Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Administration of Paclitaxel to Induce Mechanical Allodynia
2.3. Drug Administration
2.4. Assessment of Mechanical Allodynia
2.5. Molecular Docking
2.6. Statistical Analyses
3. Results
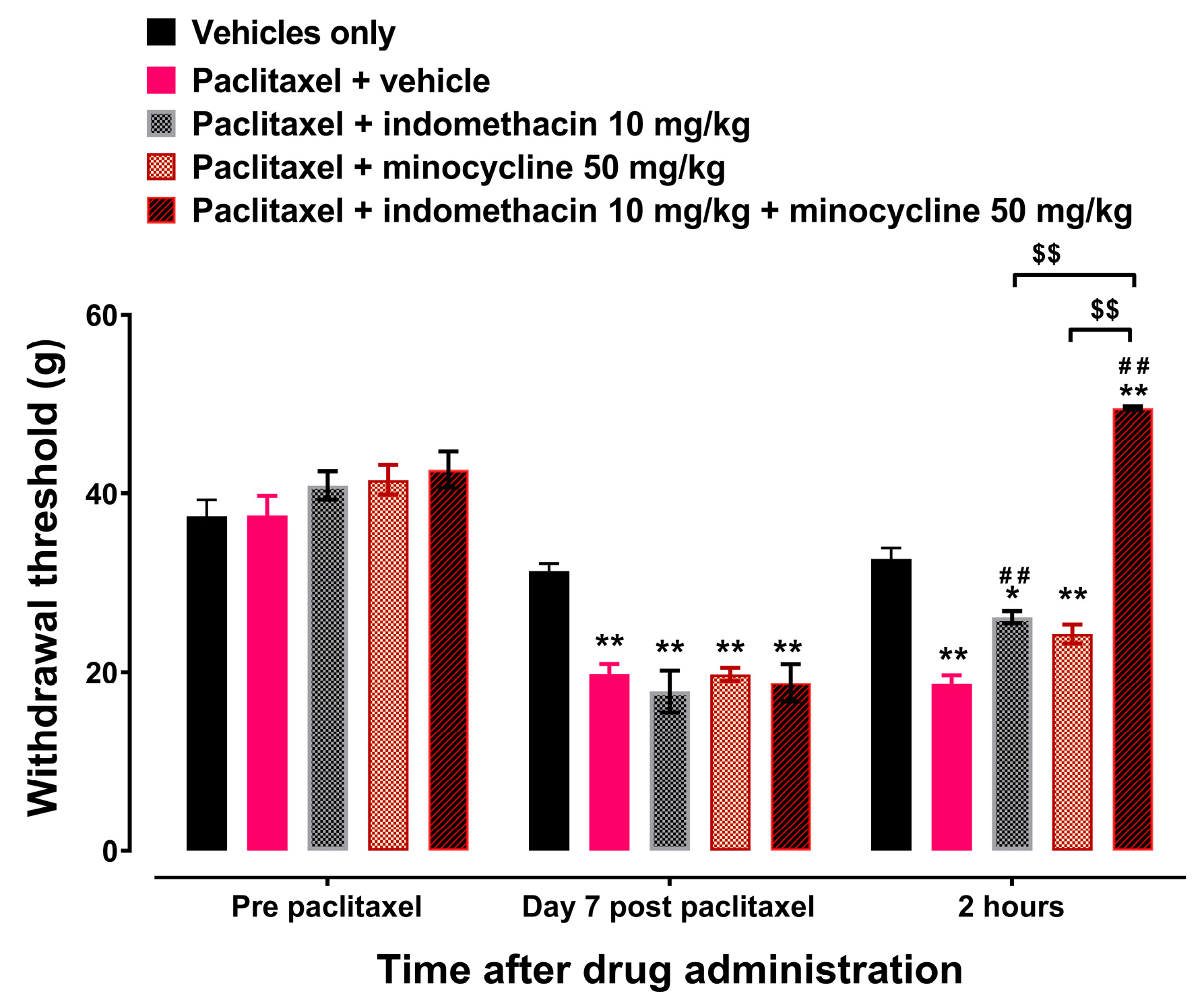
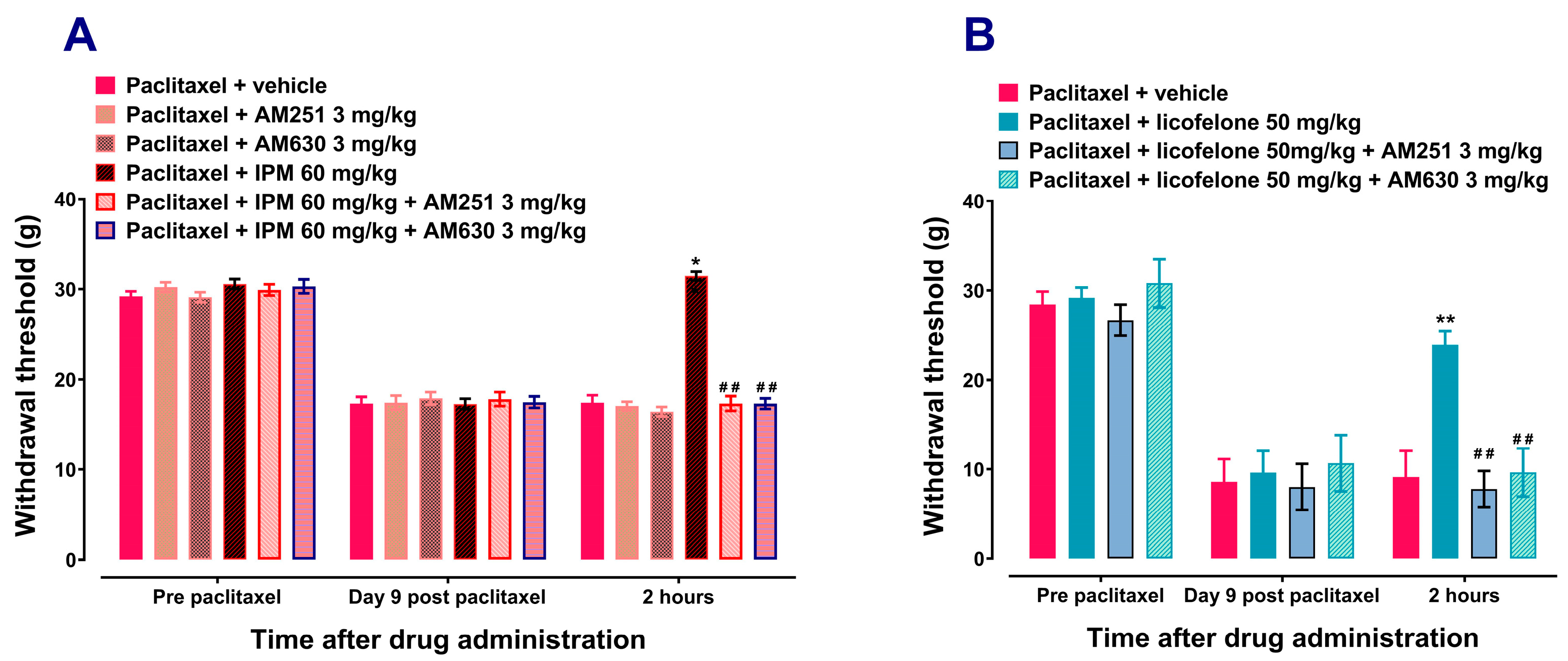
3.1. Licofelone and IPM Alleviate Paclitaxel-Induced Mechanical Allodynia in a CB Receptor-Dependent Manner
3.2. Licofelone, THC, and WIN 55,212-2 Interact with Similar CB1 and CB2 Receptor Cavities with Comparable Affinities
| Licofelone to CB1 C1 Chain A: | PHE108 ILE169 PHE170 SER173 PHE174 ASP176 PHE177 HIS178 ASP184 PHE189 LYS192 LEU193 GLY194 VAL196 THR197 ALA198 PHE200 THR201 ILE267 PHE268 PRO269 TYR275 LEU276 TRP279 TRP356 LEU359 MET363 PHE379 ALA380 SER383 MET384 LEU385 CYS386 LEU387 |
| THC to CB1 C1 Chain A: | PHE108 ILE169 PHE170 SER173 PHE174 ASP176 PHE177 HIS178 ARG182 ASP184 PHE189 LYS192 LEU193 VAL196 THR197 PHE200 ILE267 PHE268 PRO269 TRP279 TRP356 LEU359 MET363 PHE379 SER383 CYS386 |
| WIN 55,212-2 to CB1 C1 Chain A: | Chain A: PHE108 VAL110 PHE170 SER173 PHE174 PHE177 HIS178 PHE189 LYS192 LEU193 VAL196 THR197 PHE200 ILE267 PHE268 PRO269 ILE271 TYR275 LEU276 TRP279 TRP356 LEU359 MET363 LYS376 PHE379 ALA380 SER383 CYS386 |
| Licofelone to CB2 C2 Chain A: | TYR25 VAL86 PHE87 SER90 PHE91 PHE94 HIS95 PHE106 LYS109 ILE110 VAL113 THR114 PHE117 LEU182 PHE183 PRO184 TRP258 VAL261 MET265 LYS278 PHE281 ALA282 SER285 CYS288 |
| THC to CB2 C2 Chain A: | TYR25 PHE87 SER90 PHE91 PHE94 HIS95 PHE106 LYS109 ILE110 GLY111 VAL113 THR114 PHE117 LEU182 PHE183 PRO184 LEU191 TRP194 TRP258 VAL261 MET265 PHE281 ALA282 SER285 CYS288 |
| WIN 55,212-2 to CB2 C2 Chain A: | Chain A: TYR25 MET26 PHE87 SER90 PHE91 PHE94 HIS95 PHE106 LYS109 ILE110 GLY111 VAL113 THR114 PHE117 GLU181 LEU182 PHE183 PRO184 ILE186 TYR190 LEU191 TRP194 TRP258 VAL261 MET265 LYS278 PHE281 ALA282 SER285 CYS288 |
4. Discussion

5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argyriou, A.A.; Bruna, J.; Kalofonou, F.; Velasco, R.; Litsardopoulos, P.; Alemany, M.; Anastopoulou, G.G.; Kalofonos, H.P. Incidence and risk factors for developing chemotherapy-induced neuropathic pain in 500 cancer patients: A file-based observational study. J. Peripher. Nerv. Syst. 2024, 29, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Salat, K. Chemotherapy-induced peripheral neuropathy: Part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Kuroi, K.; Shimozuma, K. Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer 2004, 11, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gutierrez, G.; Sereno, M.; Miralles, A.; Casado-Saenz, E.; Gutierrez-Rivas, E. Chemotherapy-induced peripheral neuropathy: Clinical features, diagnosis, prevention and treatment strategies. Clin. Transl. Oncol. 2010, 12, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Speck, R.M.; Sammel, M.D.; Farrar, J.T.; Hennessy, S.; Mao, J.J.; Stineman, M.G.; DeMichele, A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 2013, 9, e234–e240. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Jahn, F.; Sauer, S.; Jordan, K. Prevention and Management of Chemotherapy-Induced Polyneuropathy. Breast Care 2019, 14, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W.; Thomas, A. Indomethacin plus minocycline coadministration relieves chemotherapy and antiretroviral drug-induced neuropathic pain in a cannabinoid receptors-dependent manner. J. Pharmacol. Sci. 2019, 139, 325–332. [Google Scholar] [CrossRef]
- Parvathy, S.S.; Masocha, W. Coadministration of indomethacin and minocycline attenuates established paclitaxel-induced neuropathic thermal hyperalgesia: Involvement of cannabinoid CB1 receptors. Sci. Rep. 2015, 5, 10541. [Google Scholar] [CrossRef]
- Daoud, K.F.; Jackson, C.G.; Williams, H.J. Basic therapy for rheumatoid arthritis: Nonsteroidal anti-inflammatory drugs. Compr. Ther. 1999, 25, 427–433. [Google Scholar] [CrossRef]
- Daymond, T.J.; Rowell, F.J. Reduction of prostaglandin E2 concentrations in synovial fluid of patients suffering from rheumatoid arthritis following tiaprofenic acid or indomethacin treatment. Drugs 1988, 35 (Suppl. S1), 4–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wei, E.Q.; Zhang, W.P.; Zhang, L.; Liu, J.R.; Chen, Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport 2004, 15, 2181–2184. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Patil, C.S.; Kumar, A.; Kulkarni, S.K. Potentiation of antinociceptive effect of NSAIDs by a specific lipooxygenase inhibitor, acetyl 11-keto-beta boswellic acid. Indian. J. Exp. Biol. 2006, 44, 128–132. [Google Scholar] [PubMed]
- Burnett, B.P.; Levy, R.M. 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 2012, 29, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.L.; Ethiraj, K.R.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar] [CrossRef]
