An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis
Abstract
1. Introduction
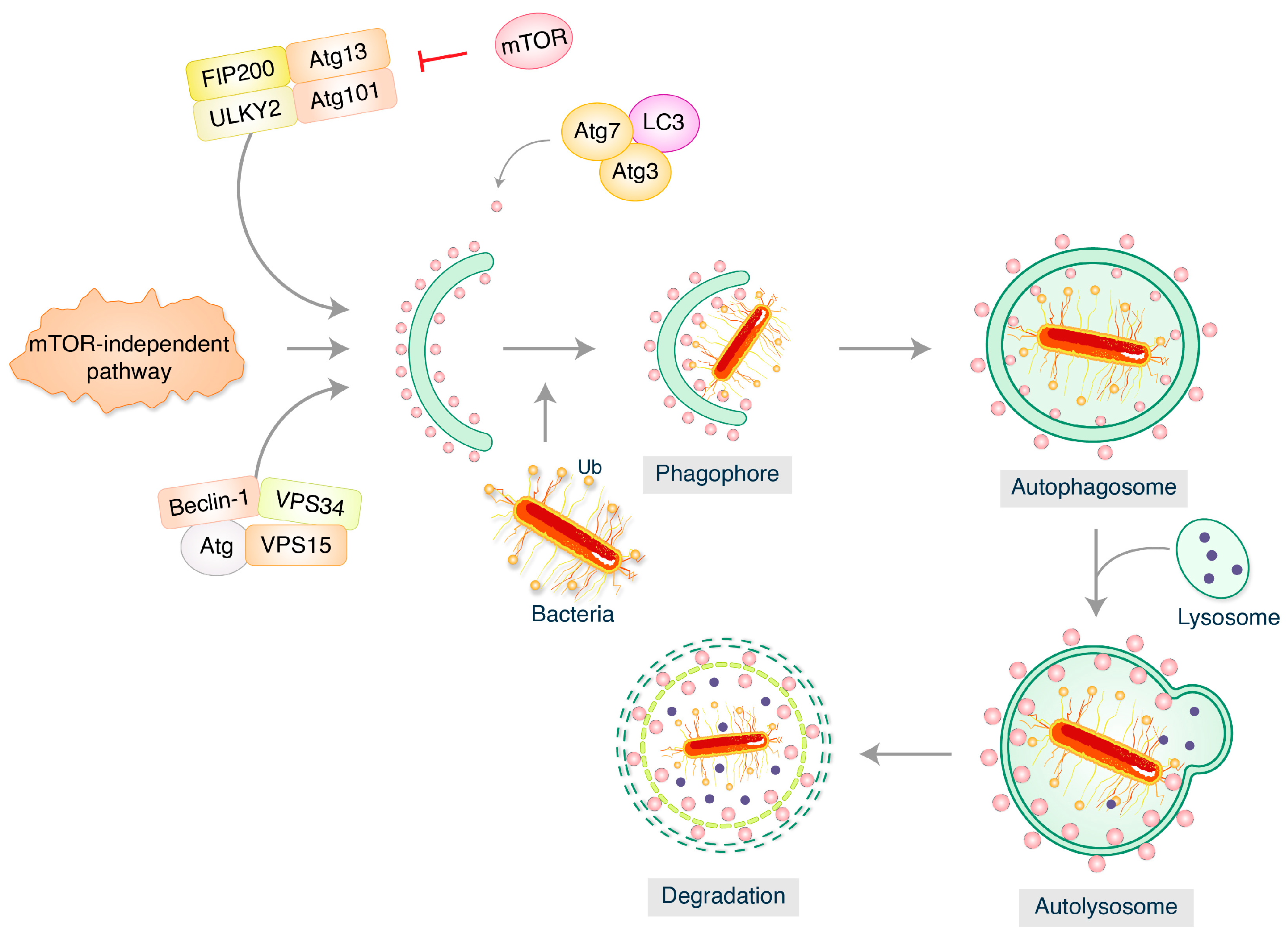
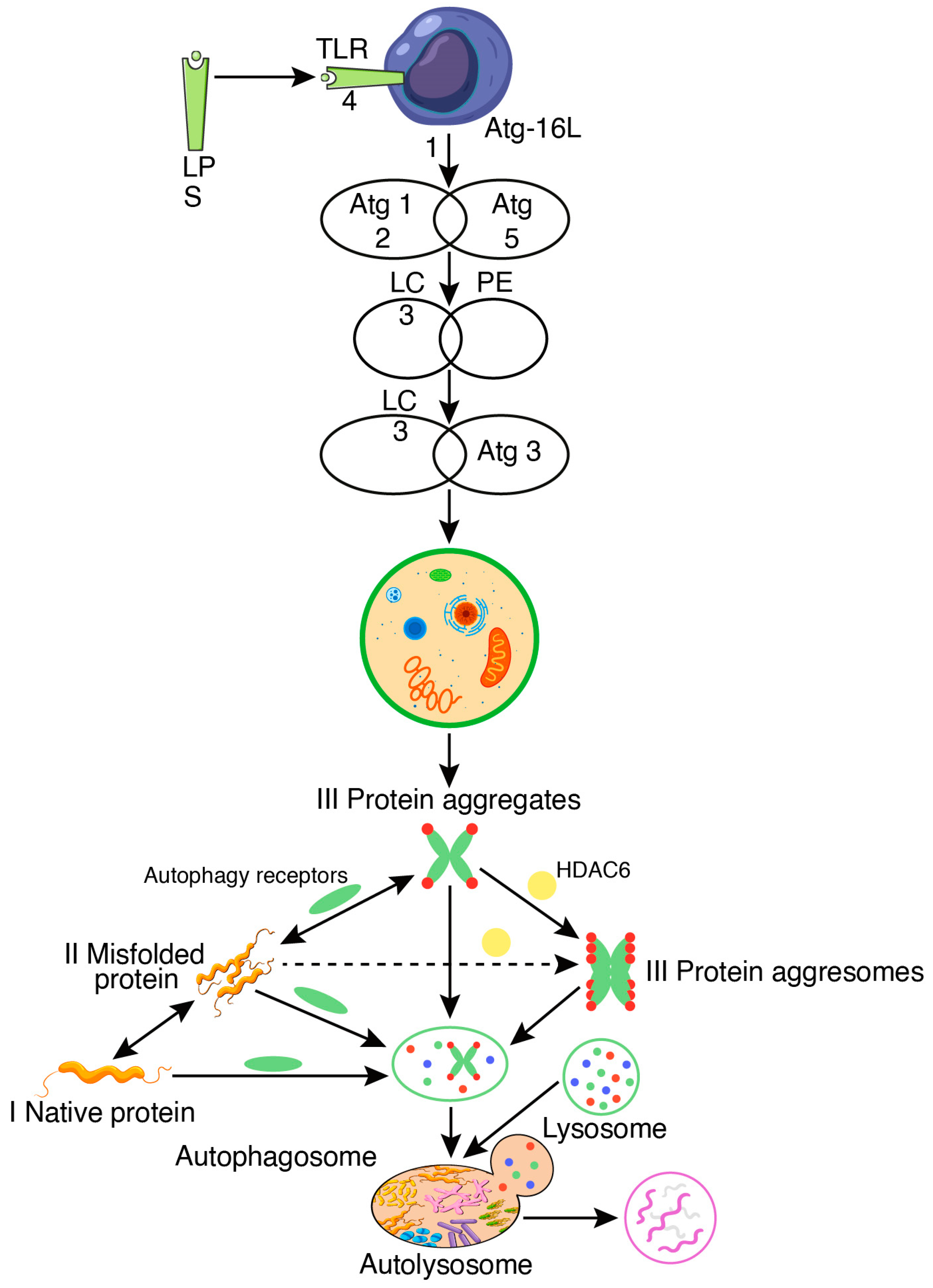
2. Molecular Mechanism of Autophagy in Perspective with Bacterial Pathogenesis
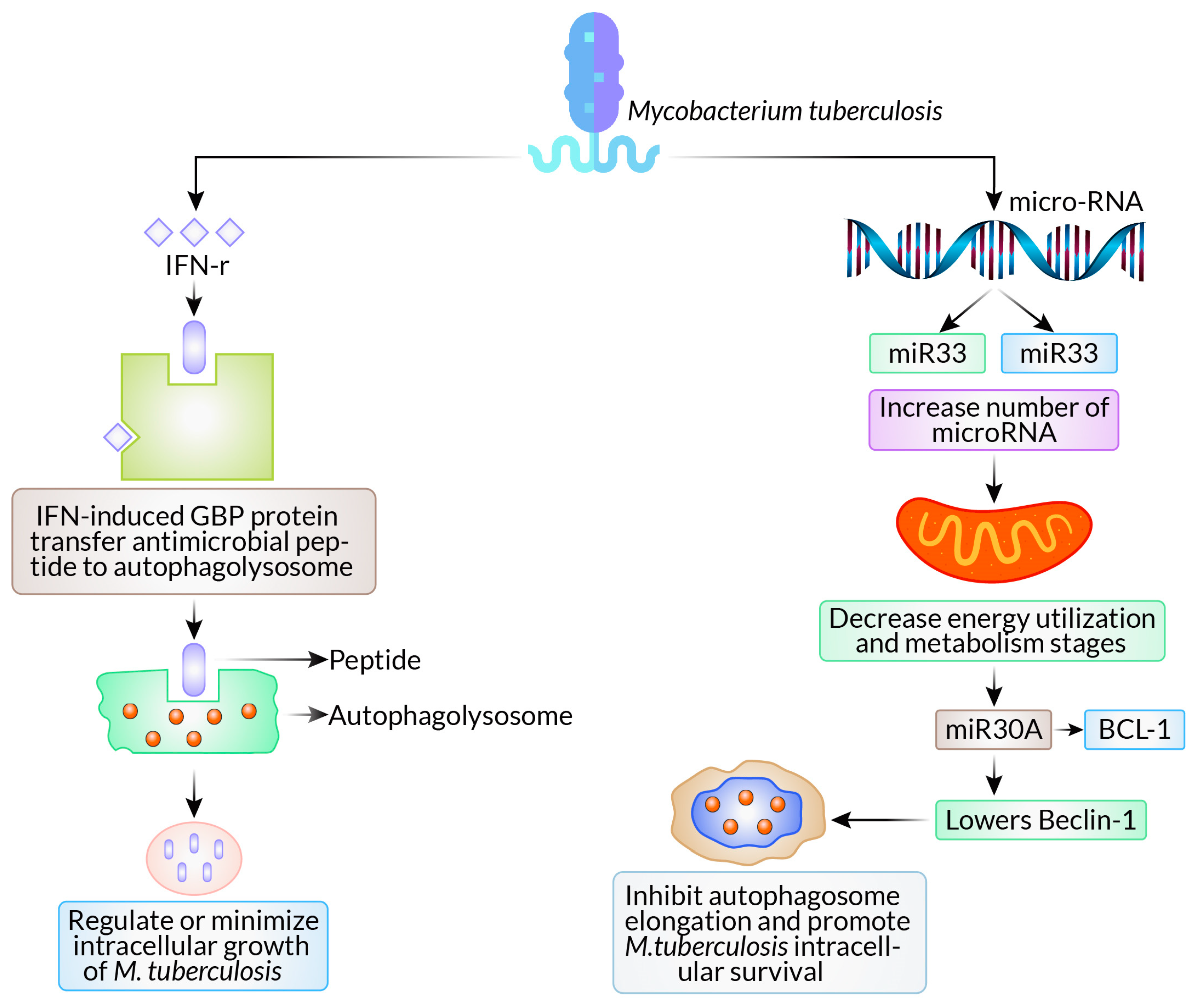
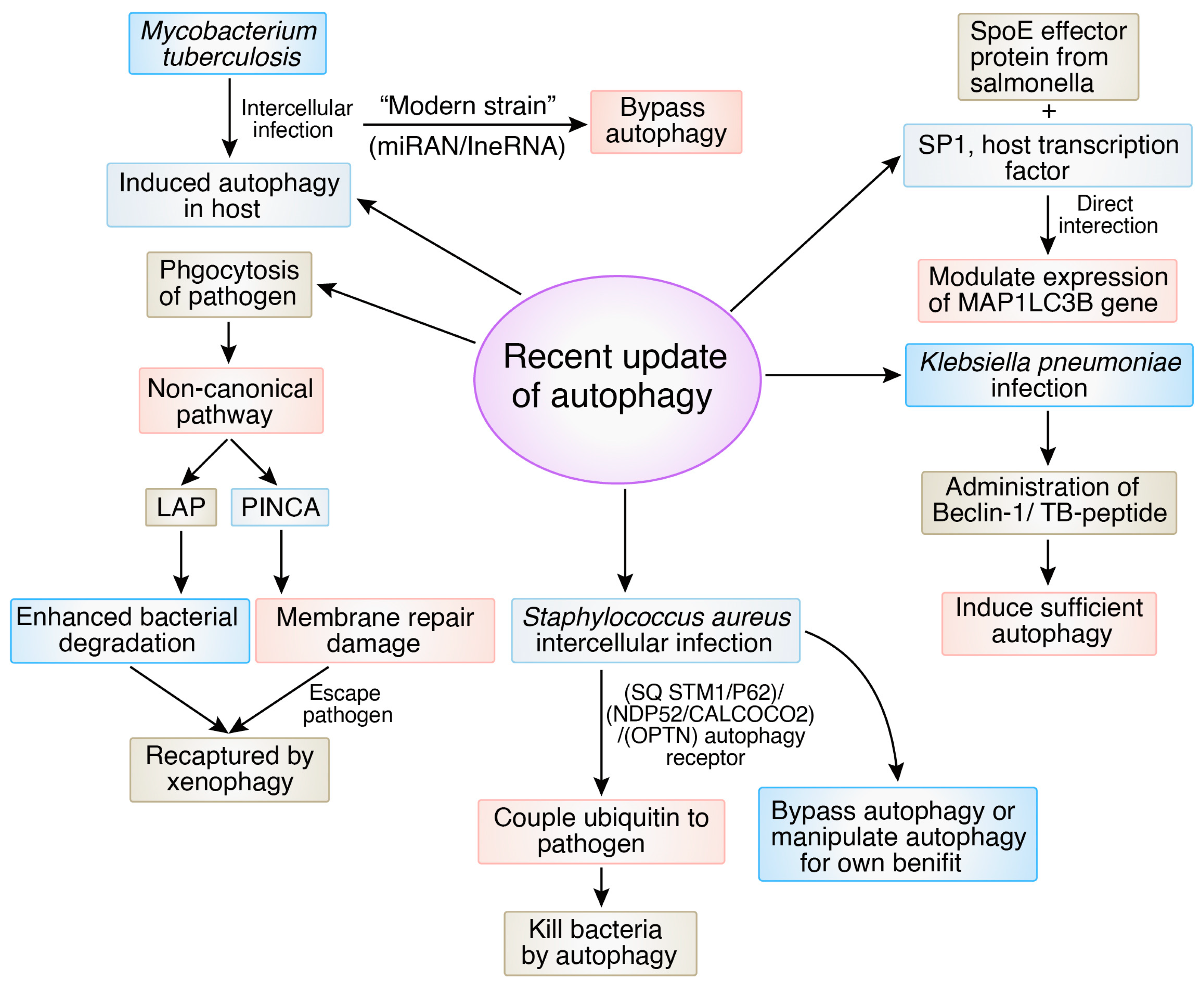
2.1. Mycobacterium tuberculosis
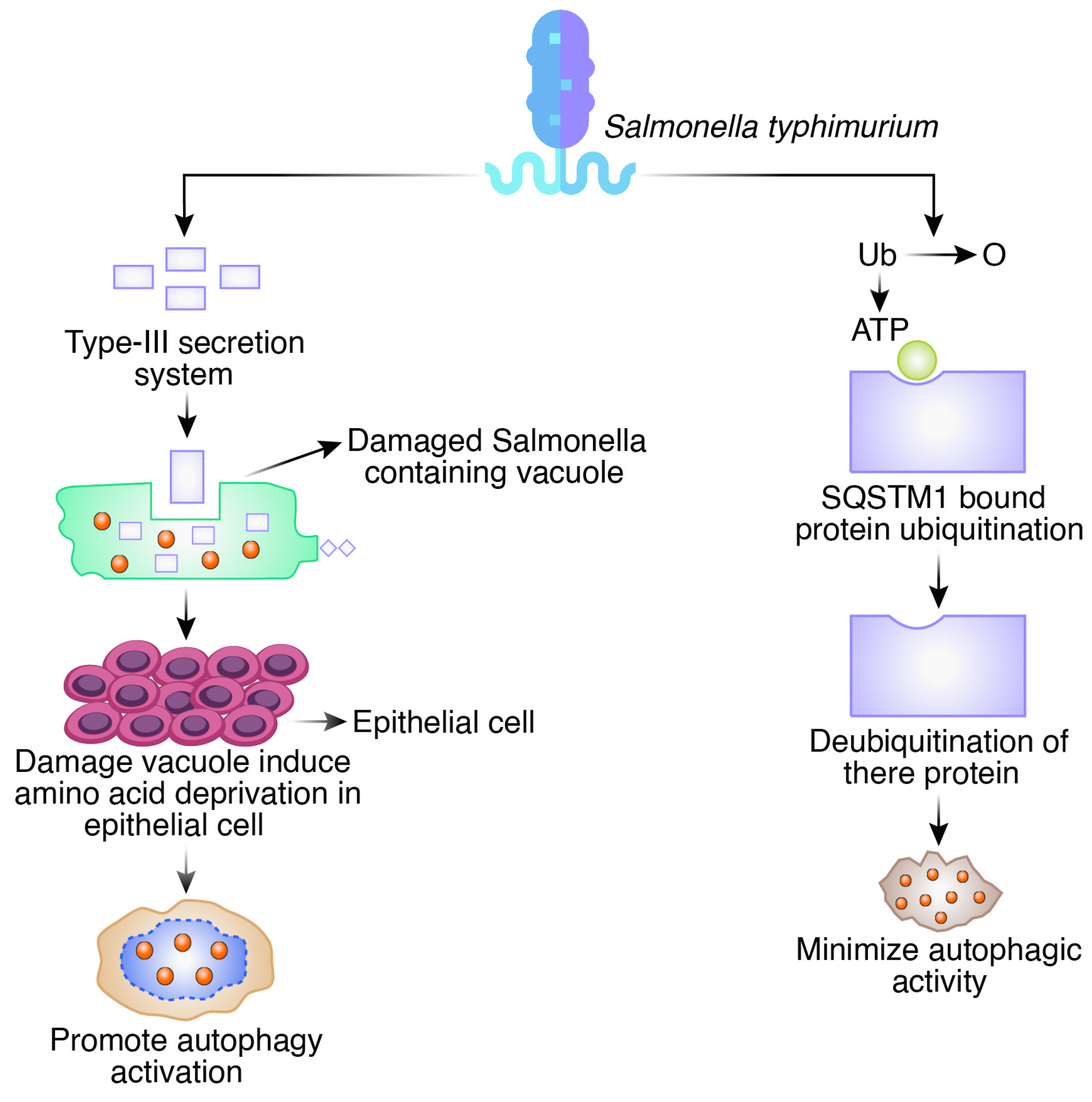
2.2. Salmonella typhimurium
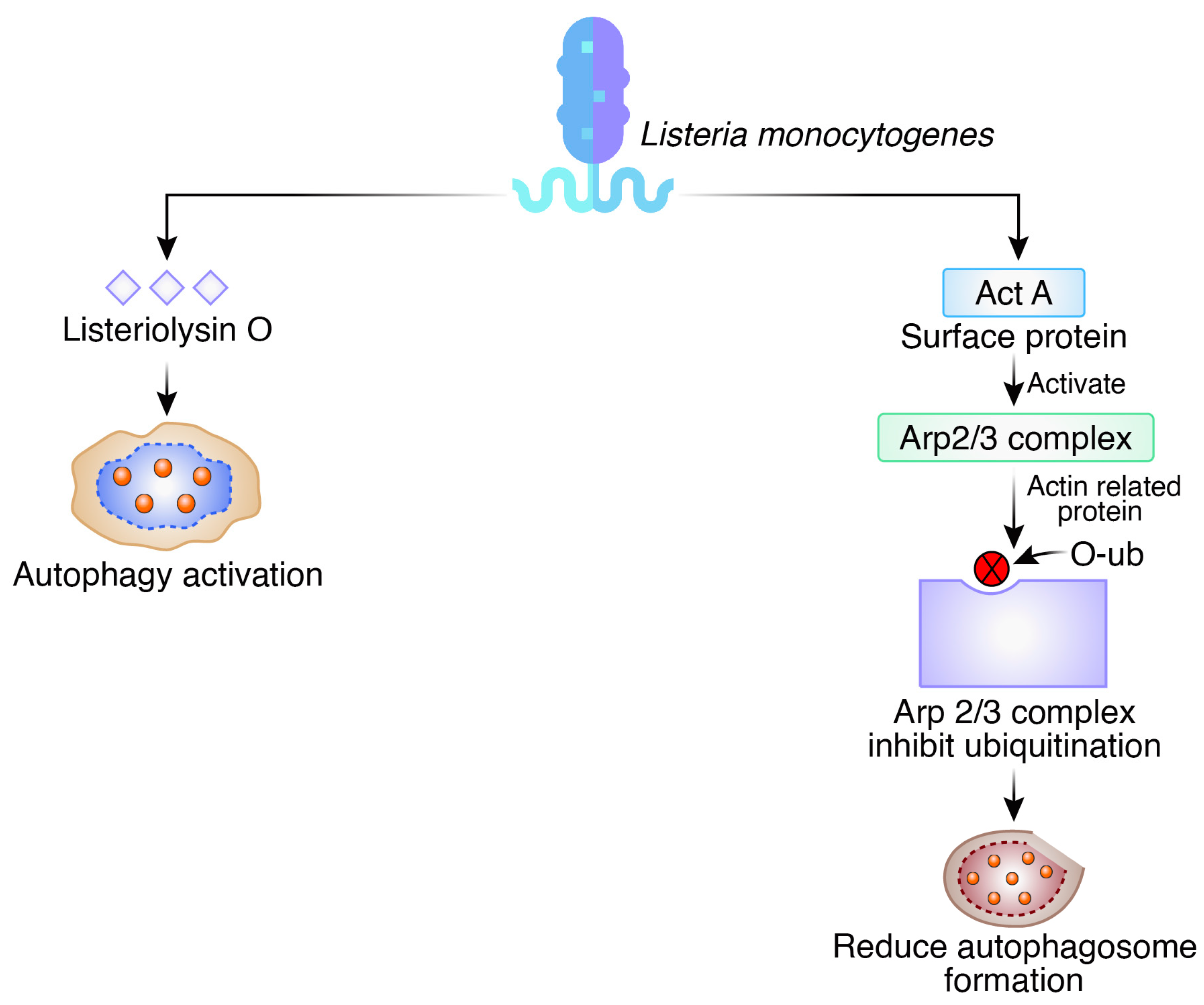
2.3. Listeria monocytogenes
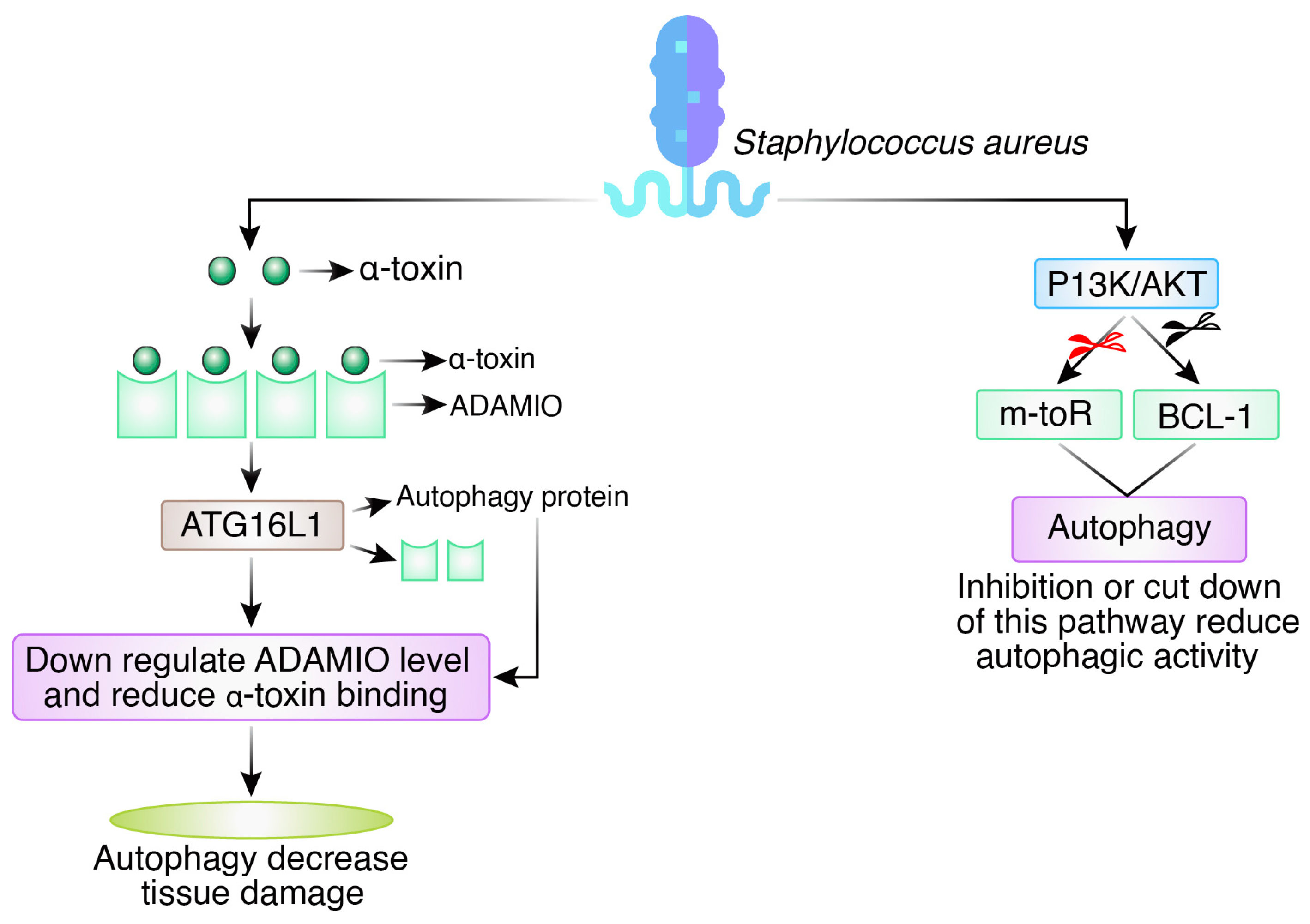
2.4. Staphylococcus aureus
3. Interplay between Autophagy and Bacterial Toxins
3.1. Interactions between Host Cell Autophagy and Bacterial Toxins Involved in Pathogenesis
3.1.1. Lipopolysaccharide (Endotoxin)
3.1.2. Bacterial Pore-Forming Toxins (PFTs)
3.1.3. Non-Pore-Forming Toxin (AB Toxin)
3.1.4. Bacterial Adenylate Cyclases (ACs)
3.2. Autophagy Evasion by a Bacterial Toxin
Evasion by Pore-Forming Toxin
4. Pharmacological Induction of Autophagy Targeting Bacterial Pathogen
4.1. Synthetic Drug
4.1.1. Autophagy Is Triggered by mTOR Signaling Inhibitors
4.1.2. Activators of AMPK Activate Autophagy
4.1.3. Autophagy Is Triggered by Blockers of Class I PI3K Signaling
4.1.4. Autophagy Is Triggered by Inhibitors of Inositol Mono-Phosphatase
4.2. Natural Compounds
4.2.1. Curcumin
4.2.2. Chloroquine
4.2.3. Chrysin
4.2.4. Oridonin
4.2.5. Quercetin
| Mechanism | Compounds | Reference |
|---|---|---|
| Activation of AMPK; inhibition of mTORC1 | Curcumin | [95] |
| Inhibiting lysosomal function | Chloroquine | [96,97] |
| Upregulating autophagy-related genes | Chrysin | [98] |
| Inhibition of the AKT/mTOR signaling pathway | Oridonin | [100] |
| Inhibiting the PI3K/Akt/mTOR signaling pathway | Quercetin | [102] |
| Inhibiting the PI3K/Akt/mTOR pathway | Morusin | [104] |
| Activating the TLR4-mediated signaling pathway | Paclitaxel | [105] |
| Upregulating the AMPK/mTOR signaling pathway | Oleanolic Acid | [106] |
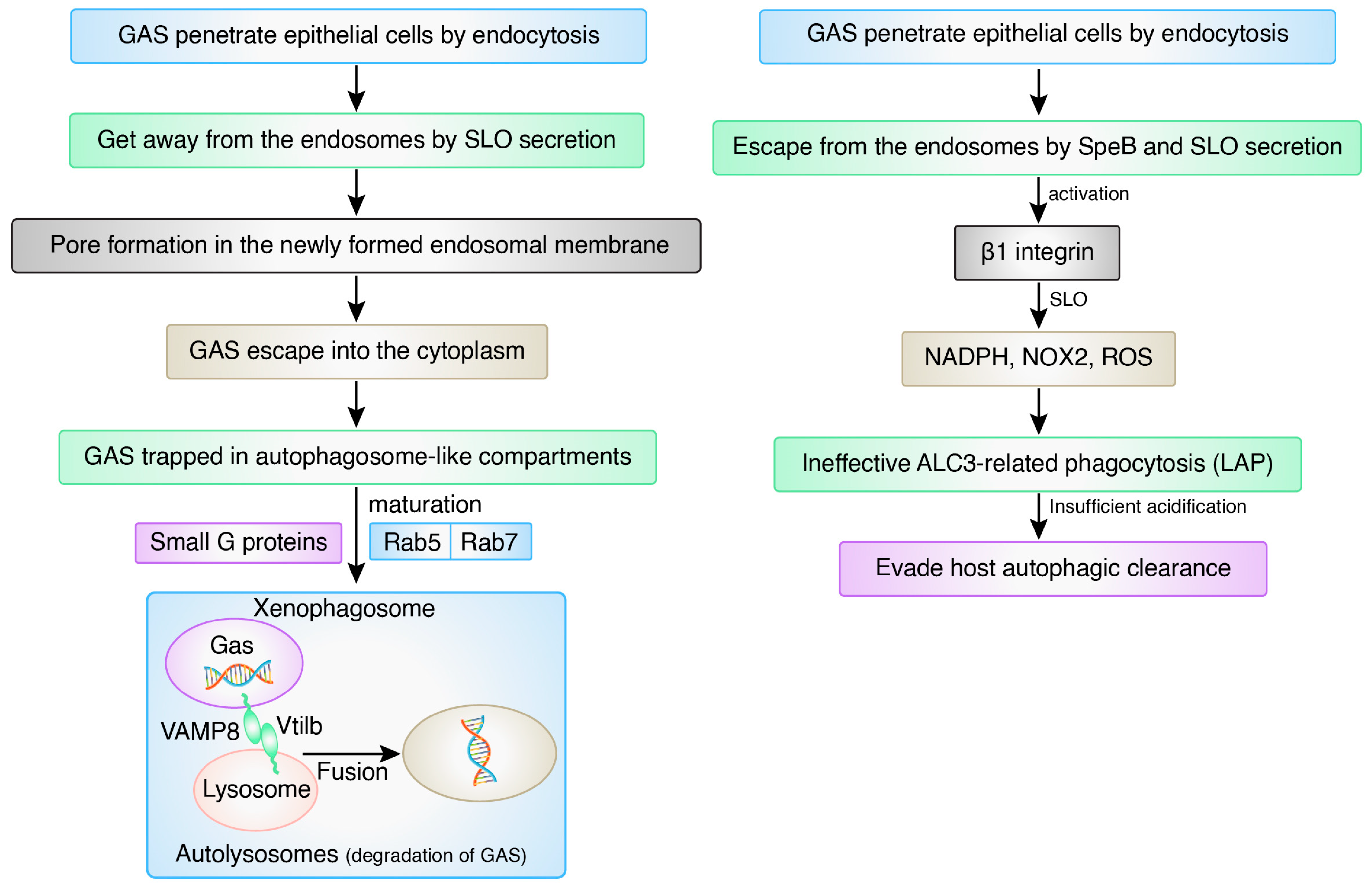
5. Recent Advances and Updates on Autophagy Signaling in Bacterial Infection
6. Limitations and Overcoming Bacterial Defense Mechanisms via Inducing Autophagy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pin, C.; David, L.; Oswald, E. Modulation of Autophagy and Cell Death by Bacterial Outer-Membrane Vesicles. Toxins 2023, 15, 502. [Google Scholar] [CrossRef]
- Nishikori, K.; Morioka, K.; Kubo, T.; Morioka, M. Age-and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J. Insect Physiol. 2009, 55, 351–357. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.H.; Mamun-Or-Rashid, A.N.M.; Hwang, H.; Chung, S.; Kim, B.; Rhim, H. Autophagy modulation in aggresome formation: Emerging implications and treatments of Alzheimer’s disease. Biomedicine 2022, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Fuzimoto, A.; Ferraresi, A.; Isidoro, C. Targeting autophagy with natural products to prevent SARS-CoV-2 infection. J. Tradit. Complement. Med. 2022, 12, 55–68. [Google Scholar] [CrossRef]
- Shariq, M.; Quadir, N.; Alam, A.; Zarin, S.; Sheikh, J.A.; Sharma, N.; Samal, J.; Ahmad, U.; Kumari, I.; Hasnain, S.E.; et al. The exploitation of host autophagy and ubiquitin machinery by Mycobacterium tuberculosis in shaping immune responses and host defense during infection. Autophag 2023, 19, 3–23. [Google Scholar] [CrossRef]
- Leong, J.X.; Raffeiner, M.; Spinti, D.; Langin, G.; Franz-Wachtel, M.; Guzman, A.R.; Kim, J.; Pandey, P.; Minina, A.E.; Macek, B.; et al. A bacterial effector counteracts host autophagy by promoting degradation of an autophagy component. EMBO J. 2022, 41, e110352. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tomás, T.; Sotiriou, A.; Tavernarakis, N. The interplay between selective types of (macro) autophagy: Mitophagy and xenophagy. Mitochondria Bact. Pathog.-Part A 2023, 130, 129. [Google Scholar]
