Role of miRNA–mRNA Interactome in Pathophysiology of Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics
2.3. Myocardial Tissue Collection
2.4. RNA Extraction
2.5. RNA-Sequencing Analysis and Bioinformatics
2.6. Statistical Analysis
3. Results
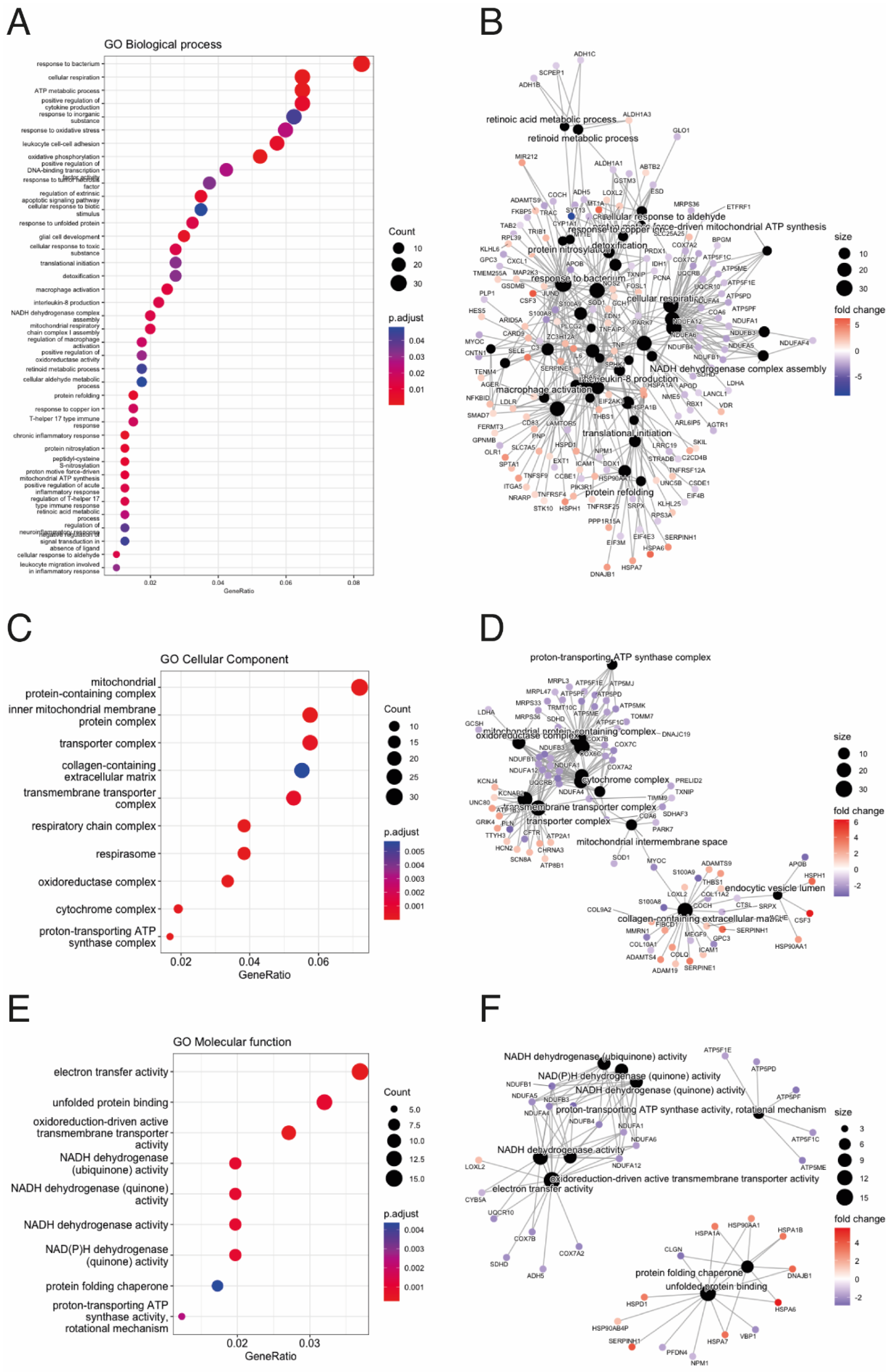
3.1. ACM and Controls mRNA and miRNA Expression Profiles
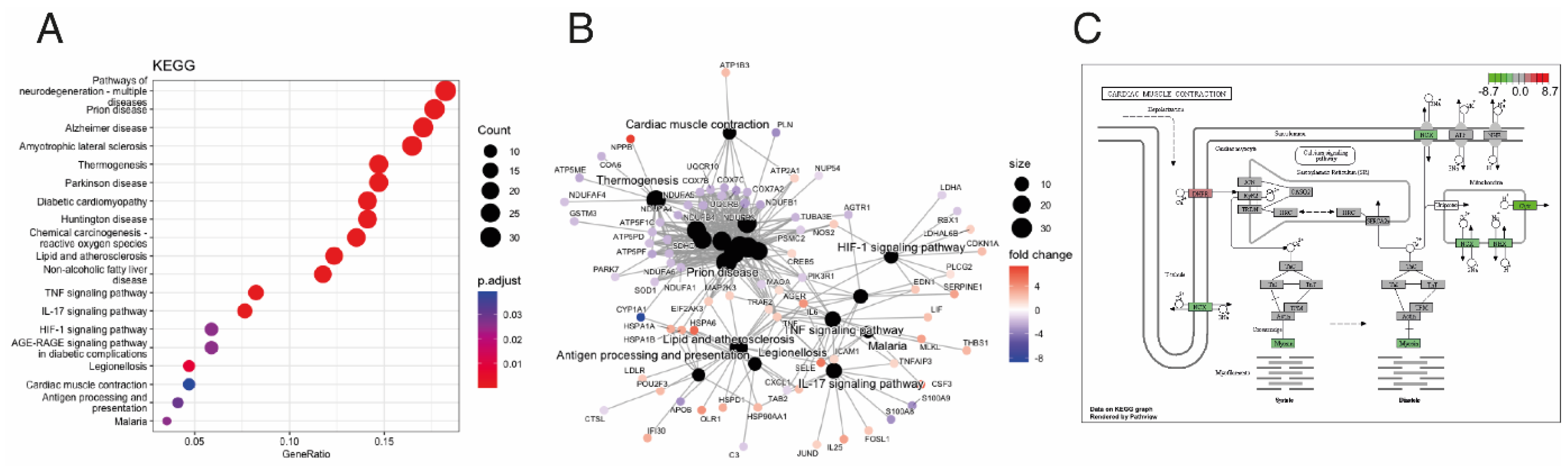
3.2. Integrative Analysis Identified miRNA–mRNA Interaction for ACM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH1 | Aldehyde dehydrogenase 1 family |
| ACM | Arrhythmogenic cardiomyopathy |
| BMPs | Bone morphogenetic proteins |
| FN1 | Fibronectin |
| ITGA5 | Integrin alpha 5 |
| LP | Likely pathogenic |
| miRNAs | MicroRNAs |
| P | Pathogenic |
| RV | Right ventricle |
| SCD | Sudden cardiac death |
| VUS | Variant of unknown significance |
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Anastasakis, A.; Basso, C.; Bauce, B.; Blomstrom-Lundqvist, C.; Bucciarelli-Ducci, C.; Cipriani, A.; De Asmundis, C.; Gandjbakhch, E.; Jimenez-Jaimez, J.; et al. Proposed diagnostic criteria for arrhythmogenic cardiomyopathy: European Task Force consensus report. Int. J. Cardiol. 2024, 395, 131447. [Google Scholar] [CrossRef] [PubMed]
- Bosman, L.P.; Te Riele, A. Arrhythmogenic right ventricular cardiomyopathy: A focused update on diagnosis and risk stratification. Heart 2022, 108, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.A.M.; Semsarian, C.; Marquez, M.F.; Sepehri Shamloo, A.; Ackerman, M.J.; Ashley, E.A.; Sternick, E.B.; Barajas-Martinez, H.; Behr, E.R.; Bezzina, C.R.; et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the State of Genetic Testing for Cardiac Diseases. Heart Rhythm. 2022, 19, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Pinamonti, B.; Brun, F.; Mestroni, L.; Sinagra, G. Arrhythmogenic right ventricular cardiomyopathy: From genetics to diagnostic and therapeutic challenges. World J. Cardiol. 2014, 6, 1234–1244. [Google Scholar] [CrossRef]
- Hoorntje, E.T.; Te Rijdt, W.P.; James, C.A.; Pilichou, K.; Basso, C.; Judge, D.P.; Bezzina, C.R.; van Tintelen, J.P. Arrhythmogenic cardiomyopathy: Pathology, genetics, and concepts in pathogenesis. Cardiovasc. Res. 2017, 113, 1521–1531. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Lacraz, G.P.A.; Vertesy, A.; van Kampen, S.J.; Perini, I.; de Ruiter, H.; Versteeg, D.; Brodehl, A.; van der Kraak, P.; Giacca, M.; et al. Spatial transcriptomics unveils ZBTB11 as a regulator of cardiomyocyte degeneration in arrhythmogenic cardiomyopathy. Cardiovasc. Res. 2023, 119, 477–491. [Google Scholar] [CrossRef]
- Sommariva, E.; Brambilla, S.; Carbucicchio, C.; Gambini, E.; Meraviglia, V.; Dello Russo, A.; Farina, F.M.; Casella, M.; Catto, V.; Pontone, G.; et al. Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur. Heart J. 2016, 37, 1835–1846. [Google Scholar] [CrossRef]
- Garcia-Gras, E.; Lombardi, R.; Giocondo, M.J.; Willerson, J.T.; Schneider, M.D.; Khoury, D.S.; Marian, A.J. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investig. 2006, 116, 2012–2021. [Google Scholar] [CrossRef]
