Abstract
Background and aims: Thromboangiitis obliterans (TAO), also known as Buerger’s disease, is a rare vasculitis. Observational epidemiology studies have suggested a relationship between the gut microbiota and TAO. However, due to confounding factors and reverse causality, the causal relationship remains unclear. Based on the assumption of their association, this study sought specific gut microbiota causally linked to TAO. Methods: The case–control study was conducted at the Xiangya Hospital of Central South University from November 2022 to January 2023 including twelve TAO patients and nine healthy controls. We conducted a Mendelian randomization (MR) study using summary statistics from a genome-wide association study (GWAS) of gut microbiota and TAO. Considering the scale and accessibility of the data, the MiBioGen consortium served as the exposure, whereas the FinnGen consortium GWAS study served as the outcome. Finally, we compared the results of the MR with those of the case–control studies. Results: The inverse variance weighted (IVW) (OR = 0.119, 95% CI: 0.021–0.688, p = 0.017) and maximum likelihood (ML) (OR = 0.121, 95% CI: 0.020–0.742, p = 0.022) estimates suggest that Ruminiclostridium 5 has a suggestive protective effect on TAO while the IVW (OR = 5.383, 95% CI: 1.128–25.693, p = 0.035) and ML (OR = 5.658, 95% CI: 1.142–28.021, p = 0.034) estimates suggest that Eubacterium (xylanophilum group) has a suggestive risk effect on TAO, and the ML (OR = 0.055, 95% CI: 0.004–0.755, p = 0.030) estimates suggest that Lachnospira has a suggestive protective effect on TAO. No significant heterogeneity of instrumental variables or horizontal pleiotropy was found. The results of the case–control study showed that the TAO had a lower relative abundance of Ruminiclostridium 5 (p = 0.015) and Lachnospira (p = 0.048), and a higher relative abundance of Eubacterium (xylanophilum group) (p = 0.029) than the healthy controls. These results were consistent with the MR analysis. Conclusions: Our study demonstrates that Ruminiclostridium 5, Lachnospira, and Eubacterium (xylanophilum group) are causally related to TAO, suggesting their potential significance for the prevention and treatment of TAO.
1. Introduction
Thromboangiitis obliterans (TAO) is a chronic inflammatory disease that primarily affects small- and medium-sized arteries and veins [1], characterized by the infiltration of inflammatory cells and thrombosis. The risk factors for TAO include smoking, periodontal disease, and genetic factors [2]. However, the exact cause of the disease remains unknown, and both the underdiagnosis and misdiagnosis of TAO continue to occur at high rates [2,3]. Although surgery is the primary treatment, long-term vascular patency rates are poor, and additional interventions are often necessary. Patients frequently suffer from symptoms such as active ulcers and resting pain, leading to a poor quality of life [4,5].
The human gastrointestinal microbiota plays a crucial role in immunological functions and metabolic processes including host immunity, food digestion, intestinal endocrine function, and intestinal permeability [6]. These microbial metabolites can enter the peripheral circulation through the portal vein [7] or are easily absorbed by the gut mucosa [8] where they can act as substrates or signaling molecules in organs. Microbiota can also exacerbate systemic vascular inflammation and generate bacterial by-products through metabolism-independent mechanisms [9]. The gut microbiota is a crucial underlying player during sepsis pathogenesis [10]. While research has paid more attention to the relationship between the gut microbiota and cardiovascular disease [11,12,13,14,15], research into the gut microbiota and TAO is limited. Studies have found that almost two-thirds of TAO patients suffer from severe periodontal disease [16]. Anaerobic DNA fragments such as Campylobacter rectus, Porphyromonas gingivalis, and Prevotella have been discovered in the diseased arteries of TAO patients [16]. However, the origins of these pathogenic bacteria need further exploration. In observational studies, the association between gut microbiota and TAO is susceptible to confounding factors such as age, environment, dietary patterns, and lifestyle [17,18,19].
Mendelian randomization (MR) offers a new approach to investigate the causal link between the gut microbiota and TAO. The principle of gut microbiota genome-wide association studies (GWASs) involves sequencing the genotype and microbial composition of large-scale samples, followed by statistical analysis of their correlation to determine the genetic basis of specific microbial populations [20]. Twin studies have shown that the abundance of various bacterial taxa has an average heritability of 20% [21,22], supporting the notion that genetic factors play a significant role in shaping the gut microbiota composition. MR employs genetic variants as instrumental variables of exposure to estimate the causal relationship between exposure and outcome [23], as genotypes are randomly allocated from the parents to offspring, lending credence to a causal sequence. MR has been extensively used to explore the causal association between the gut microbiota and several diseases including metabolic diseases [24], autoimmune diseases [25], and rheumatoid arthritis [26]. In this study, we conducted a two-sample MR analysis using GWAS summary statistics from the MiBioGen and FinnGen consortiums to assess the causal link between the gut microbiota and TAO. Additionally, we conducted a case–control study to investigate alterations in microbial abundance among TAO patients, confirming our MR results.
