Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
2.2. Study Participants
2.3. Peripheral Blood Mononuclear Cell Isolation
2.4. Ex Vivo Stimulation of PBMCs with Candida spp.
2.5. Quantification of Cytokines in Culture Supernatants
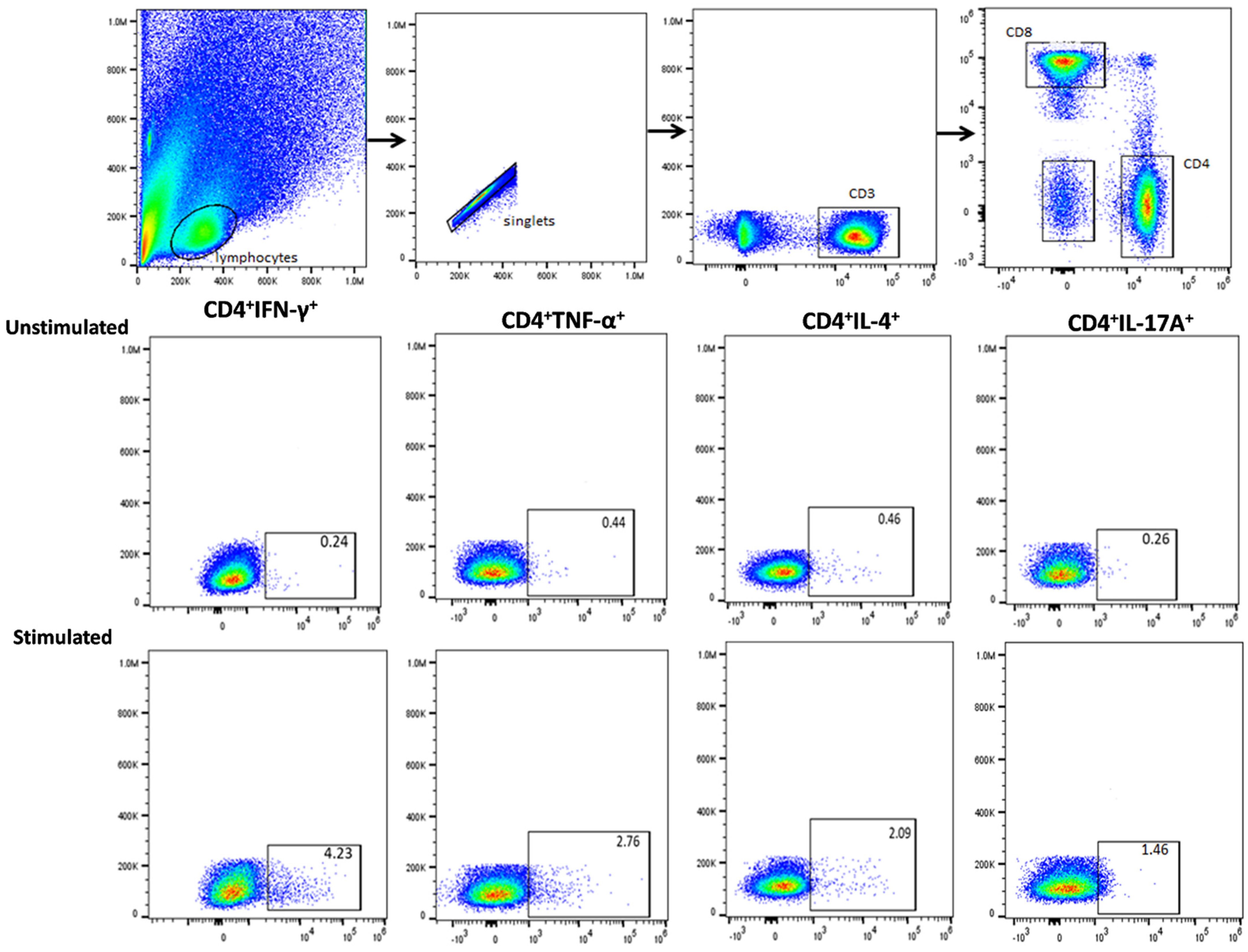
2.6. Immunostaining and Flow Cytometry
2.7. Statistical Analysis
3. Results
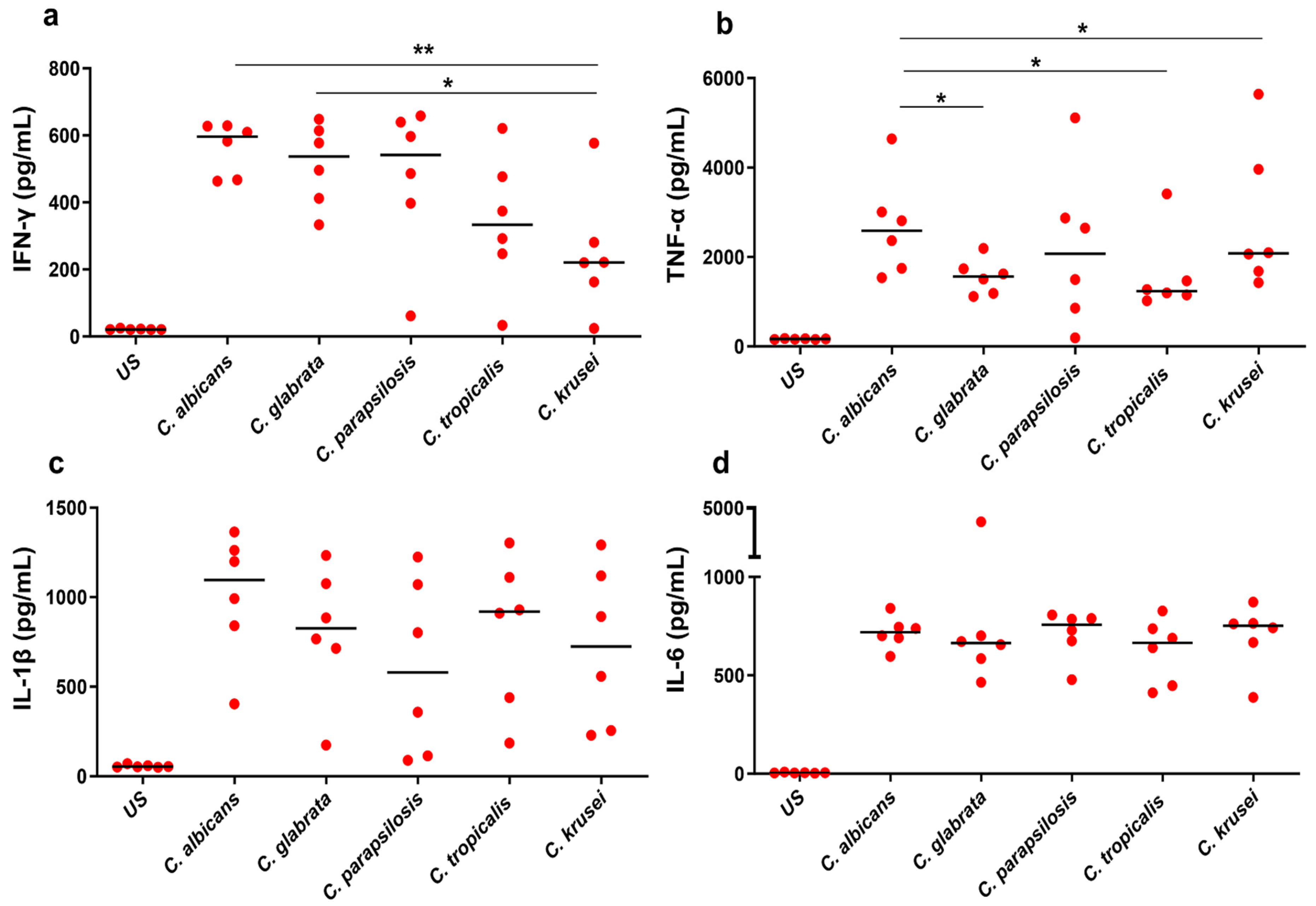
3.1. Ex Vivo Cytokine Response to Candida spp.
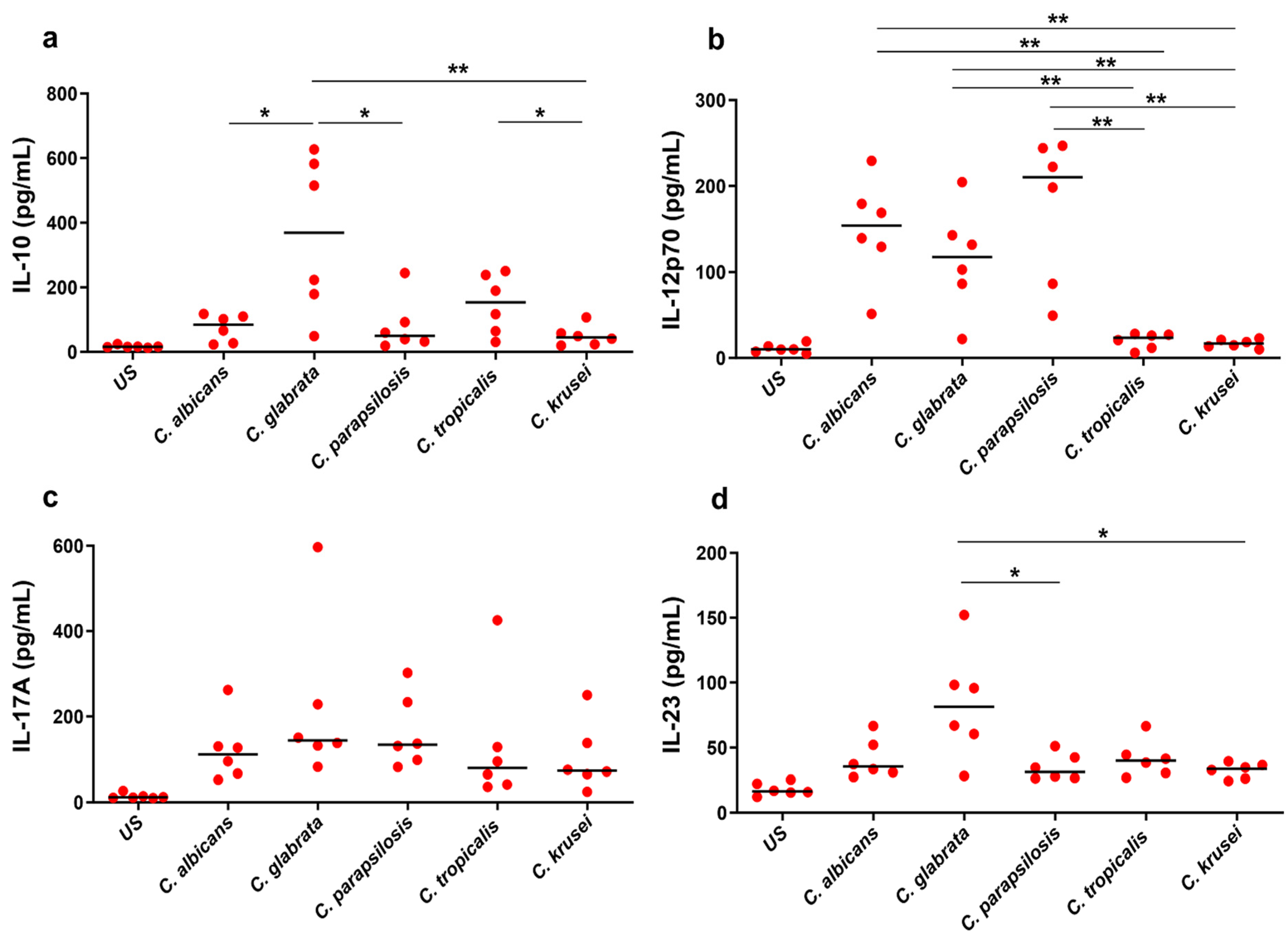
3.2. Differential Cytokine Responses to Candida spp.
3.3. Functional Signature of Candida-Specific CD4+ and CD8+ T-Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Ellis, D.; Tullio, V.; Rodloff, A.; Fu, W.; Ling, T.A.; Global Antifungal Surveillance, G. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 2010, 48, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.F.; Yu, K.W.; Tang, R.B.; Fan, Y.H.; Yang, Y.L.; Hsieh, K.S.; Ho, M.; Lo, H.J. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn. Microbiol. Infect. Dis. 2004, 48, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Quindós, G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev. Iberoam. Micol. 2014, 31, 42–48. [Google Scholar] [CrossRef]
- Ashman, R.B.; Fulurija, A.; Papadimitriou, J.M. Both CD4+ and CD8+ lymphocytes reduce the severity of tissue lesions in murine systemic cadidiasis, and CD4+ cells also demonstrate strain-specific immunopathological effects. Microbiology 1999, 145 Pt 7, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Haraguchi, N.; Ishii, Y.; Morishima, Y.; Yoh, K.; Matsuno, Y.; Kikuchi, N.; Sakamoto, T.; Takahashi, S.; Hizawa, N. Impairment of host defense against disseminated candidiasis in mice overexpressing GATA-3. Infect. Immun. 2010, 78, 2302–2311. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jäger, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef]
