Y.; Schrauwen, R.W.M.; Tan, A.C.; Bogers, S.K.; De Jong, B.; Siersema, P.D. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut 2020, 69, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Zakko, L.; Visrodia, K.H.; Leggett, C.L.; Lutzke, L.S.; Clemens, M.A.; Allen, J.D.; Anderson, M.A.; Wang, K.K. Breath Testing for Barrett’s Esophagus Using Exhaled Volatile Organic Compound Profiling with an Electronic Nose Device. Gastroenterology 2017, 152, 24–26. [Google Scholar] [CrossRef]
- Dryahina, K.; Pospíšilová, V.; Sovová, K.; Shestivska, V.; Kubišta, J.; Spesyvyi, A.; Pehal, F.; Turzíková, J.; Votruba, J.; Spaněl, P. Exhaled breath concentrations of acetic acid vapour in gastro-esophageal reflux disease. J. Breath. Res. 2014, 8, 037109. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Abellán-Alfocea, F.; Prieto-Baeza, L.; Campillo, N.; Del Val Oliver, B.; Zarauz-García, J.; Sáenz, L.; Viñas, P. Application of untargeted volatile profiling in inflammatory bowel disease research. Anal. Bioanal. Chem. 2023, 415, 3571–3579. [Google Scholar] [CrossRef]
- Smolinska, A.; Bodelier, A.G.; Dallinga, J.W.; Masclee, A.A.; Jonkers, D.M.; van Schooten, F.J.; Pierik, M.J. The potential of volatile organic compounds for the detection of active disease in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Dryahina, K.; Smith, D.; Bortlík, M.; Machková, N.; Lukáš, M.; Španěl, P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. J. Breath. Res. 2017, 12, 016002. [Google Scholar] [CrossRef] [PubMed]
- Arasaradnam, R.P.; McFarlane, M.; Daulton, E.; Skinner, J.; O’Connell, N.; Wurie, S.; Chambers, S.; Nwokolo, C.; Bardhan, K.; Savage, R.; et al. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD). Dig. Liver Dis. 2016, 48, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kurada, S.; Grove, D.; Cikach, F.; Lopez, R.; Patel, N.; Singh, A.; Alkhouri, N.; Shen, B.; Brzezinski, A.; et al. A Distinct colon-derived breath metabolome is associated with inflammatory bowel disease, but not its complications. Clin. Transl. Gastroenterol. 2016, 7, e201. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Mujagic, Z.; Smolinska, A.; Dallinga, J.W.; Jonkers, D.M.A.E.; Tigchelaar, E.F.; Dekens, J.; Zhernakova, A.; Ludwig, T.; Masclee, A.A.M.; et al. Volatile organic compounds in breath as markers for irritable bowel syndrome: A metabolomic approach. Aliment. Pharmacol. Ther. 2016, 44, 45–56. [Google Scholar] [CrossRef]
- Van Malderen, K.; Hanning, N.; Lambrechts, H.; Haverhals, T.; Van Marcke, S.; Ceuleers, H.; De Man, J.G.; De Winter, B.Y.; Lamote, K.; De Schepper, H.U. Volatile organic compound profiling as a potential biomarker in irritable bowel syndrome: A feasibility study. Front. Med. 2022, 9, 960000. [Google Scholar] [CrossRef]
- Cauchi, M.; Fowler, D.P.; Walton, C.; Turner, C.; Jia, W.; Whitehead, R.N.; Griffiths, L.; Dawson, C.; Bai, H.; Waring, R.H.; et al. Application of gas chromatography mass spectrometry (GC-MS) in conjunction with multivariate classification for the diagnosis of gastrointestinal diseases. Metabolomics 2014, 10, 1113–1120. [Google Scholar] [CrossRef]
- Patel, S.; Patel, N.; Okwu, V.; Matloob, A.; Grove, D.; Rome, E.; Dweik, R.; Alkhouri, N. Analysis of Exhaled Volatile Organic Compounds Reveals New Biomarkers for Irritable Bowel Syndrome: ACG Fellow Award: 1827. Off. J. Am. Coll. Gastroenterol. 2014, 109, S540–S541. [Google Scholar] [CrossRef]