- Tang, L.; Song, Y.; Xu, J.; Chu, Y. The role of selective autophagy in pathogen infection. Chin. Sci. Bull. 2023, 68, 886–900. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saikat, A.S.M.; Rahman, M.S.; Islam, M.; Parvez, M.A.K.; Kim, B. Recent Update and Drug Target in Molecular and Pharmacological Insights into Autophagy Modulation in Cancer Treatment and Future Progress. Cell 2023, 12, 458. [Google Scholar] [CrossRef]
- Nozawa, T.; Toh, H.; Iibushi, J.; Kogai, K.; Minowa-Nozawa, A.; Satoh, J.; Ito, S.; Murase, K.; Nakagawa, I. Rab41-mediated ESCRT machinery repairs membrane rupture by a bacterial toxin in xenophagy. Nat. Commun. 2023, 14, 6230. [Google Scholar] [CrossRef] [PubMed]
- Chand, U.; Kushawaha, P.K. Nano-immunomodulators: Prospective applications to combat drug resistant bacterial infections and related complications. J. Biomater. Sci. Polym. Ed. 2023, 34, 2577–2597. [Google Scholar] [CrossRef] [PubMed]
- Riebisch, A.K.; Mühlen, S.; Beer, Y.Y.; Schmitz, I. Autophagy—A story of bacteria interfering with the host cell degradation machinery. Pathogen 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, S.; Brumell, J.H. Mechanisms and consequences of bacterial targeting by the autophagy pathway. Curr. Opin. Microbiol. 2011, 14, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.G.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Henault, J.; Martinez, J.; Riggs, J.M.; Tian, J.; Mehta, P.; Clarke, L.; Sasai, M.; Latz, E.; Brinkmann, M.M.; Iwasaki, A.; et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity 2012, 37, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, S.; Xu, L. Autophagy in Mycobacterium tuberculosis and HIV infections. Front. Cell. Infect. Microbiol. 2015, 5, 49. [Google Scholar]
- Haibo, S.; Shufeng, W.; Liulin, L.; Qin, S.; Taiyue, L.; Huixia, M.; Yumo, H.; Jing, W.; Honghai, W.; Wenhong, Z.; et al. Mycobacterium tuberculosis hijacks host macrophages-derived interleukin 16 to block phagolysosome maturation for enhancing intracellular growth. Emerg. Microbes Infect. 2024, 13, 2322663. [Google Scholar]
- Ernst, J.D. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 1998, 66, 1277–1281. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Boisson-Dupuis, S.; Chapgier, A.; Yang, K.; Bustamante, J.; Puel, A.; Picard, C.; Abel, L.; Jouanguy, E.; Casanova, J.L. Inborn errors of interferon (IFN)-mediated immunity in humans: Insights into the respective roles of IFN-α/β, IFN-γ, and IFN-λ in host defense. Immunol. Rev. 2008, 226, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.A.; Das, P.K.; Tiwari, S.; MacMicking, J.D. A family of IFN-γ-inducible 65-kD gTPases protects against bacterial infection. Science 2011, 332, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Koster, S.; Sakowski, E.; Ramkhelawon, B.; van Solingen, C.; Oldebeken, S.; Karunakaran, D.; Portal-Celhay, C.; Sheedy, F.J.; Ray, T.D.; et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 2016, 17, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Yuk, J.-M.; Kim, S.Y.; Kim, T.S.; Jin, H.S.; Yang, C.-S.; Jo, E.-K. MicroRNA-125a inhibits autophagy activation and antimicrobial responses during mycobacterial infection. J. Immunol. 