2. Materials and Methods
2.1. Assumptions of MR
This study followed the MR reporting guidelines [27]. The MR approach is based on three primary assumptions (Figure 1) [28]. First, the instrumental variables (IVs) in the form of SNPs must exhibit a substantial correlation with the gut microbiota and achieve genome-wide significance (Assumption 1). Second, the IVs should be independent of confounding factors (Assumption 2). Third, the IVs should only affect TAO via the gut microbiota pathway while precluding other pathways (Assumption 3).
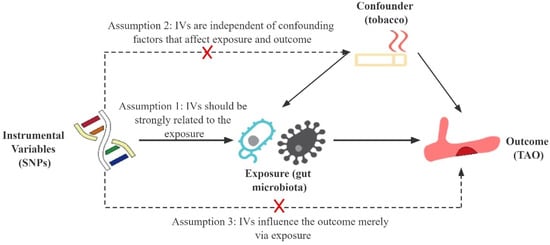
Figure 1.
Schematic representation of this Mendelian randomization study. SNPs, single nucleotide polymorphisms; IVs, instrumental variables; TAO, thromboangiitis obliterans.
2.2. Selection of Instrumental Variables
To identify the genetic variants associated with gut microbiota, the large meta-analysis of GWAS conducted by the MiBioGen consortium was utilized (https://mibiogen.gcc.rug.nl/, accessed on 15 February 2023) [20]. The study included 18,340 individuals from 24 cohorts, with the majority being of European descent (n = 13,266). They targeted variable regions V4, V3–V4, and V1–V2 of the 16S rRNA gene to profile the microbial composition and employed direct taxonomic binning to conduct the taxonomic classification. In this study, the lowest taxonomic level considered was the genus, and 131 genera with a mean abundance higher than 1% were identified including 12 unknown genera [20].
The selection criteria for the IVs were as follows. (1) The locus-wide significance threshold is more stringent compared to the genome-wide significance threshold. SNPs associated with each genus at the locus-wide significance threshold (p < 1.0 × 10−5) were chosen as potential IVs [24]. (2)The PLINK clumping method was utilized with a more stringent clumping threshold (r2 < 0.001, kb = 10,000) to prune SNPs in LD within a given window to account for residual linkage disequilibrium (LD) of the genetic variants [29]. (3) The F-statistic was calculated to evaluate the strength of the IVs using the formula . If the corresponding F-statistic was >10, no significant weak instrumental bias was assumed [30]. (4) Tobacco exposure is critical to TAO initiation, maintenance, and progression [1] and may impact the MR findings as a confounding factor. Therefore, we examined the PhenoScanner [31] (http://www.phenoscanner.medschl.cam.ac.uk/, accessed on 5 March 2023) database, which contains publicly available information on a large number of genetic variants, to identify and exclude IVs directly linked to tobacco exposure or factors affecting the gut microbiota to address the MR assumptions. (5) SNPs with a palindromic structure were automatically eliminated during the analysis.
2.3. Data Sources of Outcomes
We obtained the summary statistics for TAO from the R7 release data of the FinnGen consortium [32] (https://r7.finngen.fi/, accessed on 10 February 2023), which included a total of 16,383,294 variables from Finnish biobanks. The GWAS comprised 288,723 participants including 85 cases (53 males and 32 females) and 288,638 controls. To adjust for potential confounding factors, we controlled for sex, age, first 10 principal components, and genotyping batch during the analysis [32]. To ensure uniform reference alleles, the exposure and outcome data were combined and standardized. We then oriented the summary effect estimates in the same direction as those in the 1000 genomes reference dataset [33].