- Razavi, S.M.; Khayatan, D.; Arab, Z.N.; Momtaz, S.; Zare, K.; Jafari, R.M.; Dehpour, A.R.; Abdolghaffari, A.H. Licofelone, a potent COX/5-LOX inhibitor and a novel option for treatment of neurological disorders. Prostaglandins Other Lipid Mediat. 2021, 157, 106587. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Singh, V.P. Licofelone—A novel analgesic and anti-inflammatory agent. Curr. Top. Med. Chem. 2007, 7, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.; Smit, I.; Baier, V.; Cordes, H.; Fabry, B.; Blank, L.M.; Kuepfer, L. Using quantitative systems pharmacology to evaluate the drug efficacy of COX-2 and 5-LOX inhibitors in therapeutic situations. NPJ Syst. Biol. Appl. 2018, 4, 28. [Google Scholar] [CrossRef]
- Singh, V.P.; Patil, C.S.; Kulkarni, S.K. Effect of licofelone against mechanical hyperalgesia and cold allodynia in the rat model of incisional pain. Pharmacol. Rep. 2005, 57, 380–384. [Google Scholar]
- Dulin, J.N.; Karoly, E.D.; Wang, Y.; Strobel, H.W.; Grill, R.J. Licofelone modulates neuroinflammation and attenuates mechanical hypersensitivity in the chronic phase of spinal cord injury. J. Neurosci. 2013, 33, 652–664. [Google Scholar] [CrossRef]
- Nashawi, H.; Masocha, W.; Edafiogho, I.O.; Kombian, S.B. Paclitaxel Causes Electrophysiological Changes in the Anterior Cingulate Cortex via Modulation of the gamma-Aminobutyric Acid-ergic System. Med. Princ. Pract. 2016, 25, 423–428. [Google Scholar] [CrossRef]
- Munawar, N.; Oriowo, M.A.; Masocha, W. Antihyperalgesic Activities of Endocannabinoids in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Front. Pharmacol. 2017, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Priego, C.G.; Mendez-Mena, R.; Banos-Gonzalez, M.A.; Araiza-Saldana, C.I.; Castaneda-Corral, G.; Torres-Lopez, J.E. Antihyperalgesic Effects of Indomethacin, Ketorolac, and Metamizole in Rats: Effects of Metformin. Drug Dev. Res. 2017, 78, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.; Sagen, J. Antinociceptive effect of cannabinoid agonist WIN 55,212-2 in rats with a spinal cord injury. Exp. Neurol. 2007, 204, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, G.; Mansouri, M.T.; Naghizadeh, B.; Hemmati, A.A.; Hashemitabar, M. Potentiation of indomethacin-induced anti-inflammatory response by pioglitazone in carrageenan-induced acute inflammation in rats: Role of PPARgamma receptors. Int. Immunopharmacol. 2016, 38, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Kelly, J.P.; O’Driscoll, M.; Finn, D.P. Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats. Immunology 2008, 125, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Hajhashemi, V.; Hosseinzadeh, H. Minocycline potentiates the anti-hyperalgesic effect of ceftriaxone in CCI-induced neuropathic pain in rats. Res. Pharm. Sci. 2015, 10, 34–42. [Google Scholar] [PubMed]