2015, 194, 5355–5365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, T.; Liu, Z.; Zhang, G.; Wang, J.; Feng, S.; Liang, J. Inhibition of autophagy by MiR-30A induced by mycobacteria tuberculosis as a possible mechanism of immune escape in human macrophages. Jpn. J. Infect. Dis. 2015, 68, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Holden, D.W. Trafficking of the Salmonella vacuole in macrophages. Traffic 2002, 3, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.D.; Pypaert, M.; Flavell, R.A.; Galán, J.E. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 2003, 163, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Smith, A.C.; Bakowski, M.A.; Yoshimori, T.; Brumell, J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006, 281, 11374–11383. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Sorbara, M.T.; Vuckovic, D.; Ling, A.; Soares, F.; Carneiro, L.A.; Yang, C.; Emili, A.; Philpott, D.J.; Girardin, S.E. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 2012, 11, 563–575. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Shahnazari, S.; Brech, A.; Lamark, T.; Johansen, T.; Brumell, J.H. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009, 183, 5909–5916. [Google Scholar] [CrossRef]
- Thurston, T.L.; Ryzhakov, G.; Bloor, S.; von Muhlinen, N.; Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009, 10, 1215–1221. [Google Scholar] [CrossRef]
- Cemma, M.; Kim, P.K.; Brumell, J.H. The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 2011, 7, 341–345. [Google Scholar] [CrossRef]
- Thomas, M.; Mesquita, F.S.; Holde, D.W. The DUB-ious lack of ALIS in Salmonella infection: A Salmonella deubiquitinase regulates the autophagy of protein aggregates. Autophagy 2012, 8, 1824–1826. [Google Scholar] [CrossRef]
- Owen, K.A.; Meyer, C.B.; Bouton, A.H.; Casanova, J.E. Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 2014, 10, e1004159. [Google Scholar] [CrossRef]
- Vázquez-Boland, J.A.; Kuhn, M.; Berche, P.; Chakraborty, T.; Dominguez-Bernal, G.; Goebel, W.; González-Zorn, B.; Wehland, J.; Kreft, J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001, 14, 584–640. [Google Scholar] [CrossRef]
- Py, B.F.; Lipinski, M.M.; Yuan, J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 2007, 3, 117–125. [Google Scholar] [CrossRef]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K.; Tait, S.W.; Lamkanfi, M.; Amer, A.O.; Nunez, G.; Pagès, G.; Pouysségur, J.; McGargill, M.A.; Green, D.R.; Kanneganti, T.-D. TLR2 and RIP2 pathways mediate autophagy of Listeria monocytogenes via extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 2011, 286, 42981–42991. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P. Illuminating the landscape of host–pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2011, 108, 19484–19491. [Google Scholar] [CrossRef]
- Mostowy, S.; Sancho-Shimizu, V.; Hamon, M.A.; Simeone, R.; Brosch, R.; Johansen, T.; Cossart, P. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 2011, 286, 26987–26995. [Google Scholar] [CrossRef]
- Schnaith, A.; Kashkar, H.; Leggio, S.A.; Addicks, K.; Krönke, M.; Krut, O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 2007, 282, 2695–2706. [Google Scholar] [CrossRef]
- Maurer, K.; Reyes-Robles, T.; Alonzo, F.; Durbin, J.; Torres, V.J.; Cadwell, K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe 2015, 17, 429–440. [Google Scholar] [CrossRef]