2.4. Case–Control Study
The case–control study was conducted at Xiangya Hospital of Central South University between November 2022 and January 2023. Individuals meeting the following inclusion criteria for TAO were as follows [1]: (1) young and middle-aged individuals with a smoking history; (2) varying degrees of ischemic symptoms in the affected limb; (3) decreased or absent pulsation of the dorsalis pedis artery and/or posterior tibial artery on the affected side; (4) TAO suggested by CT angiography. We then selected a certain number of varicose vein patients as the control group and conducted physical examinations during the same period. Fecal specimens were collected after the diagnosis of TAO but before the treatment. To ensure the representativeness of fecal specimens, we controlled for confounding factors that can alter the gut microbiota community. The exclusion criteria were as follows [18,34]: (1) history of antibiotic, probiotic, hormone, and gastrointestinal disease-related drug use, alcohol and drug abuse within the last month; (2) metabolic diseases such as diabetes, rheumatoid arthritis, severe organ damage, tumors, infectious diseases, food intolerance, and a history of inflammatory bowel disease; (3) previous long-term or recent history of bloating, diarrhea, gastrointestinal discomfort such as constipation; (4) short-term change in diet structure; (5) definite autoimmune diseases, hypercoagulable blood, popliteal artery trapping syndrome, and patients with emboli of proximal origin; (6) non-Han Chinese. Demographic information was recorded for all of the study participants.
In order to minimize the potential impact of collecting samples from different fecal locations, we collected the middle feces from all participants as samples. The QIAGEN Fecal DNA Extraction Kit (QIAamp PowerFecal Pro DNA Kit) (Cat NO. 51804) was utilized to extract fecal DNA in this study. The extracted fecal DNA was qualified and sent to Nuohe Zhiyuan Company (Beijing, China) for 16s rRNA amplification and sequencing. Before sequencing, a quantitative analysis was carried out, requiring that the DNA concentration of the sample to be tested be ≥ 10 ng/μL, with a mass ≥ 500 ng. DNA amplification was followed by splicing to construct a library, which was then quantified and sequenced using a computer. After off-board data analysis, MEGAHIT assembly, QUEST evaluation, Prokka gene annotation, and MetaPhlAn2 species abundance analysis were performed. The human study was approved by the Institutional Review Board of Xiangya Hospital (Approval Number: 2022111192).
2.5. MR Analysis and Statistical Analysis
This study utilized multiple methods including the inverse variance weighted (IVW), maximum likelihood (ML), MR-Egger regression, weighted median, weighted model, and MR-PRESSO to examine the possible causal association between the gut microbiota and TAO. The IVW method employed a meta-analysis approach that combined with the Wald estimates for each SNP to obtain an overall effect estimate of the gut microbiota on TAO. The results are unbiased if horizontal pleiotropy is absent [35]. Similar to IVW, the ML method assumes no heterogeneity or horizontal pleiotropy. If these hypotheses are met, the results will be unbiased, and the standard errors will be smaller than those obtained with IVW [36]. MR-Egger regression is based on the instrument strength independent of the direct effect (InSIDE) assumption, which enables the assessment of pleiotropy by examining the intercept term. If the intercept term equals zero, it suggests that horizontal pleiotropy does not exist, and the result of the MR-Egger regression is consistent with that of IVW [37]. The weighted median method allows for the correct estimation of causal association even when up to 50% of the instrumental variables are invalid [38]. If the InSIDE assumption fails, the weighted model estimate has been shown to have greater power to detect a causal effect, less bias, and lower type I error rates than MR-Egger regression [38]. The MR-PRESSO analysis detects and attempts to reduce horizontal pleiotropy by removing significant outliers. However, the MR-PRESSO outlier test requires that at least 50% of genetic variants be valid instruments and relies on InSIDE assumptions [39].
To quantify the heterogeneity of IVs, Cochran’s IVW Q statistics were conducted. The Cochran Q test was used to test for heterogeneity, with a p-value < 0.05 indicating significantly heterogeneous. Furthermore, a “leave-one-out” analysis was carried out by excluding each instrumental SNP in turn to identify potential heterogeneous SNPs. Directional pleiotropy was assessed through visual inspection of SNP scatter plots and funnel plots, and formally tested using the Egger intercept test.
The power of the MR estimates was calculated using an online calculator tool (https://sb452.shinyapps.io/power/, accessed on 13 March 2023) provided by Stephen Burgess [40]. With Bonferroni correction, the adjusted multiple comparisons p-value < 0.00042 (0.05/119) was considered to be statistically significant. A suggestive association between the genera of gut microbiota and TAO was defined as a p-value > 0.00042, but <0.05. All statistical data analyses were conducted with R software, version 4.2.1 (http://www.r-project.org, accessed on 3 January 2023), with the TwoSampleMR (version 0.5.6) [41] and MRPRESSO packages (version 1.0) [39]. We also performed a reverse MR analysis on the bacteria that showed causality in the positive MR analysis for TAO to exclude potential reverse associations. The methodologies and configurations employed were analogous to those used in the forward MR analysis.