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; de Saedeleer, A.; Kibble, G.; et al. Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound-Toward Improved Detection of Liver Impairment. Clin. Transl. Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, A.; Pennazza, G.; Santonico, M.; Vespasiani-Gentilucci, U.; Galati, G.; Gallo, P.; Zompanti, A.; Pedone, C.; Antonelli Incalzi, R.; Picardi, A. Breath-print analysis by e-nose may refine risk stratification for adverse outcomes in cirrhotic patients. Liver Int. 2017, 37, 242–250. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, A.; Pennazza, G.; Santonico, M.; Vespasiani-Gentilucci, U.; Galati, G.; Gallo, P.; Vernile, C.; Pedone, C.; Antonelli Incalzi, R.; Picardi, A. Breath-print analysis by e-nose for classifying and monitoring chronic liver disease: A proof-of-concept study. Sci. Rep. 2016, 6, 25337. [Google Scholar] [CrossRef] [PubMed]
- Pijls, K.E.; Smolinska, A.; Jonkers, D.M.; Dallinga, J.W.; Masclee, A.A.; Koek, G.H.; van Schooten, F.J. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci. Rep. 2016, 6, 19903. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Singh, T.; Alsabbagh, E.; Guirguis, J.; Chami, T.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Isoprene in the exhaled breath is a novel biomarker for advanced fibrosis in patients with chronic liver disease: A pilot study. Clin. Transl. Gastroenterol. 2015, 6, e112. [Google Scholar] [CrossRef] [PubMed]
- Fernández del Río, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile Biomarkers in Breath Associated with Liver Cirrhosis—Comparisons of Pre- and Post-liver Transplant Breath Samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Morisco, F.; Aprea, E.; Lembo, V.; Fogliano, V.; Vitaglione, P.; Mazzone, G.; Cappellin, L.; Gasperi, F.; Masone, S.; De Palma, G.D.; et al. Rapid “Breath-Print” of Liver Cirrhosis by Proton Transfer Reaction Time-of-Flight Mass Spectrometry. A Pilot Study. PLoS ONE 2013, 8, e59658. [Google Scholar] [CrossRef] [PubMed]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath biomarkers of liver cirrhosis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 905, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Posudin, Y. Methods of Analysis of Volatile Organic Compounds. In Methods of Measuring Environmental Parameters; Wiley: Hoboken, NY, USA, 2014; pp. 229–243. [Google Scholar]
- Vera, T.; Villanueva, F.; Wimmerová, L.; Tolis, E.I. An overview of methodologies for the determination of volatile organic compounds in indoor air. Appl. Spectrosc. Rev. 2022, 57, 625–674. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-Transfer Reaction Mass Spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef] [PubMed]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass. Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Cancilla, J.C.; Torrecilla, J.S.; Pariente, E.S.; Brönstrup, G.; Christiansen, S.; Johnson, D.W.; Leja, M.; Davies, M.P.; Liran, O.; et al. Silicon Nanowire Sensors Enable Diagnosis of Patients via Exhaled Breath. ACS Nano 2016, 10, 7047–7057. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.J.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Hanevelt, J.; Schoenaker, I.J.H.; Brohet, R.M.; Schrauwen, R.W.M.; Baas, F.J.N.; Tanis, P.J.; van Westreenen, H.L.; de Vos tot Nederveen Cappel, W.H. Alteration of the Exhaled Volatile Organic Compound Pattern in Colorectal Cancer Patients after Intentional Curative Surgery—A Prospective Pilot Study. Cancers 2023, 15, 4785. [Google Scholar] [CrossRef]