- Hernández-Santos, N.; Huppler, A.R.; Peterson, A.C.; Khader, S.A.; McKenna, K.C.; Gaffen, S.L. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013, 6, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Na, L.; Fidel, P.L.; Schwarzenberger, P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004, 190, 624–631. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Joosten, L.A.; Shaw, P.J.; Smeekens, S.P.; Malireddi, R.K.; van der Meer, J.W.; Kullberg, B.J.; Netea, M.G.; Kanneganti, T.D. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. Eur. J. Immunol. 2011, 41, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- De Luca, A.; Carvalho, A.; Cunha, C.; Iannitti, R.G.; Pitzurra, L.; Giovannini, G.; Mencacci, A.; Bartolommei, L.; Moretti, S.; Massi-Benedetti, C.; et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 2013, 9, e1003486. [Google Scholar] [CrossRef]
- Nosek, J.; Holesova, Z.; Kosa, P.; Gacser, A.; Tomaska, L. Biology and genetics of the pathogenic yeast Candida parapsilosis. Curr. Genet. 2009, 55, 497–509. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Lewis, R.E.; Tarrand, J.; Hachem, R.; Rolston, K.V.; Raad, I.I.; Kontoyiannis, D.P. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): Stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009, 115, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef]
- Nucci, M.; Colombo, A.L. Candidemia due to Candida tropicalis: Clinical, epidemiologic, and microbiologic characteristics of 188 episodes occurring in tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 2007, 58, 77–82. [Google Scholar] [CrossRef]
- Abbas, J.; Bodey, G.P.; Hanna, H.A.; Mardani, M.; Girgawy, E.; Abi-Said, D.; Whimbey, E.; Hachem, R.; Raad, I. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch. Intern. Med. 2000, 160, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Prayag, P.S.; Patwardhan, S.; Panchakshari, S.; Rajhans, P.A.; Prayag, A. The Dominance of Candida auris: A Single-center Experience of 79 Episodes of Candidemia from Western India. Indian J. Crit. Care Med. 2022, 26, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Marolda, A.; Hunniger, K.; Bottcher, S.; Vivas, W.; Loffler, J.; Figge, M.T.; Kurzai, O. Candida Species-Dependent Release of IL-12 by Dendritic Cells Induces Different Levels of NK Cell Stimulation. J. Infect. Dis. 2020, 221, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.; Essig, F.; Hunniger, K.; Mokhtari, Z.; Bauer, L.; Lehnert, T.; Brandes, S.; Hader, A.; Jacobsen, I.D.; Martin, R.; et al. Neutrophil activation by Candida glabrata but not Candida albicans promotes fungal uptake by monocytes. Cell Microbiol. 2015, 17, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, T.; Carrete, L. The birth of a deadly yeast: Tracing the evolutionary emergence of virulence traits in Candida glabrata. FEMS Yeast Res. 2016, 16, fov110. [Google Scholar] [CrossRef] [PubMed]