- Maurer, K.; Torres, V.J.; Cadwell, K. Autophagy is a key tolerance mechanism during Staphylococcus aureus infection. Autophagy 2015, 11, 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fang, L.; Ding, P.; Liu, R. PI3K/Akt-Beclin1 signaling pathway positively regulates phagocytosis and negatively mediates NF-κB-dependent inflammation in Staphylococcus aureus-infected macrophages. Biochem. Biophys. Res. Commun. 2019, 510, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dickinson, M.S.; Coers, J.; Miao, E.A. Pyroptosis in defense against intracellular bacteria. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Ramon-Luing, L.A.; Palacios, Y.; Ruiz, A.; Téllez-Navarrete, N.A.; Chavez-Galan, L. Virulence Factors of Mycobacterium tuberculosis as Modulators of Cell Death Mechanisms. Pathogens 2023, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J. Interactions between autophagy and bacterial toxins: Targets for therapy? Toxins 2015, 7, 2918–2958. [Google Scholar] [CrossRef]
- Morris, M.C.; Gilliam, E.A.; Li, L. Innate immune programing by endotoxin and its pathological consequences. Front. Immunol. 2015, 5, 680. [Google Scholar] [CrossRef]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Chung, K.W.; Kim, K.M.; Choi, Y.J.; An, H.J.; Lee, B.; Kim, D.H.; Lee, E.K.; Im, E.; Lee, J.; Im, D.S.; et al. The critical role played by endotoxin-induced liver autophagy in the maintenance of lipid metabolism during sepsis. Autophagy 2017, 13, 1113–1129. [Google Scholar] [CrossRef]
- Qin, X. How NOD2 and autophagy may be related to Crohn’s disease? A view shifted from live microbes to luminal bacterial debris. J. Crohn’s Colitis 2014, 8, 88. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Lee, S.-N.; Yang, J.-Y.; Kim, D.W.; Yoon, J.-H.; Ko, H.-J.; Ogawa, M.; Sasakawa, C.; Kweon, M.-N. Autophagy controls an intrinsic host defense to bacteria by promoting epithelial cell survival: A murine model. PloS ONE 2013, 8, e81095. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, X.; Zeng, Y.; Fan, J.; Wu, J.; Li, Z.; Liu, X.; Huang, R.; Huang, F.; Yu, X.; et al. Lipopolysaccharide (LPS)-induced autophagy is involved in the restriction of Escherichia coli in peritoneal mesothelial cells. BMC Microbiol. 2013, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, Y.; Nishiumi, S.; Masuda, A.; Inoue, J.; Nguyen, N.M.T.; Irino, Y.; Komatsu, M.; Tanaka, K.; Kutsumi, H.; Azuma, T.; et al. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-κB activation. Arch. Biochem. Biophys. 2011, 506, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.-G.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Gonzalez, M.; Bischofberger, M.; Pernot, L.; Van Der Goot, F.G.; Freche, B. Bacterial pore-forming toxins: The (w) hole story? Cell. Mol. Life Sci. 2008, 65, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Kloft, N.; Neukirch, C.; Bobkiewicz, W.; Veerachato, G.; Busch, T.; von Hoven, G.; Boller, K.; Husmann, M. Pro-autophagic signal induction by bacterial pore-forming toxins. Med. Microbiol. Immunol. 2010, 199, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Campoy, E.; Colombo, M.I. Autophagy in intracellular bacterial infection. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Canadien, V.; Kaniuk, N.A.; Steinberg, B.E.; Higgins, D.E.; Brumell, J.H. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 2008, 451, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Duan, L.; Zhang, Y.; Li, J.; Zhang, S.; Wang, H. A dynamically evolving war between autophagy and pathogenic microorganisms. J. Zhejiang Univ.-Sci. B 2022, 23, 19–41. [Google Scholar] [CrossRef]
- Huang, J.; Brumell, J.H. Bacteria–autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef]