- Piquer-Gil, M.; Domenech-Dauder, S.; Sepulveda-Gomez, M.; Machi-Camacho, C.; Braza-Boils, A.; Zorio, E. Non Coding RNAs as Regulators of Wnt/beta-Catenin and Hippo Pathways in Arrhythmogenic Cardiomyopathy. Biomedicines 2022, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Gurha, P.; Lombardi, R.; Ruggiero, A.; Willerson, J.T.; Marian, A.J. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ. Res. 2014, 114, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, L.; Fan, S.; Cheedipudi, S.M.; Braza-Boils, A.; Molina, M.S.; Yao, Y.; Robertson, M.J.; Coarfa, C.; Gimeno, J.R.; Molina, P.; et al. The EP300/TP53 pathway, a suppressor of the Hippo and canonical WNT pathways, is activated in human hearts with arrhythmogenic cardiomyopathy in the absence of overt heart failure. Cardiovasc. Res. 2022, 118, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Kalayinia, S.; Arjmand, F.; Maleki, M.; Malakootian, M.; Singh, C.P. MicroRNAs: Roles in cardiovascular development and disease. Cardiovasc. Pathol. 2021, 50, 107296. [Google Scholar] [CrossRef]
- Alcalde, M.; Toro, R.; Bonet, F.; Cordoba-Caballero, J.; Martinez-Barrios, E.; Ranea, J.A.; Vallverdu-Prats, M.; Brugada, R.; Meraviglia, V.; Bellin, M.; et al. Role of microRNAs in arrhythmogenic cardiomyopathy: Translation as biomarkers into clinical practice. Transl. Res. J. Lab. Clin. Med. 2023, 259, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Rainer, J.; Meraviglia, V.; Blankenburg, H.; Piubelli, C.; Pramstaller, P.P.; Paolin, A.; Cogliati, E.; Pompilio, G.; Sommariva, E.; Domingues, F.S.; et al. The arrhythmogenic cardiomyopathy-specific coding and non-coding transcriptome in human cardiac stromal cells. BMC Genom. 2018, 19, 491. [Google Scholar] [CrossRef]
- Hall, C.L.; Gurha, P.; Sabater-Molina, M.; Asimaki, A.; Futema, M.; Lovering, R.C.; Suarez, M.P.; Aguilera, B.; Molina, P.; Zorio, E.; et al. RNA sequencing-based transcriptome profiling of cardiac tissue implicates novel putative disease mechanisms in FLNC-associated arrhythmogenic cardiomyopathy. Int. J. Cardiol. 2020, 302, 124–130. [Google Scholar] [CrossRef]
- Lin, L.; Liu, S.; Chen, Z.; Xie, J.; Fu, M.; Lu, D.; Wu, Y.; Shen, H.; Yang, P.; Qian, J. Anatomically resolved transcriptome and proteomelandscapes reveal disease-relevant molecular signaturesand systematic changes in heart function of end-stagedilated cardiomyopathy. VIEW 2023, 4, 1–18. [Google Scholar] [CrossRef]
- van Opbergen, C.J.M.; den Braven, L.; Delmar, M.; van Veen, T.A.B. Mitochondrial Dysfunction as Substrate for Arrhythmogenic Cardiomyopathy: A Search for New Disease Mechanisms. Front. Physiol. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Lippi, M.; Maione, A.S.; Chiesa, M.; Perrucci, G.L.; Iengo, L.; Sattin, T.; Cencioni, C.; Savoia, M.; Zeiher, A.M.; Tundo, F.; et al. Omics Analyses of Stromal Cells from ACM Patients Reveal Alterations in Chromatin Organization and Mitochondrial Homeostasis. Int. J. Mol. Sci. 2023, 24, 17. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Stadiotti, I.; Casella, M.; Catto, V.; Dello Russo, A.; Carbucicchio, C.; Arnaboldi, L.; De Metrio, S.; Milano, G.; Scopece, A.; et al. Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol. Med. 2021, 13, e14365. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, M.; van Opbergen, C.J.M.; Bagwan, N.; Vissing, C.R.; Marron-Linares, G.M.; Zhang, M.; Torres Vega, E.; Sorrentino, A.; Drici, L.; Sulek, K.; et al. Loss of Nuclear Envelope Integrity and Increased Oxidant Production Cause DNA Damage in Adult Hearts Deficient in PKP2: A Molecular Substrate of ARVC. Circulation 2022, 146, 851–867. [Google Scholar] [CrossRef]