- Poļaka, I.; Mežmale, L.; Anarkulova, L.; Kononova, E.; Vilkoite, I.; Veliks, V.; Ļeščinska, A.M.; Stonāns, I.; Pčolkins, A.; Tolmanis, I.; et al. The Detection of Colorectal Cancer through Machine Learning-Based Breath Sensor Analysis. Diagnostics 2023, 13, 3355. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevs, E.; Polaka, I.; Lauka, L.; Zvaigzne, L.; Ozola, A.; Jaeschke, C.; Leja, M.; Haick, H. Food ingestion influence on Sniffphone E-Nose Device breath profile. Helicobacter 2018, 23, 78. [Google Scholar] [CrossRef]
- Steenhuis, E.G.M.; Schoenaker, I.J.H.; de Groot, J.W.B.; Fiebrich, H.B.; de Graaf, J.C.; Brohet, R.M.; van Dijk, J.D.; van Westreenen, H.L.; Siersema, P.D.; de Vos tot Nederveen Cappel, W.H. Feasibility of volatile organic compound in breath analysis in the follow-up of colorectal cancer: A pilot study. Eur. J. Surg. Oncol. 2020, 46, 2068–2073. [Google Scholar] [CrossRef]
- Scheepers, M.; Al-Difaie, Z.; Brandts, L.; Peeters, A.; van Grinsven, B.; Bouvy, N.D. Diagnostic Performance of Electronic Noses in Cancer Diagnoses Using Exhaled Breath: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2219372. [Google Scholar] [CrossRef]
- Hvid-Jensen, F.; Pedersen, L.; Drewes, A.M.; Sørensen, H.T.; Funch-Jensen, P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 2011, 365, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Singh, S.; Anshasi, A.; El-Serag, H.B. Prevalence of Barrett’s Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Wani, S.; Gyawali, C.P.; Komanduri, S. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 2696–2706.e2691. [Google Scholar] [CrossRef] [PubMed]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e332. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.M.; Esteban, J.M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Thrift, A.P.; Rugge, M.; El-Serag, H.B. Prevalence of Barrett’s esophagus and performance of societal screening guidelines in an unreferred primary care population of U.S. veterans. Gastrointest. Endosc. 2021, 93, 409–419.e401. [Google Scholar] [CrossRef]
- Hammad, T.A.; Thrift, A.P.; El-Serag, H.B.; Husain, N.S. Missed Opportunities for Screening and Surveillance of Barrett’s Esophagus in Veterans with Esophageal Adenocarcinoma. Dig. Dis. Sci. 2019, 64, 367–372. [Google Scholar] [CrossRef]
- Sawas, T.; Majzoub, A.M.; Haddad, J.; Tielleman, T.; Nayfeh, T.; Yadlapati, R.; Singh, S.; Kolb, J.; Vajravelu, R.K.; Katzka, D.A.; et al. Magnitude and Time-Trend Analysis of Postendoscopy Esophageal Adenocarcinoma: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e31–e50. [Google Scholar] [CrossRef] [PubMed]
- Vajravelu, R.K.; Kolb, J.M.; Thanawala, S.U.; Scott, F.I.; Han, S.; Singal, A.G.; Falk, G.W.; Katzka, D.A.; Wani, S. Characterization of Prevalent, Post-Endoscopy, and Incident Esophageal Cancer in the United States: A Large Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1739–1747. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Celiac disease and selected long-term health issues. Maturitas 2012, 73, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sanchez, M.I.; Silvester, J.A.; Lebwohl, B.; Leffler, D.A.; Anderson, R.P.; Therrien, A.; Kelly, C.P.; Verdu, E.F. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Tigchelaar, E.; Smolinska, A.; Dallinga, J.W.; Moonen, E.J.; Dekens, J.A.; Wijmenga, C.; Zhernakova, A.; van Schooten, F.J. Profile of volatile organic compounds in exhaled breath changes as a result of gluten-free diet. J. Breath. Res. 2013, 7, 037104. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Cappellin, L.; Gasperi, F.; Morisco, F.; Lembo, V.; Rispo, A.; Tortora, R.; Vitaglione, P.; Caporaso, N.; Biasioli, F. Application of PTR-TOF-MS to investigate metabolites in exhaled breath of patients affected by coeliac disease under gluten free diet. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 966, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e113. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, V.R.Y.; Ramachandran, G.K.; Loo, E.X.L.; Soh, A.Y.S.; Yong, W.P.; Siah, K.T.H. Volatile organic compounds as potential biomarkers of irritable bowel syndrome: A systematic review. Neurogastroenterol. Motil. 2023, 35, e14536. [Google Scholar] [CrossRef]
- Walsh, C.M.; Fadel, M.G.; Jamel, S.H.; Hanna, G.B. Breath Testing in the Surgical Setting: Applications, Challenges, and Future Perspectives. Eur. Surg. Res. 2023, 64, 315–322. [Google Scholar] [CrossRef]
- Moura, P.C.; Raposo, M.; Vassilenko, V. Breath biomarkers in Non-Carcinogenic diseases. Clin. Chim. Acta 2024, 552, 117692. [Google Scholar] [CrossRef] [PubMed]
- Van Malderen, K.; De Winter, B.Y.; De Man, J.G.; De Schepper, H.U.; Lamote, K. Volatomics in inflammatory bowel disease and irritable bowel syndrome. EBioMedicine 2020, 54, 102725. [Google Scholar] [CrossRef]
- Weston, S.R.; Leyden, W.; Murphy, R.; Bass, N.M.; Bell, B.P.; Manos, M.M.; Terrault, N.A. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology 2005, 41, 372–379. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014, 61, 642–659. [Google Scholar] [CrossRef]
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major. Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Christensen, E. Prognostic models including the Child-Pugh, MELD and Mayo risk scores--where are we and where should we go? J. Hepatol. 2004, 41, 344–350. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M. Hepatic Encephalopathy. N. Engl. J. Med. 2016, 375, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Khalid, T.Y.; Costello, B.D.L.; Ewen, R.; White, P.; Stevens, S.; Gordon, F.; Collins, P.; McCune, A.; Shenoy, A.; Shetty, S.; et al. Breath volatile analysis from patients diagnosed with harmful drinking, cirrhosis and hepatic encephalopathy: A pilot study. Metabolomics 2013, 9, 938–948. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.; Ling, K.; Wurie, S.; O’Connell, N.; Nwokolo, C.U.; Bardhan, K.D.; Skinner, J.; Savage, R.S.; Covington, J.A. Breathomics—Exhaled volatile organic compound analysis to detect hepatic encephalopathy: A pilot study. J. Breath Res. 2016, 10, 016012. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.E.; Fernández Del Río, R.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Limonene in exhaled breath is elevated in hepatic encephalopathy. J. Breath Res. 2016, 10, 046010. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, G.; Huang, H.; Liu, T.; Wang, J.; Sun, J.; Wang, H. A semi-packed micro GC column for separation of the NAFLD exhaled breath VOCs. Surf. Coat. Technol. 2019, 363, 322–329. [Google Scholar] [CrossRef]