- Mika, J.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur. J. Pharmacol. 2007, 560, 142–149. [Google Scholar] [CrossRef]
- Thangamani, D.; Edafiogho, I.O.; Masocha, W. The anticonvulsant enaminone E139 attenuates paclitaxel-induced neuropathic pain in rodents. Sci. World J. 2013, 2013, 240508. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y. Protein-Ligand Blind Docking Using CB-Dock2. Methods Mol. Biol. 2024, 2714, 113–125. [Google Scholar] [CrossRef]
- Abu-Ghefreh, A.A.; Masocha, W. Enhancement of antinociception by coadministration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naive mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet. Disord. 2010, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cicuttini, F. Licofelone (Merckle). IDrugs 2003, 6, 802–808. [Google Scholar] [PubMed]
- Alhouayek, M.; Muccioli, G.G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef]
- Hampson, A.J.; Hill, W.A.; Zan-Phillips, M.; Makriyannis, A.; Leung, E.; Eglen, R.M.; Bornheim, L.M. Anandamide hydroxylation by brain lipoxygenase:metabolite structures and potencies at the cannabinoid receptor. Biochim. Biophys. Acta 1995, 1259, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Fiorucci, L.; Erba, F.; Bari, M.; Finazzi-Agro, A.; Ascoli, F. Human mast cells take up and hydrolyze anandamide under the control of 5-lipoxygenase and do not express cannabinoid receptors. FEBS Lett. 2000, 468, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Gaw, A.G.; Okine, B.N.; Woodhams, S.G.; Wong, A.; Kendall, D.A.; Chapman, V. Dynamic regulation of the endocannabinoid system: Implications for analgesia. Mol. Pain. 2009, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Gonsiorek, W.; Lunn, C.; Fan, X.; Narula, S.; Lundell, D.; Hipkin, R.W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol. Pharmacol. 2000, 57, 1045–1050. [Google Scholar]
- Grueter, B.A.; Brasnjo, G.; Malenka, R.C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 2010, 13, 1519–1525. [Google Scholar] [CrossRef]