3. Results
3.1. Genetic Associations between Gut Microbiota and TAO
Supplementary Table S1 details the selected IVs. As presented in Table 1 and Figure 2, we identified three bacterial genera, namely Ruminiclostridium 5, Eubacterium (xylanophilum group), and Lachnospira, as linked to TAO. The IVW (OR = 0.119, 95% CI: 0.021–0.688, p = 0.017) and ML (OR = 0.121, 95% CI: 0.020–0.742, p = 0.022) estimates indicated a suggestive protective effect of Ruminiclostridium 5 on TAO. The IVW (OR = 5.383, 95% CI: 1.128–25.693, p = 0.035) and ML (OR = 5.658, 95% CI: 1.142–28.021, p = 0.034) estimates suggested a suggestive risk effect of Eubacterium (xylanophilum group) on TAO. Although the IVW estimates did not support the causal association of Lachnospira on TAO, the ML estimates suggested a possible relationship (OR = 0.055, 95% CI: 0.004–0.755, p = 0.030). The reverse MR did not yield positive results. F-statistics of the IVs for these three causal associations ranged from 12.03 to 26.97, which eliminated the bias of weak IVs. The Cochran’s IVW Q test results showed no significant heterogeneity among these IVs (Supplementary Table S2).

Table 1.
MR estimates for the association between gut microbiota and TAO.
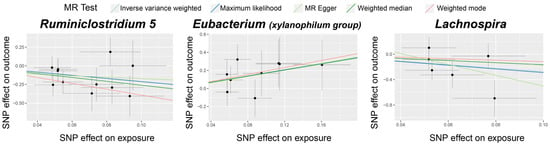
Figure 2.
Scatter plots for the causal association between gut microbiota and TAO. The dots in the graph represent SNPs.
Visual inspection of the funnel plots (Figure 3) and leave-one-out plots (Figure 4) revealed potential outliers for the IVs of Eubacterium (xylanophilum group) and Lachnospira. However, no instrumental variables significantly influenced the overall results. The MR-PRESSO analysis did not identify any significant outliers (Global test p > 0.05, Supplementary Table S3). The MR-Egger regression intercept analysis (Supplementary Table S4) did not detect significant horizontal pleiotropy.
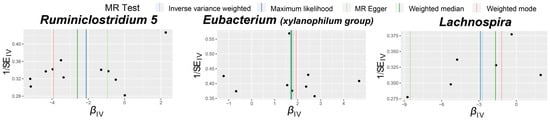
Figure 3.
Funnel plots for the causal association between gut microbiota and TAO. The dots in the graph represent SNPs.
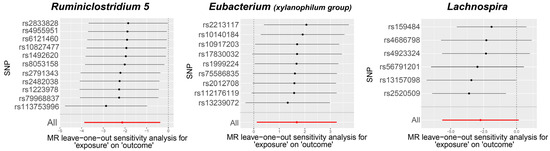
Figure 4.
Leave-one-out plots for the causal association between gut microbiota and TAO.
3.2. Case–Control Study
A case–control study was conducted, and the baseline demographic and clinicopathologic features were compared and presented in Table 2.

Table 2.
Demographic and clinicopathologic features of the participants in the case–control study.
A total of 5076 operational taxonomic units (OTUs) were detected in the TAO patients, while 3583 OTUs belonged to the gut microbiota in the controls (Figure 5A). Among these, 3054 OTUs constituted common microbiota, which showed lower relative abundance in TAO patients (Figure 5B). α-Diversity and β-diversity were used to describe the microbial composition and distribution. Sequences with ≥ 100% similarity were classified as the same feature. The Chao1 index, Shannon index, and Simpson index to were used to describe the α-diversity further. The violin plot of the Chao1 index revealed statistically significant differences between the TAO patients and controls (p = 0.029) (Figure 5C). Additionally, the violin plots of the Shannon index suggested statistical significance differences between the TAO patients and controls (p = 0.004), while the Simpson index suggested statistical significance among the TAO patients versus controls (p = 0.006) (Figure 5D,E). Principal coordinates analysis (PCoA) based on the weighted UniFrac distance to assess β-diversity indicated that the two groups differed in significance in the gut microbiological composition (R2 = 0.145, p = 0.010) (Figure 5F).
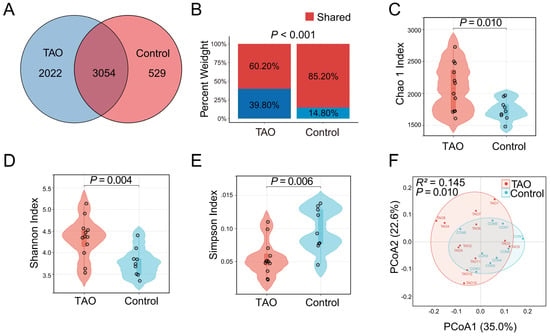
Figure 5.