- Pitsch, M.; Kant, S.; Mytzka, C.; Leube, R.E.; Krusche, C.A. Autophagy and Endoplasmic Reticulum Stress during Onset and Progression of Arrhythmogenic Cardiomyopathy. Cells 2021, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Aliyari Ghasabeh, M.; Te Riele, A.; James, C.A.; Chen, H.S.V.; Tichnell, C.; Murray, B.; Eng, J.; Kral, B.G.; Tandri, H.; Calkins, H.; et al. Epicardial Fat Distribution Assessed with Cardiac CT in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Radiology 2018, 289, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Peng, Y.; Peng, Y.; Peng, J.; Jiang, S. miR-135a-5p inhibits 3T3-L1 adipogenesis through activation of canonical Wnt/beta-catenin signaling. J. Mol. Endocrinol. 2014, 52, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, N.; Zhao, S.; Chen, Z.; Zhang, W.; Yan, F.; Liao, H.; Chi, K. FBXW7 promotes pathological cardiac hypertrophy by targeting EZH2-SIX1 signaling. Exp. Cell Res. 2020, 393, 112059. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, J.; Zhang, P.; Zhao, X.; Li, Q.; Jiang, A.; Jiang, D.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Mediates 3T3-L1 Preadipocyte Proliferation and Differentiation. Molecules 2018, 23, 317. [Google Scholar] [CrossRef]
- Vaccari, T.; Moroni, A.; Rocchi, M.; Gorza, L.; Bianchi, M.E.; Beltrame, M.; DiFrancesco, D. The human gene coding for HCN2, a pacemaker channel of the heart. Biochim. Biophys. Acta 1999, 1446, 419–425. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, N.W.E.; Kawasaki, M.; Fabrizi, B.; Nariswari, F.A.; Verduijn, A.C.; Neefs, J.; Wesselink, R.; Al-Shama, R.F.M.; van der Wal, A.C.; de Boer, O.J.; et al. Epicardial and endothelial cell activation concurs with extracellular matrix remodeling in atrial fibrillation. Clin. Transl. Med. 2021, 11, e558. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.; Jian Motamedi, F.; Weerasinghe Arachchige, L.C.; Tison, A.; Bradford, S.T.; Lefebvre, J.; Dolle, P.; Ghyselinck, N.B.; Wagner, K.D.; Schedl, A. Retinoic acid signaling is directly activated in cardiomyocytes and protects mouse hearts from apoptosis after myocardial infarction. eLife 2021, 10, e68280. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, D.; Huang, W.; Wang, T.; Li, W. miR-135a-5p affects adipogenic differentiation of human adipose-derived mesenchymal stem cellsby promoting the Hippo signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 1347–1355. [Google Scholar] [PubMed]
- Chen, Y.; Tang, B.; Jiang, W.; Sun, M.; Zhang, H.; Tao, Y.; Wang, H.; Xiang, D.; Bai, H.; Guo, M.; et al. miR-486-5p Attenuates Steroid-Induced Adipogenesis and Osteonecrosis of the Femoral Head Via TBX2/P21 Axis. Stem Cells 2023, 41, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dube, K.N.; Vieira, J.M.; Zhou, B.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; Davidson, S.; Yellon, D.; et al. Myocardial regeneration: Expanding the repertoire of thymosin beta4 in the ischemic heart. Ann. N. Y. Acad. Sci. 2012, 1269, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cunado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; von Gise, A.; Ma, Q.; Hu, Y.W.; Pu, W.T. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev. Biol. 2010, 338, 251–261. [Google Scholar] [CrossRef]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef]