- Hillard, C.J.; Manna, S.; Greenberg, M.J.; DiCamelli, R.; Ross, R.A.; Stevenson, L.A.; Murphy, V.; Pertwee, R.G.; Campbell, W.B. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J. Pharmacol. Exp. Ther. 1999, 289, 1427–1433. [Google Scholar] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Savinainen, J.R.; Jarvinen, T.; Laine, K.; Laitinen, J.T. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001, 134, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Stella, N.; Schweitzer, P.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Jakowiecki, J.; Filipek, S. Hydrophobic Ligand Entry and Exit Pathways of the CB1 Cannabinoid Receptor. J. Chem. Inf. Model. 2016, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Lesniak, A.; Bujalska-Zadrozny, M.; Pawinski, T.; Sulkowska, J.I. Identification of Novel CB2 Ligands through Virtual Screening and In Vitro Evaluation. J. Chem. Inf. Model. 2023, 63, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- El-Atawneh, S.; Goldblum, A. Candidate Therapeutics by Screening for Multitargeting Ligands: Combining the CB2 Receptor With CB1, PPARgamma and 5-HT4 Receptors. Front. Pharmacol. 2022, 13, 812745. [Google Scholar] [CrossRef]
- Anthony, A.T.; Rahmat, S.; Sangle, P.; Sandhu, O.; Khan, S. Cannabinoid Receptors and Their Relationship with Chronic Pain: A Narrative Review. Cureus 2020, 12, e10436. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. A physiological role for endocannabinoid-derived products of cyclooxygenase-2-mediated oxidative metabolism. Br. J. Pharmacol. 2008, 153, 1341–1343. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Aly, E.; Khajah, M.A.; Masocha, W. beta-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain. Molecules 2019, 25, 106. [Google Scholar] [CrossRef]
- Milligan, A.L.; Szabo-Pardi, T.A.; Burton, M.D. Cannabinoid Receptor Type 1 and Its Role as an Analgesic: An Opioid Alternative? J. Dual Diagn. 2020, 16, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Pascual, D.; Goicoechea, C.; Suardiaz, M.; Martin, M.I. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 2005, 118, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Fonseca, F.; Del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol. Alcohol. 2005, 40, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Okine, B.N.; Finn, D.P.; Masocha, W. Peripheral deficiency and antiallodynic effects of 2-arachidonoyl glycerol in a mouse model of paclitaxel-induced neuropathic pain. Biomed. Pharmacother. 2020, 129, 110456. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007, 132 (Suppl. S1), S26–S45. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Araldi, D.; Green, P.G.; Levine, J.D. Marked sexual dimorphism in neuroendocrine mechanisms for the exacerbation of paclitaxel-induced painful peripheral neuropathy by stress. Pain 2020, 161, 865–874. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Kim, E.S.; Kim, C.H.; Kwon, J.Y.; Kim, H.K. Gender differences in paclitaxel-induced neuropathic pain behavior and analgesic response in rats. Korean J. Anesthesiol. 2012, 62, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Legakis, L.P.; Bigbee, J.W.; Negus, S.S. Lack of paclitaxel effects on intracranial self-stimulation in male and female rats: Comparison to mechanical sensitivity. Behav. Pharmacol. 2018, 29, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef] [PubMed]
- Huss, M.K.; Pacharinsak, C. A Review of Long-acting Parenteral Analgesics for Mice and Rats. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 595–602. [Google Scholar] [CrossRef]
- Oh, S.S.; Narver, H.L. Mouse and Rat Anesthesia and Analgesia. Curr. Protoc. 2024, 4, e995. [Google Scholar] [CrossRef]


| Group | Nature of Experiment | Number of Animals per Group |
|---|---|---|
| Group 1 | Effects of licofelone on paclitaxel-induced mechanical allodynia. | 32 |
| Group 2 | Effects of indomethacin plus minocycline combination (IPM) on paclitaxel-induced mechanical allodynia. | 40 |
| Group 3 | Effects of cannabinoid receptor antagonists on indomethacin plus minocycline combination (IPM)’s antiallodynic activities | 45 |
| Group 4 | Effects of cannabinoid receptor antagonists on licofelone’s antiallodynic activities | 20 |
| CurPocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center (x, y, z) | Docking Size (x, y, z) | Contact Residues |
|---|---|---|---|---|---|
| C1 | −8.1 | 3456 | −39, −168, 310 | 29, 21, 21 | Chain A: PHE108 ILE169 PHE170 SER173 PHE174 ASP176 PHE177 HIS178 ASP184 PHE189 LYS192 LEU193 GLY194 VAL196 THR197 ALA198 PHE200 THR201 ILE267 PHE268 PRO269 TYR275 LEU276 TRP279 TRP356 LEU359 MET363 PHE379 ALA380 SER383 MET384 LEU385 CYS386 LEU387 |
| C2 | −8.1 | 889 | −41, −111, 245 | 21, 21, 21 | Chain A: SER1010 THR1011 THR1012 ASN1014 THR1015 SER1058 THR1059 TRP1060 GLY1061 ASP1062 CYS1093 GLY1094 ASP1095 TRP1098 TYR1100 GLY1128 ASP1129 |
| C3 | −7.3 | 881 | −46, −138, 299 | 31, 21, 21 | Chain A: ARG150 PRO151 SER152 TYR153 HIS154 PHE155 ILE156 LEU209 ALA210 ALA211 ASP213 ARG214 TYR224 VAL228 THR229 ARG230 ALA233 PHE237 TYR294 THR344 ILE396 TYR397 ARG400 SER401 LYS402 ASP403 |
| C4 | −6.3 | 395 | −41, −135, 285 | 21, 21, 21 | Chain A: ARG214 SER217 ILE218 HIS219 PRO221 MET295 ILE297 LEU298 TRP299 LYS300 ALA301 HIS302 HIS304 ALA305 MET337 ASP338 LEU341 ALA342 LYS343 VAL346 |