- Kammer, P.; McNamara, S.; Wolf, T.; Conrad, T.; Allert, S.; Gerwien, F.; Hunniger, K.; Kurzai, O.; Guthke, R.; Hube, B.; et al. Survival Strategies of Pathogenic Candida Species in Human Blood Show Independent and Specific Adaptations. mBio 2020, 11, 02435-20. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal Nomenclature: Managing Change is the Name of the Game. Open Forum Infect. Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef] [PubMed]
- Whibley, N.; Gaffen, S.L. Beyond Candida albicans: Mechanisms of immunity to non-albicans Candida species. Cytokine 2015, 76, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii Complex and Candida auris: Biology, Virulence Factors, Immune Response, and Multidrug Resistance. Infect. Drug Resist. 2023, 16, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Csonka, K.; Jacobs, C.; Vágvölgyi, C.; Nosanchuk, J.D.; Netea, M.G.; Gácser, A. Candida albicans and Candida parapsilosis induce different T-cell responses in human peripheral blood mononuclear cells. J. Infect. Dis. 2013, 208, 690–698. [Google Scholar] [CrossRef]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.P.; Moyes, D.L. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Balish, E.; Wagner, R.D.; Vázquez-Torres, A.; Pierson, C.; Warner, T. Candidiasis in interferon-gamma knockout (IFN-gamma-/-) mice. J. Infect. Dis. 1998, 178, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, L.M.; Schopf, L.R.; Chung, C.L.; Maylor, R.; Sypek, J.P. The role of recombinant murine IL-12 and IFN-gamma in the pathogenesis of a murine systemic Candida albicans infection. J. Immunol. 