- Phillips, M.D.; Mukhopadhyay, M.; Poscablo, C.; Westphal, H. Dkk1 and Dkk2 regulate epicardial specification during mouse heart development. Int. J. Cardiol. 2011, 150, 186–192. [Google Scholar] [CrossRef]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Francou, A.; De Bono, C.; Kelly, R.G. Epithelial Properties of the Second Heart Field. Circ. Res. 2018, 122, 142–154. [Google Scholar] [CrossRef]
- Yuan, P.; Cheedipudi, S.M.; Rouhi, L.; Fan, S.; Simon, L.; Zhao, Z.; Hong, K.; Gurha, P.; Marian, A.J. Single-Cell RNA Sequencing Uncovers Paracrine Functions of the Epicardial-Derived Cells in Arrhythmogenic Cardiomyopathy. Circulation 2021, 143, 2169–2187. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Rayburn, H.; Hynes, R.O. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 1993, 119, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Pulina, M.; Hou, S.Y.; Astrof, S. Fibronectin and integrin alpha 5 play essential roles in the development of the cardiac neural crest. Mech. Dev. 2010, 127, 472–484. [Google Scholar] [CrossRef]
- Mittal, A.; Pulina, M.; Hou, S.Y.; Astrof, S. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev. Biol. 2013, 381, 73–82. [Google Scholar] [CrossRef]
- Ong, L.P.; Bargehr, J.; Knight-Schrijver, V.R.; Lee, J.; Colzani, M.; Bayraktar, S.; Bernard, W.G.; Marchiano, S.; Bertero, A.; Murry, C.E.; et al. Epicardially secreted fibronectin drives cardiomyocyte maturation in 3D-engineered heart tissues. Stem Cell Rep. 2023, 18, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Liang, J.; Sun, Z.; Wu, Q.; Liu, Y.; Sun, C. The Mechanism of Leptin on Inhibiting Fibrosis and Promoting Browning of White Fat by Reducing ITGA5 in Mice. Int. J. Mol. Sci. 2021, 22, 12353. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.H.; Lee, S.Y.; Shin, K.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev. 2012, 21, 1749–1760. [Google Scholar] [CrossRef]
- Morandi, E.M.; Verstappen, R.; Zwierzina, M.E.; Geley, S.; Pierer, G.; Ploner, C. ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells. Sci. Rep. 2016, 6, 28889. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, J.; Cai, T.; Du, W.; Zhang, Y.; Zhu, Q.; Liu, Z.; Huang, J.A. Suppression of non-small cell lung cancer migration and invasion by hsa-miR-486-5p via the TGF-beta/SMAD2 signaling pathway. J. Cancer 2019, 10, 6014–6024. [Google Scholar] [CrossRef] [PubMed]