- Shahnazari, S.; Namolovan, A.; Mogridge, J.; Kim, P.K.; Brumell, J.H. Bacterial toxins can inhibit host cell autophagy through cAMP generation. Autophagy 2011, 7, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.-M.; Yoshimori, T.; Jo, E.-K. Autophagy and bacterial infectious diseases. Exp. Mol. Med. 2012, 44, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Kaur, G.; Agrewala, J.N. Revolutionizing medicine with toll-like receptors: A path to strengthening cellular immunity. Int. J. Biol. Macromol. 2023, 253, 127252. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Yao, X.; Zhang, Y.; Wang, Y.; Han, Y.; Wu, Y.; Xu, Z.; Lan, J.; Han, S.; et al. The Aldose Reductase Inhibitor Epalrestat Maintains Blood–Brain Barrier Integrity by Enhancing Endothelial Cell Function during Cerebral Ischemia. Mol. Neurobiol. 2023, 60, 3741–3757. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.F.; Dekonenko, A.; Arko-Mensah, J.; Mandell, M.; Dupont, N.; Jiang, S.; Delgado-Vargas, M.; Timmins, G.; Bhattacharya, D.; Yang, H.; et al. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, E3168–E3176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rhim, H. Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 2017, 50, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Rahman, M.A.; Kabir, M.T.; Behl, T.; Mathew, B.; Perveen, A.; Barreto, G.E.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of Alzheimer’s disease. Iubmb Life 2020, 72, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Cho, Y.; Nam, G.; Rhim, H. Antioxidant compoun oxyresveratro inhibits APP production through the AMPK/ULK1/mTOR-mediated autophagy pathway in mouse cortical astrocytes. Antioxidants 2021, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Bishayee, K.; Sadra, A.; Huh, S.O. Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 23–36. [Google Scholar] [CrossRef]
- Xu, M.; Zhong, X.Z.; Huang, P.; Jaślan, D.; Wang, P.; Sun, X.; Dong, X.P. TRPML3/BK complex promotes autophagy and bacterial clearance by providing a positive feedback regulation of mTOR via PI3P. Proc. Natl. Acad. Sci. USA 2023, 120, e2215777120. [Google Scholar] [CrossRef]
- Deretic, V.; Delgado, M.; Vergne, I.; Master, S.; De Haro, S.; Ponpuak, M.; Singh, S. Autophagy in immunity against mycobacterium tuberculosis: A model system to dissect immunological roles of autophagy. In Autophagy in Infection and Immunity; Springer: Berlin/Heidelberg, Germany, 2009; pp. 169–188. [Google Scholar]
- Leng, J.; Denkers, E.Y. Toxoplasma gondii inhibits covalent modification of histone H3 at the IL-10 promoter in infected macrophages. PLoS ONE 2009, 4, e7589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahman, M.A.; Hwang, H.; Nah, S.Y.; Rhim, H. Gintonin stimulates autophagic flux in primary cortical astrocytes. J. Ginseng Res. 2020, 44, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Novita, B.D.; Ali, M.; Pranoto, A.; Soediono, E.I.; Mertaniasih, N.M. Metformin induced autophagy in diabetes mellitus–Tuberculosis co-infection patients: A case study. Indian J. Tuberc. 2019, 66, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Frita, R.; Carapau, D.; Mota, M.M.; Hänscheid, T. In Vivo Hemozoin Kinetics after Clearance of Plasmodium berghei Infection in Mice. Malar. Res. Treat. 2012, 2012, 373086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hideshima, T.; Catley, L.; Yasui, H.; Ishitsuka, K.; Raje, N.; Mitsiades, C.; Podar, K.; Munshi, N.C.; Chauhan, D.; Richardson, P.G.; et al. Perifosin an oral bioactive novel alkylphospholipi inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 2006, 107, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zou, Z.; Becker, N.; Anderson, M.; Sumpter, R.; Xiao, G.; Kinch, L.; Koduru, P.; Christudass, C.S.; Veltri, R.W.; et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppressio tumor progressio and tumor chemoresistance. Cell 2013, 154, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Endicott, S.J.; Ziemba, Z.J.; Beckmann, L.J.; Boynton, D.N.; Miller, R.A. Inhibition of class I PI3K enhances chaperone-mediated autophagy. J. Cell Bio. 2020, 219, e202001031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z. Autophagy and cell death: Antitumor drugs targeting autophagy. In Programmed Cell Death; IntechOpen: London, UK, 2019. [Google Scholar]