- Verdam, F.J.; Dallinga, J.W.; Driessen, A.; Jonge, C.D.; Moonen, E.J.C.; Van Berkel, J.B.N.; Luijk, J.; Bouvy, N.D.; Buurman, W.A.; Rensen, S.S.; et al. Non-alcoholic steatohepatitis: A non-invasive diagnosis by analysis of exhaled breath. J. Hepatol. 2013, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Haworth, J.J.; Pitcher, C.K.; Ferrandino, G.; Hobson, A.R.; Pappan, K.L.; Lawson, J.L.D. Breathing new life into clinical testing and diagnostics: Perspectives on volatile biomarkers from breath. Crit. Rev. Clin. Lab. Sci. 2022, 59, 353–372. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath. Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- McKay, L.F.; Holbrook, W.P.; Eastwood, M.A. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol. Microbiol. Immunol. Scand. B 1982, 90, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Gaude, E.; Nakhleh, M.K.; Patassini, S.; Boschmans, J.; Allsworth, M.; Boyle, B.; van der Schee, M.P. Targeted breath analysis: Exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J. Breath. Res. 2019, 13, 032001. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perdoni, F.; Infantino, V.; Faliva, M.A.; Peroni, G.; Iannello, G.; Nichetti, M.; Alalwan, T.A.; Perna, S.; Cocuzza, C. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J. Anal. Methods Chem. 2019, 2019, 7247802. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Akter, S.; Han, S.; Shin, Y.; Choi, T.G.; Kang, I.; Kim, S.S. Diagnosis by Volatile Organic Compounds in Exhaled Breath in Exhaled Breath from Patients with Gastric and Colorectal Cancers. Int. J. Mol. Sci. 2022, 24, 129. [Google Scholar] [CrossRef]
- van Vorstenbosch, R.; Cheng, H.R.; Jonkers, D.; Penders, J.; Schoon, E.; Masclee, A.; van Schooten, F.J.; Smolinska, A.; Mujagic, Z. Systematic Review: Contribution of the Gut Microbiome to the Volatile Metabolic Fingerprint of Colorectal Neoplasia. Metabolites 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tao, J.; Li, J.; Tao, S.; Carrera, P. Volatile organic compounds analysis as a potential novel screening tool for colorectal cancer: A systematic review and meta-analysis. Medicine 2020, 99, E20937. [Google Scholar] [CrossRef]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef]
- Strauss, A.L.; Falk, G.W. New Techniques to Screen for Barrett Esophagus. Gastroenterol. Hepatol. 2023, 19, 383–390. [Google Scholar]
- Sami, S.S.; Moriarty, J.P.; Rosedahl, J.K.; Borah, B.J.; Katzka, D.A.; Wang, K.K.; Kisiel, J.B.; Ragunath, K.; Rubenstein, J.H.; Iyer, P.G. Comparative Cost Effectiveness of Reflux-Based and Reflux-Independent Strategies for Barrett’s Esophagus Screening. Am. J. Gastroenterol. 2021, 116, 1620–1631. [Google Scholar] [CrossRef]
- Chin, S.T.; Romano, A.; Doran, S.L.F.; Hanna, G.B. Cross-platform mass spectrometry annotation in breathomics of oesophageal-gastric cancer. Sci. Rep. 2018, 8, 5139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Pang, K.; Min, Y.; Wu, D. Prospect and Challenges of Volatile Organic Compound Breath Testing in Non-Cancer Gastrointestinal Disorders. Biomedicines 2024, 12, 1815. https://doi.org/10.3390/biomedicines12081815
Zheng W, Pang K, Min Y, Wu D. Prospect and Challenges of Volatile Organic Compound Breath Testing in Non-Cancer Gastrointestinal Disorders. Biomedicines. 2024; 12(8):1815. https://doi.org/10.3390/biomedicines12081815
Chicago/Turabian StyleZheng, Weiyang, Ke Pang, Yiyang Min, and Dong Wu. 2024. "Prospect and Challenges of Volatile Organic Compound Breath Testing in Non-Cancer Gastrointestinal Disorders" Biomedicines 12, no. 8: 1815. https://doi.org/10.3390/biomedicines12081815
APA StyleZheng, W., Pang, K., Min, Y., & Wu, D. (2024). Prospect and Challenges of Volatile Organic Compound Breath Testing in Non-Cancer Gastrointestinal Disorders. Biomedicines, 12(8), 1815. https://doi.org/10.3390/biomedicines12081815