- Flum, M.; Dicks, S.; Teng, Y.H.; Schrempp, M.; Nystrom, A.; Boerries, M.; Hecht, A. Canonical TGFbeta signaling induces collective invasion in colorectal carcinogenesis through a Snail1- and Zeb1-independent partial EMT. Oncogene 2022, 41, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Afrakhte, M.; Moren, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Vizi, D.; Khammy, O.; Mariani, J.A.; Kaye, D.M. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur. J. Heart Fail. 2016, 18, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Chouvarine, P.; Legchenko, E.; Geldner, J.; Riehle, C.; Hansmann, G. Hypoxia drives cardiac miRNAs and inflammation in the right and left ventricle. J. Mol. Med. 2019, 97, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Heinke, J.; Wehofsits, L.; Zhou, Q.; Zoeller, C.; Baar, K.M.; Helbing, T.; Laib, A.; Augustin, H.; Bode, C.; Patterson, C.; et al. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ. Res. 2008, 103, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.; Umulis, D.; Ralston, A.; Chen, J.; Olson, D.J.; Avanesov, A.; Othmer, H.; O’Connor, M.B.; Blair, S.S. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 2008, 14, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.; Ren, R.; Pi, X.; Wu, Y.; Moreno, I.; Willis, M.; Moser, M.; Ross, M.; Podkowa, M.; Attisano, L.; et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 2009, 184, 597–609. [Google Scholar] [CrossRef]
- Dyer, L.; Wu, Y.; Moser, M.; Patterson, C. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev. Biol. 2014, 386, 385–394. [Google Scholar] [CrossRef]
- Helbing, T.; Rothweiler, R.; Heinke, J.; Goetz, L.; Diehl, P.; Zirlik, A.; Patterson, C.; Bode, C.; Moser, M. BMPER is upregulated by statins and modulates endothelial inflammation by intercellular adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Lockyer, P.; Dyer, L.A.; Schisler, J.C.; Russell, B.; Carey, S.; Sweet, D.T.; Chen, Z.; Tzima, E.; Willis, M.S.; et al. Bmper inhibits endothelial expression of inflammatory adhesion molecules and protects against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Miralles, I.; Ren, R.; Moser, M.; Hartnett, M.E.; Patterson, C. Bone morphogenetic protein endothelial cell precursor-derived regulator regulates retinal angiogenesis in vivo in a mouse model of oxygen-induced retinopathy. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2216–2222. [Google Scholar] [CrossRef]
- Dyer, L.; Lockyer, P.; Wu, Y.; Saha, A.; Cyr, C.; Moser, M.; Pi, X.; Patterson, C. BMPER Promotes Epithelial-Mesenchymal Transition in the Developing Cardiac Cushions. PLoS ONE 2015, 10, e0139209. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef]
- Perez, L.M.; de Lucas, B.; Galvez, B.G. BMPER is upregulated in obesity and seems to have a role in pericardial adipose stem cells. J. Cell. Physiol. 2021, 236, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hill, S.F.; Skidmore, J.M.; Sperry, E.D.; Swiderski, D.L.; Sanchez, G.J.; Bartels, C.F.; Raphael, Y.; Scacheri, P.C.; Iwase, S.; et al. CHD7 represses the retinoic acid synthesis enzyme ALDH1A3 during inner ear development. JCI Insight 2018, 3, e97440. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Nakamachi, K.; Hosoe, Y.; Nakajima, N. Identification of a novel retinoic acid-responsive element within the lamin A/C promoter. Biochem. Biophys. Res. Commun. 2000, 269, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Colbert, M.C.; Hall, D.G.; Kimball, T.R.; Witt, S.A.; Lorenz, J.N.; Kirby, M.L.; Hewett, T.E.; Klevitsky, R.; Robbins, J. Cardiac compartment-specific overexpression of a modified retinoic acid receptor produces dilated cardiomyopathy and congestive heart failure in transgenic mice. J. Clin. Investig. 1997, 100, 1958–1968. [Google Scholar] [CrossRef]