- Yang, C.; Zhu, B.; Zhan, M.; Hua, Z.-C. Lithium in Cancer Therapy: Friend or Foe? Cancer 2023, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Fan, X.D.; Hunter, S.W.; Brennan, P.J.; Bloom, B.R. Lipoarabinomanna a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 1991, 59, 1755–1761. [Google Scholar] [CrossRef]
- Deretic, V.; Kimura, T.; Timmins, G.; Moseley, P.; Chauhan, S.; Mandell, M. Immunologic manifestations of autophagy. J. Clin. Investig. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Singh, S.B.; Davis, A.S.; Taylor, G.A.; Deretic, V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006, 313, 1438–1441. [Google Scholar] [CrossRef]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I. Temsirolimu an inhibitor of mammalian target of rapamycin. Clin. Cancer Res. 2008, 14, 1286–1290. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcut and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Lampada, A.; O’Prey, J.; Szabadkai, G. mTORC1-independent autophagy regulates receptor tyrosine kinase phosphorylation in colorectal cancer cells via an mTORC2-mediated mechanism. Cell Death Differ. 2017, 24, 1045–1062. [Google Scholar] [CrossRef]
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.-E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol Activates Neuronal Autophagy Through AMPK in the Ischemic Brain. Mol. Neurobiol. 2020, 57, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Lopus, M.; Naik, P.K. Taking aim at a dynamic target: Noscapinoids as microtubule-targeted cancer therapeutics. Pharmacol. Rep. 2015, 67, 56–62. [Google Scholar] [CrossRef]
- Hahn, Y.I.; Kim, S.-J.; Choi, B.-Y.; Cho, K.-C.; Bandu, R.; Kim, K.P.; Kim, D.-H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci. Rep. 2018, 8, 6409. [Google Scholar] [CrossRef]
- Lee, J.E.; Yoon, S.S.; Moon, E.Y. Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells. Biomol. Ther. 2019, 27, 484–491. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases. Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Zhu, X.; Zhang, J.; Cheng, Z.; Wu, W.; Wang, P. Enhanced autophagy promotes the clearance of Pseudomonas aeruginosa in diabetic rats with wounds. Ann. Transl. Med. 2020, 8, 1362. [Google Scholar] [CrossRef]
- Hussain, T.; Zhao, D.; Shah, S.Z.A.; Sabir, N.; Wang, J.; Liao, Y.; Song, Y.; Mangi, M.H.; Yao, J.; Dong, H.; et al. PP2Ac Modulates AMPK-Mediated Induction of Autophagy in Mycobacterium bovis-Infected Macrophages. Int. J. Mol. Sci. 2019, 20, 6030. [Google Scholar] [CrossRef]
- Wu, M.; Cudjoe, O.; Shen, J.; Chen, Y.; Du, J. The Host Autophagy During Toxoplasma Infection. Front. Microbiol. 2020, 11, 589604. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of Quercetin against Salmonella Typhimurium Biofilm Formation on Food-Contact Surfaces and Molecular Mechanism Pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef]
- Wang, L.; Ou, J.H. Hepatitis C virus and autophagy. Biol. Chem. 2015, 396, 1215–1222. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, S.-H. Induction of cytoprotective autophagy by morusin via AMP-activated protein kinase activation in human non-small cell lung cancer cells. Nutr. Res. Pract. 2020, 14, 478–489. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Yang, F.; Li, C.-G.; Xu, L.-H.; He, X.-H.; Mai, F.-Y.; Zeng, C.-Y.; Zhang, C.-C.; Zha, Q.-B.; Ouyang, D.-Y. Paclitaxel Enhances the Innate Immunity by Promoting NLRP3 Inflammasome Activation in Macrophages. Front. Immunol. 2019, 10, 72. [Google Scholar] [CrossRef]