1998, 160, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mencacci, A.; Del Sero, G.; Cenci, E.; d’Ostiani, C.F.; Bacci, A.; Montagnoli, C.; Kopf, M.; Romani, L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J. Exp. Med. 1998, 187, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.; Ferreira, P.; Arala-Chaves, M. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J. Infect. Dis. 2000, 182, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to Candida albicans: Th1, Th2 cells and beyond. Curr. Opin. Microbiol. 1999, 2, 363–367. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Ye, X.Q.; Iwakura, Y. Interleukin-17 family members in health and disease. Int. Immunol. 2021, 33, 723–729. [Google Scholar] [CrossRef]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santos, N.; Gaffen, S.L. Th17 cells in immunity to Candida albicans. Cell Host Microbe 2012, 11, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Rocker, M.; Blango, M.G.; Kaufmann, S.; Rohmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e15. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 2012, 484, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Mata, E.; Navarro-Arias, M.J.; Perez-Garcia, L.A.; Mellado-Mojica, E.; Lopez, M.G.; Csonka, K.; Gacser, A.; Mora-Montes, H.M. Members of the Candida parapsilosis Complex and Candida albicans are Differentially Recognized by Human Peripheral Blood Mononuclear Cells. Front. Microbiol. 2015, 6, 1527. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Front. Immunol. 2017, 8, 629. [Google Scholar] [CrossRef]
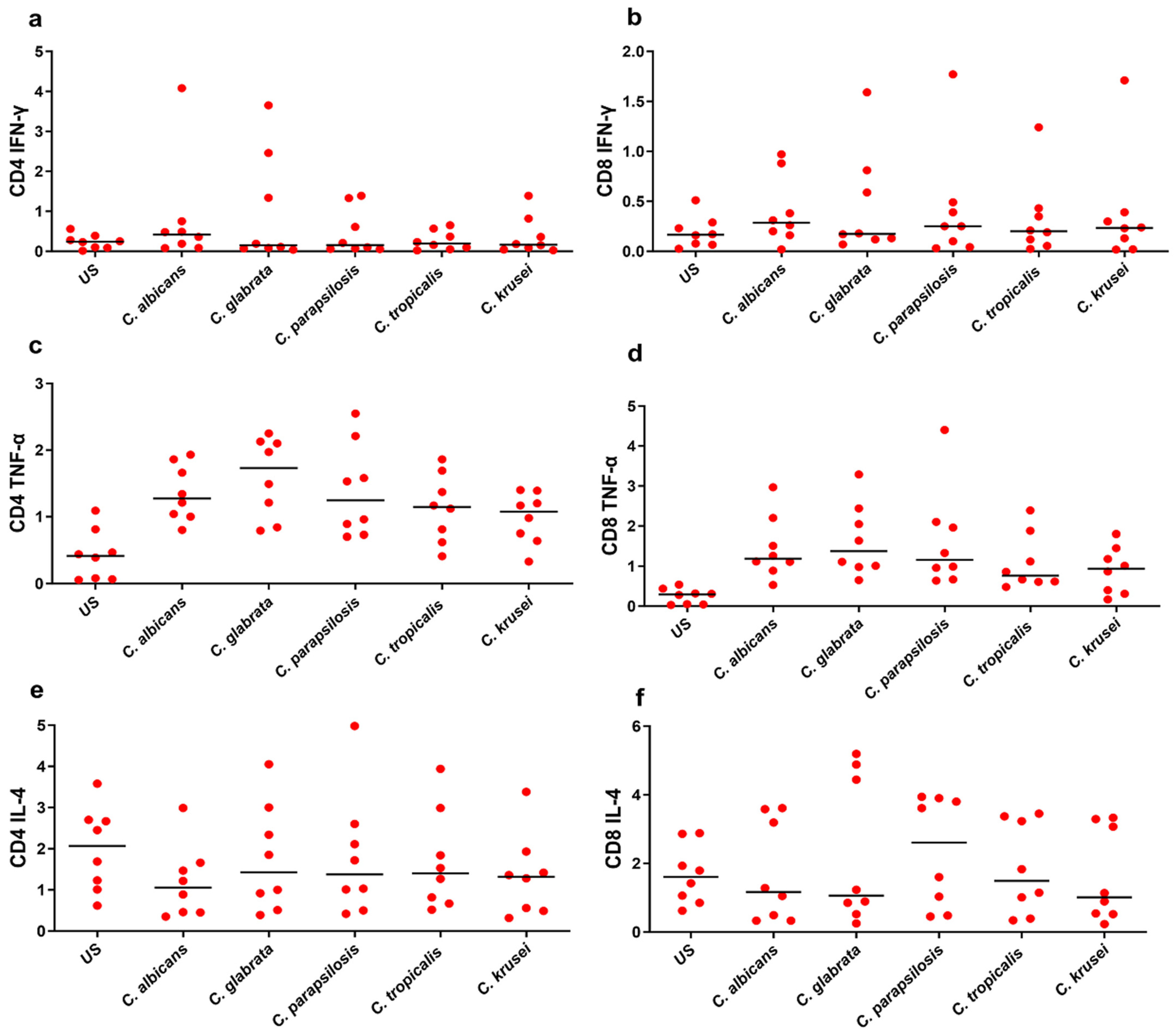
| Phenotype | US | C. albicans | C. glabrata | C. parapsilosis | C. tropicalis | C. krusei |
|---|---|---|---|---|---|---|
| CD4 IFN-γ | 0.24 | 0.42 | 0.15 | 0.155 | 0.195 | 0.165 |