Differences in gut microbiome composition and distribution in the TAO patients and controls. (A) The numbers of OTUs in the TAO patients, controls, and shared OTUs in both groups in a scaled Venn diagram. (B) Total abundance of OTUs in the TAO patients and controls. α−Diversity was described by the Chao1 index (C), Shannon index (D), and Simpson (E). β−Diversity was described by principal coordinates analysis (PCoA) based on the weighted UniFrac distance metric (F).
To validate the findings of the MR, the relative abundance of Ruminiclostridium 5, Eubacterium (xylanophilum group), and Lachnospira was compared between the TAO patients and controls using violin plots. TAO patients had a lower relative abundance of Ruminiclostridium 5 (p = 0.015) and Lachnospira (p = 0.048), but a higher relative abundance of Eubacterium (xylanophilum group) (p = 0.029) than the controls (Figure 6). These results were consistent with the MR findings, indicating that the gut microbiota may have risky or protective characteristics for TAO. These results are consistent with the conclusions drawn from the MR. To predict microbial functional, we utilized the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) and Clusters of Orthologous Genes (COG) database [42,43]. COG entries meeting a p-value of less than 0.05 and |log2 (fold change (FC))| > 1 were defined as differential abundant proteins. We discovered that proteins such as cAMP phosphodiesterase, ABC-type sulfate transport system (periplasmic component), DNA-binding transcriptional regulator (MltR family), and DNA-binding transcriptional regulator SgrR of sgrS sRNA (contains a MarR-type HTH domain and a periplasmic-type solute-binding domain) differed between the TAO patients and controls (Supplementary Figure S1). These components are involved in regulating intracellular signaling and gene expression, which are associated with vascular inflammatory responses [44,45].
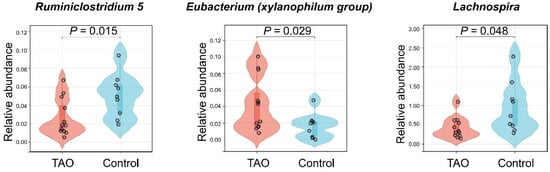
Figure 6.
Violin plots to validate MR. Comparative relative abundance of Ruminiclostridium 5, Eubacterium (xylanophilum group), and Lachnospira in TAO and controls.
4. Discussion
4.1. Principal Findings
In our investigation, we employed summary statistics from the largest GWAS meta-analysis of gut microbiota conducted by the MiBioGen consortium as well as summary statistics of TAO from the FinnGen consortium R7 release data to conduct a two-sample MR and an analysis of human fecal specimens. Our objective was to evaluate the causal relationship between the gut microbiota and TAO. To our knowledge, this is the first to apply the concept to investigate the causality between the gut microbiota and TAO. Our findings revealed that Ruminiclostridium 5 and Lachnospira had protective effects, while Eubacterium (xylanophilum group) had risk effects on TAO.
4.2. Comparison with Other Studies and Possible Mechanisms
The gut microbiome is a vast collection of microorganisms in the human digestive tract that can influence the physiological functions of the gastrointestinal tract [46] and interact with almost all human cells [47]. It plays important physiological functions in the human organism including bile acid metabolism [46], synthesis of essential vitamins [48], and regulation of the immune system [49]. Currently, exploring the gut–vascular axis provides a unique insight into cardiovascular disease [13]. Ruminiclostridium 5, known as a probiotic [50,51], has been reported to have a protective effect on cardiovascular health [52]. Furthermore, correlation analysis showed that Ruminiclostridium 5 was significantly related to several intestinal permeability characteristics such as fecal and serum lipopolysaccharide (LPS) and serum proinflammatory cytokines [52]. Dysregulation of the gut microbiome can lead to high levels of microbiota-associated molecular patterns (MAMPs), resulting in the induction of hepatocarcinogenesis by diethylnitrosamine (DEN) and carbon tetrachloride (CCl4) binding to LPS and their receptors, Toll-like receptor 4 (TLR4) [53]. Studies have also demonstrated that the oxidative stress levels in TAO patients are significantly higher than their antioxidant capacity [54]. The interaction of LPS with TLR4 can activate the NF-κB pathway and three MAPK pathways, producing inflammatory mediators such as IL-6 and IL-8 [55,56]. Another study has shown that TLR4 levels are significantly higher in patients with acute TAO than in patients with quiescent TAO [57]. Vitamin D supplementation has been found to increase the abundance of Lachnospira in human feces [58]. Patients with atrial fibrillation shown an increase in intestinal symbiotic bacteria Lachnospira after catheter ablation, suggesting a beneficial effect of gut microbiota. The immune system and inflammation are proposed to play a central role in TAO pathogenesis [59]. Further clarification is needed to determine whether gut microbiota is involved in the development of TAO through LPS/TLR4.