- Hu, C.; Cao, Y.; Li, P. Oleanolic Acid Induces Autophagy and Apoptosis via the AMPK-mTOR Signaling Pathway in Colon Cancer. J. Oncol. 2021, 2021, 8281718. [Google Scholar] [CrossRef]
- Niu, H.; Deng, M. The role of autophagy in infectious diseases. Front. Cell. Infect. Microbiol. 2022, 12, 1465. [Google Scholar] [CrossRef]
- Pellegrini, J.M.; Tateosian, N.L.; Morelli, M.P.; García, V.E. Shedding light on autophagy during human tuberculosis. A long way to go. Front. Cell. Infect. Microbiol. 2022, 11, 820095. [Google Scholar] [CrossRef]
- Romagnoli, A.; Petruccioli, E.; Palucci, I.; Camassa, S.; Carata, E.; Petrone, L.; Mariano, S.; Sali, M.; Dini, L.; Girardi, E.; et al. Clinical isolates of the modern Mycobacterium tuberculosis lineage 4 evade host defense in human macrophages through eluding IL-1β-induced autophagy. Cell Death Dis. 2018, 9, 624. [Google Scholar] [CrossRef]
- Kundu, M.; Basu, J. The role of microRNAs and long non-coding RNAs in the regulation of the immune response to my-cobacterium tuberculosis infection. Front. Immunol. 2021, 12, 687962. [Google Scholar] [CrossRef]
- Demeter, A.; Jacomin, A.C.; Gul, L.; Lister, A.; Lipscombe, J.; Invernizzi, R.; Branchu, P.; Macaulay, I.; Nezis, I.P.; Kingsley, R.A.; et al. Computational prediction and experimental validation of Salmonella Typhimurium SopE-mediated fine-tuning of autophagy in intestinal epithelial cells. Front. Cell. Infect. Microbiol. 2022, 12, 834895. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Ligeon, L.-A.; Pena-Francesch, M.; Vanoaica, L.D.; Núñez, N.G.; Talwar, D.; Dick, T.P.; Münz, C. Oxidation inhibits autophagy protein deconjugation from phagosomes to sustain MHC class II restricted antigen presentation. Nat. Commun. 2021, 12, 1508. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Farid, A.; Krönke, M. When the Phagosome Gets Leaky: Pore-Forming Toxin-Induced Non-Canonical Autophagy (PINCA). Front. Cell. Infect. Microbiol. 2022, 12, 834321. [Google Scholar] [CrossRef]
- Nikouee, A.; Kim, M.; Ding, X.; Sun, Y.; Zang, Q.S. Beclin-1–Dependent Autophagy Improves Outcomes of Pneumonia-Induced Sepsis. Front. Cell. Infect. Microbiol. 2021, 11, 706637. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, Y.; Qian, S.; Chiou, H.; Zang, Q.S. Beclin-1 improves mitochondria-associated membranes in the heart during endotoxemia. FASEB BioAdvances 2021, 3, 123. [Google Scholar] [CrossRef]
- Neumann, Y.; Bruns, S.A.; Rohde, M.; Prajsnar, T.K.; Foster, S.J.; Schmitz, I. Intracellular Staphylococcus aureus eludes selective autophagy by activating a host cell kinase. Autophagy 2016, 12, 2069–2084. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Z.; Han, H. Autophagy in Staphylococcus aureus infection. Front. Cell. Infect. Microbiol. 2021, 11, 750222. [Google Scholar] [CrossRef]
- Gauron, M.C.; Newton, A.C.; Colombo, M.I. PKCα is recruited to Staphylococcus aureus-Containing phagosomes and impairs bacterial replication by inhibition of autophagy. Front. Immunol. 2021, 12, 662987. [Google Scholar] [CrossRef]
- Saini, S.; Gangwar, A.; Sharma, R. Harnessing Host-Pathogen Interactions for Innovative Drug Discovery and Host-Directed Therapeutics to tackle tuberculosis. Microbiol. Res. 2023, 275, 127466. [Google Scholar] [CrossRef]
- Kim, J.; Cho, B.-H.; Jang, Y.-S. Understanding the roles of host defense peptides in immune modulation: From antimicrobial action to potential as adjuvants. J. Microbiol. Biotechnol. 2023, 33, 288. [Google Scholar] [CrossRef]