- Bost, F.; Caron, L.; Marchetti, I.; Dani, C.; Le Marchand-Brustel, Y.; Binetruy, B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem. J. 2002, 361, 621–627. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.; Suh, Y.; Ko, J.K.; Lee, K. Transdifferentiation of Myoblasts Into Adipocytes by All-Trans-Retinoic Acid in Avian. Front. Cell Dev. Biol. 2022, 10, 856881. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflügers Arch. -Eur. J. Physiol. 2020, 472, 931–951. [Google Scholar] [CrossRef] [PubMed]
- Oshita, K.; Itoh, M.; Hirashima, S.; Kuwabara, Y.; Ishihara, K.; Kuwahara, K.; Nakao, K.; Kimura, T.; Nakamura, K.; Ushijima, K.; et al. Ectopic automaticity induced in ventricular myocytes by transgenic overexpression of HCN2. J. Mol. Cell Cardiol. 2015, 80, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Oshita, K.; Kozasa, Y.; Nakagawa, Y.; Kuwabara, Y.; Kuwahara, K.; Nakagawa, T.; Nakashima, N.; Hiraki, T.; Takano, M. Overexpression of the HCN2 channel increases the arrhythmogenicity induced by hypokalemia. J. Physiol. Sci. 2019, 69, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, C.K.; Grant, R.; Hagaman, J.R.; Hiller, S.; Li, F.; Xu, L.; Chang, A.S.; Madden, V.J.; Bagnell, C.R.; Rojas, M.; et al. Endothelin-1 critically influences cardiac function via superoxide-MMP9 cascade. Proc. Natl. Acad. Sci. USA 2015, 112, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Spaich, S.; Will, R.D.; Just, S.; Spaich, S.; Kuhn, C.; Frank, D.; Berger, I.M.; Wiemann, S.; Korn, B.; Koegl, M.; et al. F-box and leucine-rich repeat protein 22 is a cardiac-enriched F-box protein that regulates sarcomeric protein turnover and is essential for maintenance of contractile function in vivo. Circ. Res. 2012, 111, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hou, Y.; Cai, X. MiR-210-3p Enhances Cardiomyocyte Apoptosis and Mitochondrial Dysfunction by Targeting the NDUFA4 Gene in Sepsis-Induced Myocardial Dysfunction. Int. Heart J. 2021, 62, 636–646. [Google Scholar] [CrossRef]
- Bayraktar, E.; Bayraktar, R.; Oztatlici, H.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Targeting miRNAs and Other Non-Coding RNAs as a Therapeutic Approach: An Update. Noncoding RNA 2023, 9, 27. [Google Scholar] [CrossRef]



| Sample | Phenotype | Gene | Protein | Nucleotide | dbSNP | gnomAD | ClinVar | ACMG |
|---|---|---|---|---|---|---|---|---|
| A1.R | ACM | FLNC | p.Arg1370Ter | c.4108C > T | rs1342121466 | 4/1450766 (0.0002%) | P | P |
| A2.R | ACM | PKP2 DSG2 | p.Lys678ArgfsTer12 - | c.1881del c.523 + 2dup | rs764817683 rs2073126642 | NA 2/1451740 (0.0001%) | P VUS | P LP |
| A4.R | ACM | PKP2 | p.Arg79Ter | c.235C > T | rs121434420 | 22/1577370 (0.001%) | LP | LP |
| A6.R | ACM | TMEM43 | p.Ser358Leu | c.1073C > T | rs63750743 | 2/1461886 (0.0001%) | P | P |
| B1.R | Control | - | - | - | - | - | - | - |
| B2.R | Control | - | - | - | - | - | - | - |
| B3.R | Control | - | - | - | - | - | - | - |
| B5.R | Control | - | - | - | - | - | - | - |
| Counts | |||||||
|---|---|---|---|---|---|---|---|
| Sample A1R | Sample A2R | Sample A4R | Sample A6R | Sample B1R | Sample B2R | Sample B5R | Gene Symbol |