| CD4 TNF-α | 0.415 | 1.275 | 1.73 | 1.245 | 1.145 | 1.075 |
| CD4 IL-4 | 2.07 | 1.055 | 1.425 | 1.375 | 1.4 | 1.32 |
| CD4 IL-17A | 0.235 | 0.4 | 0.645 | 0.51 | 0.455 | 0.505 |
| CD4 IL-22 | 0.115 | 0.22 | 0.24 | 0.2 | 0.175 | 0.19 |
| CD8 IFN-γ | 0.165 | 0.285 | 0.175 | 0.25 | 0.2 | 0.235 |
| CD8 TNF-α | 0.295 | 1.19 | 1.375 | 1.16 | 0.765 | 0.94 |
| CD8 IL-4 | 1.605 | 1.165 | 1.06 | 2.605 | 1.49 | 1.015 |
| CD8 IL-17A | 0.14 | 0.585 | 0.445 | 0.48 | 0.375 | 0.345 |
| CD8 IL-22 | 0.1105 | 0.17 | 0.205 | 0.0565 | 0.17 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathakumari, B.; Liu, W.; Wang, Q.; Kong, X.; Liang, G.; Chokkakula, S.; Pathakamuri, V.; Nunna, V. Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells. Biomedicines 2024, 12, 1487. https://doi.org/10.3390/biomedicines12071487
Pathakumari B, Liu W, Wang Q, Kong X, Liang G, Chokkakula S, Pathakamuri V, Nunna V. Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells. Biomedicines. 2024; 12(7):1487. https://doi.org/10.3390/biomedicines12071487
Chicago/Turabian StylePathakumari, Balaji, Weida Liu, Qiong Wang, Xue Kong, Guanzhao Liang, Santosh Chokkakula, Vasundhara Pathakamuri, and Venkatrao Nunna. 2024. "Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells" Biomedicines 12, no. 7: 1487. https://doi.org/10.3390/biomedicines12071487
APA StylePathakumari, B., Liu, W., Wang, Q., Kong, X., Liang, G., Chokkakula, S., Pathakamuri, V., & Nunna, V. (2024). Comparative Evaluation of Candida Species-Specific T-Cell Immune Response in Human Peripheral Blood Mononuclear Cells. Biomedicines, 12(7), 1487. https://doi.org/10.3390/biomedicines12071487