Considerable knowledge has already been accumulated regarding the role of intestinal microorganisms in immune-inflammatory diseases. Lachnospira has been identified as significantly downregulated in many immune-inflammatory diseases [60]. When a drug was used to treat acute coronary syndrome, the levels of the intestinal metabolite ethanolamine (EA) were significantly altered, with Eubacterium (xylanophilum group) significantly correlated with the levels of EA [61]. The metabolites or metabolomes of the gut microbiome can partially predict the etiology and progression of TAO. Certain short-chain-fatty acids (SCFAs) produced by probiotics are the primary energy sources of intestinal epithelial cells and participate in cell proliferation and differentiation, thereby maintaining cell homeostasis through anti-inflammatory and antioxidant effects [62,63]. Therefore, probiotics and SCFAs may help TAO patients maintain intestinal barrier function and prevent systemic or vascular inflammation caused by the migration of pathogenic bacteria to reduce the risk of TAO. With a focus on the gut microbiota involved in this study, further exploration is needed to understand the complex interplay between the gut microbiota and TAO.
4.3. Strengths and Limitations
This study has several strengths. First, the MR design reduced the confounding bias and reverse causation, which are not well-controlled in traditional observational epidemiology. The consistency of effects across the MR models and sensitivity analysis enhanced the robustness of the results. Second, we established a population cohort and confirmed the MR results well, with differences in gut microbiota abundance. Third, we obtained genetic variants of gut microbiota from the largest available GWAS meta-analysis, ensuring strong instruments. Finally, it is worth mentioning that the three major assumptions of MR were well-adhered to. However, with the increase in future research, the IVs may undergo changes, potentially affecting the main results.
Several limitations should be considered when interpreting the results. As we used summary statistics rather than raw data, distinguishing TAO with varying degrees of ischemia or gender or exploring quantitative relationships was not possible. The lowest taxonomic level available in the exposure dataset was the genus, restricting our ability to explore the causal association between the gut microbiota and TAO at the species level. Given the absence of significant horizontal pleiotropy and the effectiveness of instrumental variables, IVW and ML were appropriate methods in our study. Although these results were not highly significant, they reflect the real scenario. Furthermore, currently, the data have their own limitations, and methods with a higher resolution for species identification are worth considering, although such methods require more resources. To detect horizontal pleiotropy and conduct sensitivity analysis, more genetic variations need to be included as IVs; therefore, the SNPs used in the analysis did not reach the traditional GWAS significance threshold (p < 5 × 10−8) [64,65]. Although most participants in the GWAS meta-analysis for gut microbiota data were of European descent, interference from population stratification may still exist, and the findings may not be entirely applicable to subjects of non-European descent. The inconsistency in the ancestry of the exposed, outcome, and validation dataset populations warrants a cautious interpretation of the results. Additionally, the relatively low sample size of the analyses may limit the statistical power to detect genetic associations. The lower sample size in the case–control study also suggests that the current conclusions need to be interpreted with caution. Lifestyle factors such as smoking, diet, and physical activity may play a role in the current associations, which require further analysis. Large-scale studies in diverse populations are necessary to establish the consistency of the results across different ethnic groups.
5. Conclusions
In conclusion, this study involved both two-sample MR and case–control analyses and demonstrated a causal link between Ruminiclostridium 5, Lachnospira, and Eubacterium (xylanophilum group) with TAO. These gut microbes could serve as potential markers or a therapeutic target for TAO. Additional randomized controlled trials are necessary to clarify the impact of probiotics on TAO and its specific mechanism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12071459/s1, Table S1. Instrumental variables used in MR analysis of the association between gut microbiota and TAO. Table S2. The heterogeneity of gut microbiota instrumental variables. Table S3. MR-PRESSO analysis for the association between gut microbiota and TAO. Table S4. Directional horizontal pleiotropy assessed by intercept term in MR Egger regression of the association between TAO and gut microbiota. Figure S1. Functional alterations in gut microbiota in TAO patients.
Author Contributions
C.S.: Data curation, formal analysis, investigation, methodology, writing—original draft. W.H.: Conceptualization, methodology. M.L.: Supervision, writing—review and editing. P.Y.: Supervision, writing—review and editing. M.L. and P.Y. are listed as corresponding authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of Hunan Province (2023JJ30961), the National Natural Scientific Foundation of China (82074000), the National Key Research and Development Program of China (No. 2023YFC3405200), and the Scientific Research Project of Furong Laboratory of Central South University (2023SK2083).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Xiangya Hospital (Approval Number: 2022111192). Informed consent was obtained from all subjects involved in the study. All participants provided informed consent according to the original GWAS protocols, and ethical approvals for the GWAS were obtained by the original authors.