| C5 | −5.5 | 236 | −42, −132, 273 | 21, 21, 21 | Chain A: TRP299 LYS300 HIS302 SER303 HIS304 ALA305 VAL306 ALA1002 LYS1003 ALA1029 GLY1030 TYR1031 GLU1032 ASP1034 GLU1048 GLY1049 PHE1050 ASP1051 LEU1052 ARG1086 LYS1087 ILE1148 MET337 |
| CurPocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center (x, y, z) | Docking Size (x, y, z) | Contact Residues |
|---|---|---|---|---|---|
| C1 | −8.1 | 1967 | −3, −18, 16 | 21, 32, 21 | Chain A: ASP1009 GLU1010 TYR1017 ASP1019 THR1020 GLU1021 TYR1023 THR1025 ILE1028 GLY1029 HIS1030 LEU1031 LYS1034 ASP1069 VAL1102 PHE1103 GLN1104 MET1105 GLY1106 GLU1107 GLN1140 THR1141 ARG1144 |
| C2 | −9.4 | 910 | 9, 2, −47 | 21, 21, 21 | Chain A: TYR25 VAL86 PHE87 SER90 PHE91 PHE94 HIS95 PHE106 LYS109 ILE110 VAL113 THR114 PHE117 LEU182 PHE183 PRO184 TRP258 VAL261 MET265 LYS278 PHE281 ALA282 SER285 CYS288 |
| C3 | −5.8 | 875 | 15, −8, −10 | 21, 21, 21 | Chain A: HIS62 GLN63 ARG66 LYS67 PRO68 SER69 TYR70 ARG131 CYS134 PRO138 PRO139 TYR141 LYS142 ALA143 LEU145 THR146 ARG147 ALA235 ARG236 MET237 ARG238 LEU239 ASP240 LEU243 GLU305 |
| C4 | −6.1 | 423 | 10, 9, −18 | 21, 21, 21 | Chain A: LEU49 GLU50 ASN51 VAL52 ALA53 VAL54 TYR56 LEU57 SER60 PRO296 VAL297 ILE298 ALA300 LEU301 ILE306 ARG307 SER309 ALA310 HIS311 CYS313 LEU314 |
| C5 | −5.4 | 312 | 18, 3, −57 | 21, 21, 21 | Chain A: ALA19 PRO20 PRO21 MET22 LYS23 MET26 HIS98 VAL100 ASP101 SER102 LYS103 CYS174 PRO184 LEU185 |
| CurPocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center (x, y, z) | Docking Size (x, y, z) | Contact Residues |
|---|---|---|---|---|---|
| C1 | −11.2 | 3456 | −39, −168, 310 | 29, 22, 22 | Chain A: PHE108 ILE169 PHE170 SER173 PHE174 ASP176 PHE177 HIS178 ARG182 ASP184 PHE189 LYS192 LEU193 VAL196 THR197 PHE200 ILE267 PHE268 PRO269 TRP279 TRP356 LEU359 MET363 PHE379 SER383 CYS386 |
| C2 | −7.7 | 889 | −41, −111, 245 | 22, 22, 22 | Chain A: THR1012 ASN1014 SER1058 THR1059 TRP1060 GLY1061 ASP1062 SER1064 ILE1065 GLU1066 CYS1093 GLY1094 ASP1095 TRP1098 TYR1100 GLY1128 ASP1129 PRO1130 |
| C3 | −8.9 | 881 | −46, −138, 299 | 31, 22, 22 | Chain A: SER152 TYR153 HIS154 PHE155 ILE156 LEU209 ALA210 ALA211 ASP213 ARG214 TYR224 ILE227 VAL228 THR229 ARG230 LYS232 ALA233 ALA236 PHE237 TYR294 GLU340 LEU341 THR344 LEU345 ILE348 ILE396 TYR397 ALA398 ARG400 SER401 ARG405 |
| C4 | −6.0 | 395 | −41, −135, 285 | 22, 22, 22 | Chain A: ARG214 SER217 ILE218 PRO221 MET295 ILE297 LEU298 TRP299 LYS300 ALA301 HIS302 HIS304 ALA305 VAL306 MET337 ASP338 ILE339 GLU340 LEU341 ALA342 LYS343 THR344 LEU345 VAL346 ILE348 ILE396 TYR397 ARG400 |