| 268,147 | 333,296 | 319,027 | 39,769 | 149,975 | 223,451 | 826,406 | hsa-miR-1-3p |
| 110,587 | 91,623 | 77,600 | 49,265 | 38,476 | 47,353 | 180,699 | hsa-miR-143-3p |
| 26,647 | 70,761 | 71,180 | 51,234 | 63,687 | 81,431 | 160,503 | hsa-let-7a-5p |
| 27,074 | 45,652 | 39,339 | 18,103 | 25,298 | 41,027 | 111,000 | hsa-let-7f-5p |
| 25,882 | 52,083 | 43,790 | 25,771 | 21,360 | 25,545 | 85,002 | hsa-miR-26a-5p |
| 28,171 | 45,954 | 34,588 | 8582 | 17,237 | 12,243 | 56,518 | hsa-miR-30d-5p |
| 13,406 | 53,514 | 23,570 | 6424 | 8590 | 6284 | 34,382 | hsa-miR-133a-3p |
| 17,567 | 26,356 | 25,662 | 9669 | 11,579 | 12,041 | 39,905 | hsa-miR-24-3p |
| 17,871 | 16,799 | 14,542 | 4624 | 5552 | 4409 | 25,811 | hsa-miR-30a-5p |
| 10,120 | 14,886 | 12,774 | 4398 | 5066 | 5808 | 20,783 | hsa-miR-126-3p |
| 8811 | 13,206 | 12,865 | 4841 | 5804 | 6031 | 20,016 | hsa-miR-3074-5p |
| 4907 | 18,329 | 10,663 | 3449 | 6000 | 6092 | 18,876 | hsa-miR-30c-5p |
| 4371 | 12,644 | 10,991 | 5905 | 5633 | 5314 | 18,177 | hsa-miR-125b-5p |
| 4524 | 11,010 | 10,114 | 5501 | 6172 | 6933 | 18,603 | hsa-let-7g-5p |
| 6351 | 9710 | 7213 | 3066 | 3287 | 5284 | 15,622 | hsa-miR-27b-3p |
| 2106 | 7043 | 6079 | 2008 | 5542 | 7547 | 16,297 | hsa-miR-125a-5p |
| 3642 | 10,930 | 6973 | 2556 | 3627 | 4263 | 12,207 | hsa-miR-23b-3p |
| 4042 | 10,743 | 6644 | 1492 | 2689 | 2598 | 10,319 | hsa-miR-378a-3p |
| 2029 | 6048 | 4382 | 2687 | 4514 | 3126 | 9480 | hsa-miR-92a-3p |
| 2838 | 7362 | 5436 | 3769 | 2454 | 2178 | 6331 | hsa-miR-23a-3p |
| 3568 | 4394 | 3894 | 2565 | 3678 | 2126 | 9118 | hsa-miR-16-5p |
| 5040 | 4232 | 4240 | 1244 | 2229 | 2111 | 8984 | hsa-miR-22-3p |
| 2482 | 5222 | 4175 | 2261 | 1938 | 3021 | 8180 | hsa-miR-26b-5p |
| 2953 | 4191 | 2876 | 2977 | 4368 | 1119 | 7761 | hsa-miR-451a |
| 1496 | 3889 | 2983 | 1273 | 2556 | 2826 | 10,222 | hsa-miR-486-5p |
| 1496 | 3889 | 2983 | 1273 | 2556 | 2826 | 10,222 | hsa-miR-486-3p |
| 1872 | 3873 | 2903 | 2441 | 2029 | 2386 | 8070 | hsa-let-7i-5p |
| 1796 | 4135 | 3928 | 3380 | 1426 | 1829 | 7013 | hsa-miR-199a-3p |
| 817 | 2593 | 2470 | 3058 | 2414 | 3316 | 6456 | hsa-let-7c-5p |
| 3914 | 3111 | 3522 | 2173 | 1043 | 1457 | 5324 | hsa-miR-21-5p |
| 1840 | 3173 | 2807 | 1327 | 1658 | 1086 | 4991 | hsa-miR-103a-3p |
| 1840 | 3173 | 2807 | 1327 | 1658 | 1086 | 4991 | hsa-miR-103b |
| 2528 | 2397 | 2317 | 272 | 1096 | 1841 | 6253 | hsa-miR-499b-3p |
| 2528 | 2397 | 2317 | 272 | 1096 | 1840 | 6253 | hsa-miR-499a-5p |
| 1664 | 3486 | 2368 | 1272 | 1048 | 1185 | 5165 | hsa-miR-99b-5p |
| 1518 | 3079 | 2508 | 2021 | 848 | 946 | 4498 | hsa-miR-99a-5p |
| 2306 | 2956 | 2142 | 874 | 1256 | 1034 | 4838 | hsa-miR-181a-5p |
| 764 | 2217 | 1593 | 2737 | 1488 | 1875 | 4178 | hsa-let-7b-5p |
| 3068 | 2358 | 2571 | 523 | 976 | 664 | 4526 | hsa-miR-30e-5p |
| 1377 | 3945 | 2391 | 2752 | 671 | 508 | 2690 | hsa-miR-145-5p |
| 894 | 2055 | 1956 | 1684 | 712 | 909 | 3489 | hsa-miR-199b-3p |
| 1378 | 2869 | 1863 | 519 | 888 | 720 | 3044 | hsa-miR-30e-3p |
| 927 | 1861 | 1642 | 873 | 897 | 963 | 3769 | hsa-miR-423-3p |
| 927 | 1861 | 1642 | 873 | 897 | 963 | 3769 | hsa-miR-3184-5p |
| 912 | 1552 | 1243 | 2236 | 719 | 516 | 2638 | hsa-miR-100-5p |