| Bacterium | Bacterial Toxin | The Function of Toxin in Host | The Link between Autophagy and Toxin | References |
|---|---|---|---|---|
| Escherichia coli | Colicins | Extensive vacuolation in epithelial cells. | In response to starvation, toxins activate the pathway that blocks translation and proceeds to autophagy. | [57] |
| Vibrio cholerae | Cytolysin (VCC) | Depending on the toxin dose and cell type, this toxin either forms vacuoles or causes lysis of the cell. | Vacuolization brought on by this exotoxin is connected to autophagy via the autophagic cell response after VCC intoxication. | [58] |
| Streptococcus pyogenes | Streptolysin O (SLO) | Induces AMP-activated protein kinase (AMPK) phosphorylation in epithelial cells. | Preventing TORC1 (target of rapamycin complex 1) AMPK inducing autophagy after decreasing the ATP/AMP ratio. | [57] |
| Listeria monocytogenes | Listeriolysin O (LLO) | Blocks phagosome–lysosome fusion by generating tiny pores and allowing Listeria to escape from phagosomes. | Targets damaged phagosomes to prevent bacterial escape and clear Listeria monocytogenes by activating autophagy genes. | [47,59] |
| Staphylococcus aureus | Alpha-toxin | Formation and binding of oligomers into lipid bilayers to form pores and decrease intracellular ATP levels. | Causes a drop in the intracellular cyclic adenosine monophosphate (cAMP) levels and favors a nontraditional autophagic process. | [47,60] |
| Serratia marcescens | Sh1A | Elicit an autophagic response in epithelial cells. | Unknown. | [47] |
| Salmonella enterica serovar Typhimurium | InvA and SipB | Damages Salmonella-containing vacuoles. | Restrict the intracellular growth by colocalizing with polyubiquitinated proteins. | [30,47] |
| Helicobacter pylori | VacA | Causes cytotoxic effects in impaired cells by vacuole formation. | Autophagy can degrade VacA in the early phase but this toxin impairs the autophagy process later. | [47] |
| Mechanism | Compounds | References |
|---|---|---|
| Inhibition of mTORC1 | Rapamycin | [85,86] |
| Activates AMPK | Metformin | [87,88] |
| Inhibitor of AKT pathway | Perifosine | [77] |
| Inhibiting inositol mono-phosphatase | Lithium | [82,83,84] |
| Inhibiting the function of L-type calcium channels | Verapamil | [89] |
| Inhibiting the mTORC1 pathway | Nimodipine | [90] |
| Upregulating the AMPK pathway | Nitrendipine | [91] |
| Inhibiting microtubule polymerization | Noscapine | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Sarker, A.; Ayaz, M.; Shatabdy, A.R.; Haque, N.; Jalouli, M.; Rahman, M.H.; Mou, T.J.; Dey, S.K.; Hoque Apu, E.; et al. An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis. Biomedicines 2024, 12, 1757. https://doi.org/10.3390/biomedicines12081757
Rahman MA, Sarker A, Ayaz M, Shatabdy AR, Haque N, Jalouli M, Rahman MH, Mou TJ, Dey SK, Hoque Apu E, et al. An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis. Biomedicines. 2024; 12(8):1757. https://doi.org/10.3390/biomedicines12081757
Chicago/Turabian StyleRahman, Md Ataur, Amily Sarker, Mohammed Ayaz, Ananya Rahman Shatabdy, Nabila Haque, Maroua Jalouli, MD. Hasanur Rahman, Taslin Jahan Mou, Shuvra Kanti Dey, Ehsanul Hoque Apu, and et al. 2024. "An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis" Biomedicines 12, no. 8: 1757. https://doi.org/10.3390/biomedicines12081757
APA StyleRahman, M. A., Sarker, A., Ayaz, M., Shatabdy, A. R., Haque, N., Jalouli, M., Rahman, M. H., Mou, T. J., Dey, S. K., Hoque Apu, E., Zafar, M. S., & Parvez, M. A. K. (2024). An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis. Biomedicines, 12(8), 1757. https://doi.org/10.3390/biomedicines12081757