| C5 | −5.7 | 236 | −42, −132, 273 | 22, 22, 22 | Chain A: ILE297 LEU298 TRP299 ALA301 HIS302 SER303 HIS304 ALA305 VAL306 ALA1002 LYS1003 ALA1004 ALA1029 GLY1030 TYR1031 GLU1032 VAL1033 ASP1034 GLY1049 PHE1050 ASP1051 LEU1052 ARG1145 ILE1148 MET337 ASP338 ILE339 ALA342 |
| CurPocket ID | Vina Score (kcal/mol) | Cavity Volume (Å3) | Center (x, y, z) | Docking Size (x, y, z) | Contact Residues |
|---|---|---|---|---|---|
| C1 | −7.7 | 1967 | −3, −18, 16 | 22, 32, 22 | Chain A: ASP1009 GLU1010 TYR1017 LYS1018 ASP1019 THR1020 GLU1021 TYR1023 THR1025 ILE1028 GLY1029 HIS1030 LEU1031 LYS1034 ASP1069 ALA1072 ALA1073 VAL1102 PHE1103 GLN1104 MET1105 GLY1106 GLU1107 GLN1140 THR1141 ARG1144 |
| C2 | −11.5 | 910 | 9, 2, −47 | 22, 22, 22 | Chain A: TYR25 PHE87 SER90 PHE91 PHE94 HIS95 PHE106 LYS109 ILE110 GLY111 VAL113 THR114 PHE117 LEU182 PHE183 PRO184 LEU191 TRP194 TRP258 VAL261 MET265 PHE281 ALA282 SER285 CYS288 |
| C3 | −5.9 | 875 | 15, −8, −10 | 22, 22, 22 | Chain A: HIS62 GLN63 ARG66 LYS67 PRO68 SER69 TYR70 ARG131 CYS134 TYR141 LYS142 ALA143 LEU145 THR146 ARG147 ALA235 ARG236 MET237 ARG238 LEU239 ASP240 LEU243 GLU305 |
| C4 | −6.8 | 423 | 10, 9, −18 | 22, 22, 22 | Chain A: LEU46 LEU49 GLU50 VAL52 ALA53 VAL54 TYR56 LEU57 SER60 PRO296 VAL297 TYR299 ALA300 LEU301 ILE306 ARG307 ALA310 CYS313 LEU314 ALA315 |
| C5 | −5.3 | 312 | 18, 3, −57 | 22, 22, 22 | Chain A: ALA19 PRO20 PRO21 MET22 LYS23 MET26 HIS98 VAL100 ASP101 SER102 LYS103 PRO184 LEU185 |
| Ligand | Vina Scores (kcal/mol) | |
|---|---|---|
| CB1 | CB2 | |
| Licofelone | −8.1 | −9.4 |
| THC | −11.2 | −11.5 |
| WIN 55,212-2 | −10.6 | −13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masocha, W.; Aly, E.; Albaloushi, A.; Al-Romaiyan, A. Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner. Biomedicines 2024, 12, 1545. https://doi.org/10.3390/biomedicines12071545
Masocha W, Aly E, Albaloushi A, Al-Romaiyan A. Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner. Biomedicines. 2024; 12(7):1545. https://doi.org/10.3390/biomedicines12071545
Chicago/Turabian StyleMasocha, Willias, Esraa Aly, Aisha Albaloushi, and Altaf Al-Romaiyan. 2024. "Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner" Biomedicines 12, no. 7: 1545. https://doi.org/10.3390/biomedicines12071545
APA StyleMasocha, W., Aly, E., Albaloushi, A., & Al-Romaiyan, A. (2024). Licofelone, a Dual COX/LOX Inhibitor, Ameliorates Paclitaxel-Induced Mechanical Allodynia in Rats in a Cannabinoid Receptor-Dependent Manner. Biomedicines, 12(7), 1545. https://doi.org/10.3390/biomedicines12071545