Data Availability Statement
All data and materials supporting the findings of this work are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy concerns and restrictions imposed by the ethical review board.
Acknowledgments
The authors express their gratitude to the participants and investigators of the FinnGen study. The authors also appreciate the MiBioGen consortium for releasing the gut microbiota GWAS summary statistics. In addition, we acknowledge the work of Nuohe Zhiyuan Company (Beijing, China).
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
| TAO | Thromboangiitis obliterans |
| MR | Mendelian randomization |
| GWAS | Genome-wide association study |
| SNPs | Single nucleotide polymorphisms |
| IVs | Instrumental variables |
| IVW | Inverse-variance weighted |
| ML | Maximum likelihood |
| LPS | Lipopolysaccharide |
| mbQTL | Microbiota quantitative trait loci |
| LD | Linkage disequilibrium |
| ICD-10 | International Classification of Diseases 10th version |
| InSIDE | Instrument strength independent of direct effect |
| OR | Odds ratio |
| CI | Confidence interval |
| PCoA | Principal coordinates analysis |
| MAMPs | Microbiota-associated molecular patterns |
| TLR4 | Toll-like receptor 4 |
| EA | Metabolite ethanolamine |
| SCFAs | Short-chain-fatty acids |
References
- Piazza, G.; Creager, M.A. Thromboangiitis obliterans. Circulation 2010, 121, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Wang, Y.F.; Yang, M.W.; Hong, F.F.; Yang, S.L. Risk Factors, Mechanisms and Treatments of Thromboangiitis Obliterans: An Overview of Recent Research. Curr. Med. Chem. 2020, 27, 6057–6072. [Google Scholar] [CrossRef] [PubMed]
- Shepard, Z.; Skorupa, T.; Espinoza, L.; Erlandson, K.; Damioli, L. Coxiella burnetii Infection Associated With Thromboangiitis Obliterans-like Phenomena With Digital Autoamputation: A Case Report and Review of Q Fever-Associated Autoimmunity. Open Forum Infect. Dis. 2022, 9, ofab637. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Liakopoulos, D.; Chamzin, A.; Sigala, F.; Filis, K. A systematic review and meta-analysis of early and late outcomes after endovascular angioplasty among patients with thromboangiitis obliterans and chronic limb ischemia. J. Vasc. Surg. 2022, 77, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Cacione, D.G.; Macedo, C.R.; do Carmo Novaes, F.; Baptista-Silva, J.C. Pharmacological treatment for Buerger’s disease. Cochrane Database Syst. Rev. 2020, 5, CD011033. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J. Gut microbial metabolites in health and disease. Gut Microbes 2016, 7, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Xu, J.; Deng, X.; Zhou, S. A Mendelian Randomization Study: Roles of Gut Microbiota in Sepsis—Who is the Angle? Pol. J. Microbiol. 2024, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.L.; Shen, B.; Yuan, Y.; Liu, C.; Xie, Q.W.; Hu, T.T.; Yao, Q.; Wu, Q.Q.; Tang, Q.Z. The effect of HMGA1 in LPS-induced Myocardial Inflammation. Int. J. Biol. Sci. 2020, 16, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Hogue, T. Exercise and the Microbiome: Mechanistic Perspectives of the Impact of Exercise on the Gut-Vascular Axis. mSystems 2021, 6, e0065021. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc. Ther. 2019, 2019, 5164298. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhai, W.; Yang, C.; Li, Z.; Mao, L.; Zhao, M.; Wu, X. The Relationship among Physical Activity, Intestinal Flora, and Cardiovascular Disease. Cardiovasc. Ther. 2021, 2021, 3364418. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Inoue, Y.; Umeda, M.; Huang, Y.; Kurihara, N.; Koike, M.; Ishikawa, I. Oral bacteria in the occluded arteries of patients with Buerger disease. J. Vasc. Surg. 2005, 42, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Raygoza Garay, J.A.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 2000, 29, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021, 12, 746998. [Google Scholar] [CrossRef] [PubMed]
- Inamo, J. Non-causal association of gut microbiome on the risk of rheumatoid arthritis: A Mendelian randomisation study. Ann. Rheum. Dis. 2021, 80, e103. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.M.; Davies, N.M.; Hemani, G.; Zheng, J.; Haycock, P.C.; Gaunt, T.R.; Davey Smith, G.; Martin, R.M. Using the MR-Base platform to investigate risk factors and drug targets for thousands of phenotypes. Wellcome Open Res. 2019, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.; Staiger, D. Instrumental Variables Regression with Weak Instruments. Econometrica 1997, 65, 557–586. [Google Scholar]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J.; et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics 2016, 32, 3207–3209. [Google Scholar] [CrossRef] [PubMed]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv 2022. [Google Scholar] [CrossRef]