| 951 | 2629 | 1462 | 1280 | 536 | 278 | 1718 | hsa-miR-140-3p |
| 1289 | 1841 | 1288 | 706 | 619 | 593 | 1990 | hsa-miR-27a-3p |
| 928 | 1654 | 1220 | 515 | 378 | 367 | 1917 | hsa-miR-151a-3p |
| 729 | 1841 | 1080 | 386 | 513 | 522 | 1785 | hsa-miR-30a-3p |
| 1095 | 1195 | 1101 | 748 | 560 | 487 | 1617 | hsa-miR-29a-3p |
| 913 | 1935 | 1215 | 466 | 387 | 249 | 1449 | hsa-miR-30b-5p |
| 603 | 1136 | 945 | 598 | 764 | 562 | 1959 | hsa-miR-191-5p |
| 267 | 588 | 593 | 469 | 840 | 1560 | 2217 | hsa-let-7e-5p |
| 543 | 1081 | 910 | 461 | 539 | 605 | 1712 | hsa-miR-151a-5p |
| 71 | 203 | 353 | 759 | 862 | 416 | 2886 | hsa-miR-10a-5p |
| 267 | 768 | 737 | 486 | 849 | 777 | 1490 | hsa-let-7d-5p |
| 431 | 821 | 735 | 377 | 481 | 704 | 1800 | hsa-miR-98-5p |
| 96 | 163 | 236 | 1239 | 872 | 287 | 1942 | hsa-miR-10b-5p |
| 495 | 983 | 831 | 383 | 366 | 320 | 1333 | hsa-miR-148a-3p |
| Pathway | This Study | Reported Literature |
|---|---|---|
| Adipogenesis | ✓ | [27,28] |
| Apoptosis | ✓ | [29] |
| Cardiac Electrophysiology | ✓ | [30,31] |
| Cardiac Muscle Contraction | ✓ | [10,12] |
| Cardiovascular System Development | [20] | |
| Cell–Cell Adhesion | ✓ | [19,20,22] |
| Chromatin Organization | [22] | |
| Circulatory System Development | [20] | |
| EMT Process | ✓ | [32] |
| ER Stress | ✓ | [25] |
| Extracellular Matrix | ✓ | [19,20] |
| Inflammation | ✓ | [19,20] |
| Lipid and Atherosclerosis | ✓ | [19] |
| Lipid Metabolism | ✓ | [33] |
| Mitochondrial Respiration | ✓ | [22] |
| Oxidative Stress | ✓ | [23] |
| Oxidized LDL-Dependent Pathway | [23] | |
| Platelet Degranulation | [19] | |
| Regulation of Protein Secretion | [22] | |
| Retinoic Acid Metabolic Process | ✓ | [34] |
| TGF-ß Signalling | ✓ | [35] |
| Tissue Development | [20] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonet, F.; Campuzano, O.; Córdoba-Caballero, J.; Alcalde, M.; Sarquella-Brugada, G.; Braza-Boïls, A.; Brugada, R.; Hernández-Torres, F.; Quezada-Feijoo, M.; Ramos, M.; et al. Role of miRNA–mRNA Interactome in Pathophysiology of Arrhythmogenic Cardiomyopathy. Biomedicines 2024, 12, 1807. https://doi.org/10.3390/biomedicines12081807
Bonet F, Campuzano O, Córdoba-Caballero J, Alcalde M, Sarquella-Brugada G, Braza-Boïls A, Brugada R, Hernández-Torres F, Quezada-Feijoo M, Ramos M, et al. Role of miRNA–mRNA Interactome in Pathophysiology of Arrhythmogenic Cardiomyopathy. Biomedicines. 2024; 12(8):1807. https://doi.org/10.3390/biomedicines12081807
Chicago/Turabian StyleBonet, Fernando, Oscar Campuzano, José Córdoba-Caballero, Mireia Alcalde, Georgia Sarquella-Brugada, Aitana Braza-Boïls, Ramon Brugada, Francisco Hernández-Torres, Maribel Quezada-Feijoo, Monica Ramos, and et al. 2024. "Role of miRNA–mRNA Interactome in Pathophysiology of Arrhythmogenic Cardiomyopathy" Biomedicines 12, no. 8: 1807. https://doi.org/10.3390/biomedicines12081807
APA StyleBonet, F., Campuzano, O., Córdoba-Caballero, J., Alcalde, M., Sarquella-Brugada, G., Braza-Boïls, A., Brugada, R., Hernández-Torres, F., Quezada-Feijoo, M., Ramos, M., Mangas, A., Ranea, J. A. G., & Toro, R. (2024). Role of miRNA–mRNA Interactome in Pathophysiology of Arrhythmogenic Cardiomyopathy. Biomedicines, 12(8), 1807. https://doi.org/10.3390/biomedicines12081807