- Fairley, S.; Lowy-Gallego, E.; Perry, E.; Flicek, P. The International Genome Sample Resource (IGSR) collection of open human genomic variation resources. Nucleic Acids Res. 2020, 48, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Pierce, B.L.; Burgess, S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 2014, 43, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Z.; Jin, Y.; Lu, J.; Feng, D.; Peng, R.; Sun, H.; Mu, X.; Li, C.; Chen, Y. Hepatic stellate cell activation and senescence induced by intrahepatic microbiota disturbances drive progression of liver cirrhosis toward hepatocellular carcinoma. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Kristensen, D.M.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Microbial genome analysis: The COG approach. Brief. Bioinform. 2019, 20, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hong, Z.; Huang, L.S.; Tsukasaki, Y.; Nepal, S.; Di, A.; Zhong, M.; Wu, W.; Ye, Z.; Gao, X.; et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020, 130, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, P. Regulation of vascular inflammation and remodeling by ETS factors. Circ. Res. 2006, 99, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Neish, A.S. Gut Microbiota in Intestinal and Liver Disease. Annu. Rev. Pathol. 2021, 16, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Long-Smith, C.M.; Carbia, C.; Bastiaanssen, T.F.S.; van de Wouw, M.; Wiley, N.; Strain, C.R.; Fouhy, F.; Stanton, C.; Cryan, J.F.; et al. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology 2021, 238, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.S.; Gaffney, M.; Hopkins, S.; Kelley, T.; Gonzalez, A.; Bowers, S.J.; Vitaterna, M.H.; Turek, F.W.; Foxx, C.L.; Lowry, C.A.; et al. Ruminiclostridium 5, Parabacteroides distasonis, and bile acid profile are modulated by prebiotic diet and associate with facilitated sleep/clock realignment after chronic disruption of rhythms. Brain Behav. Immun. 2021, 97, 150–166. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Du, Y.; Liu, X.; Chen, G.; Xiang, P.; Wu, H.; Liu, C.; Wang, D. Brussels Chicory Stabilizes Unstable Atherosclerotic Plaques and Reshapes the Gut Microbiota in Apoe-/- Mice. J. Nutr. 2022, 152, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Greten, T.F. Gut microbiome in HCC—Mechanisms, diagnosis and therapy. J. Hepatol. 2020, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sharebiani, H.; Fazeli, B.; Maniscalco, R.; Ligi, D.; Mannello, F. The Imbalance among Oxidative Biomarkers and Antioxidant Defense Systems in Thromboangiitis Obliterans (Winiwarter-Buerger Disease). J. Clin. Med. 2020, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, L.; Ling, S.; Xu, J.W. Levistilide A Ameliorates NLRP3 Expression Involving the Syk-p38/JNK Pathway and Peripheral Obliterans in Rats. Mediators Inflamm. 2018, 2018, 7304096. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Takahashi, M.; Naruse, T.; Nakajima, T.; Chen, Y.W.; Inoue, Y.; Ishikawa, I.; Iwai, T.; Kimura, A. Synergistic contribution of CD14 and HLA loci in the susceptibility to Buerger disease. Hum. Genet. 2007, 122, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Mohareri, M.; Mirhosseini, A.; Mehraban, S.; Fazeli, B. Thromboangiitis obliterans episode: Autoimmune flare-up or reinfection? Vasc. Health Risk Manag. 2018, 14, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, B.; Ligi, D.; Keramat, S.; Maniscalco, R.; Sharebiani, H.; Mannello, F. Recent Updates and Advances in Winiwarter-Buerger Disease (Thromboangiitis Obliterans): Biomolecular Mechanisms, Diagnostics and Clinical Consequences. Diagnostics 2021, 11, 1736. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ma, Y.; Liang, X.; Zhang, Y.; Hong, D.; Wang, Y.; Bai, D. Efficacy and Mechanism of Qianshan Huoxue Gao in Acute Coronary Syndrome via Regulation of Intestinal Flora and Metabolites. Drug Des. Devel Ther. 2023, 17, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; Willeit, P.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bäck, M.; Rees, J.M.B.; Mason, A.M.; Burgess, S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: A Mendelian randomization study. Eur. Heart J. 2020, 41, 221–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).