Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future
Abstract
1. Introduction
2. Materials and Methods
3. SGLT2 Inhibitors
3.1. Effects of SGLT2 Inhibitors on Vascular Function
3.2. Effects of SGLT2 Inhibitors on Mechanisms in Atherosclerosis
- SGLT2 inhibitors reduce vascular inflammation.
- 2.
- SGLT2 inhibitors attenuate oxidative stress and atherosclerosis
- 3.
- SGLT2 inhibitors reduce thrombosis
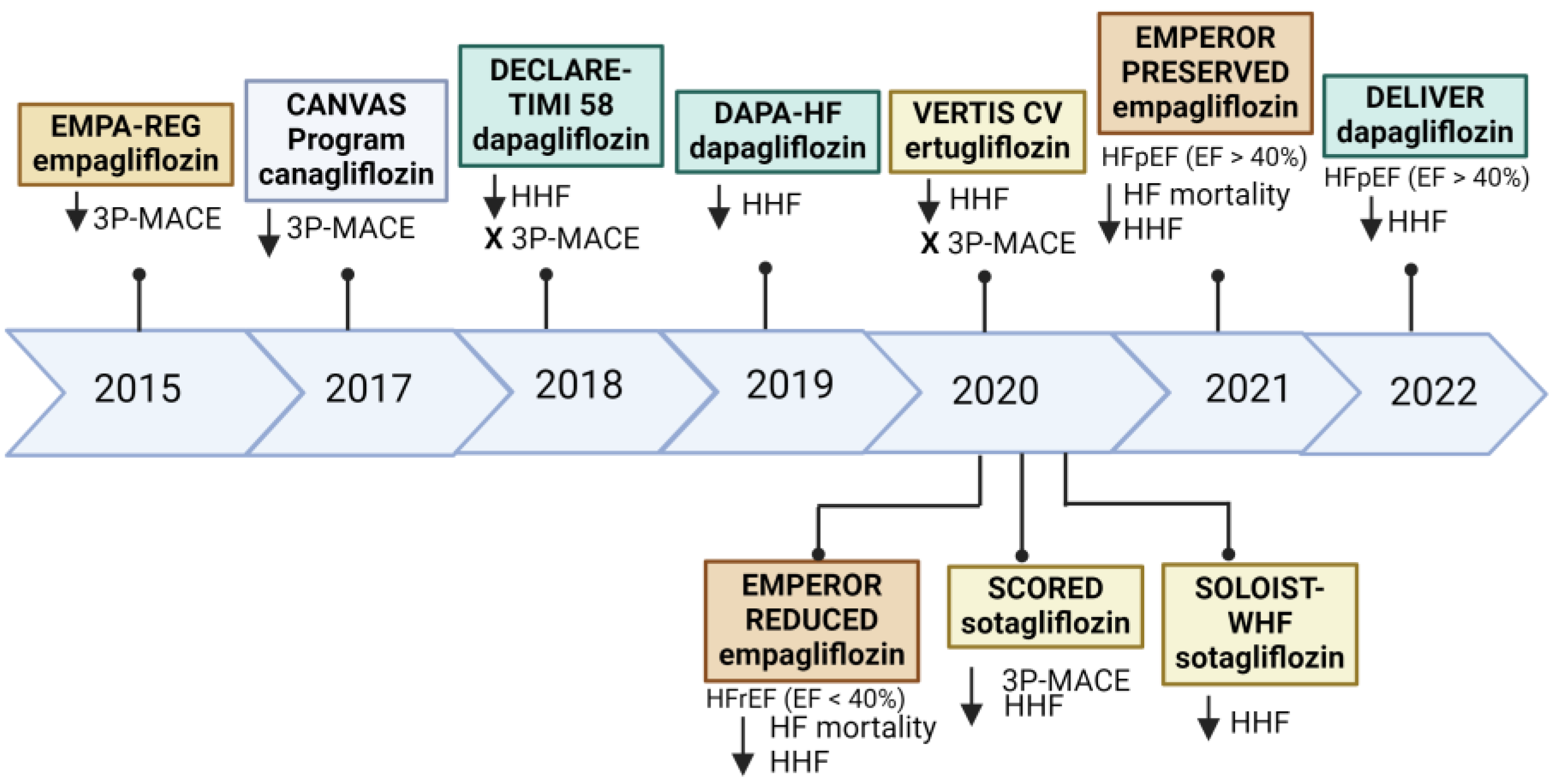
3.3. SGLT2 Inhibitors and Heart Failure
- Clinical trials and cardiac markers
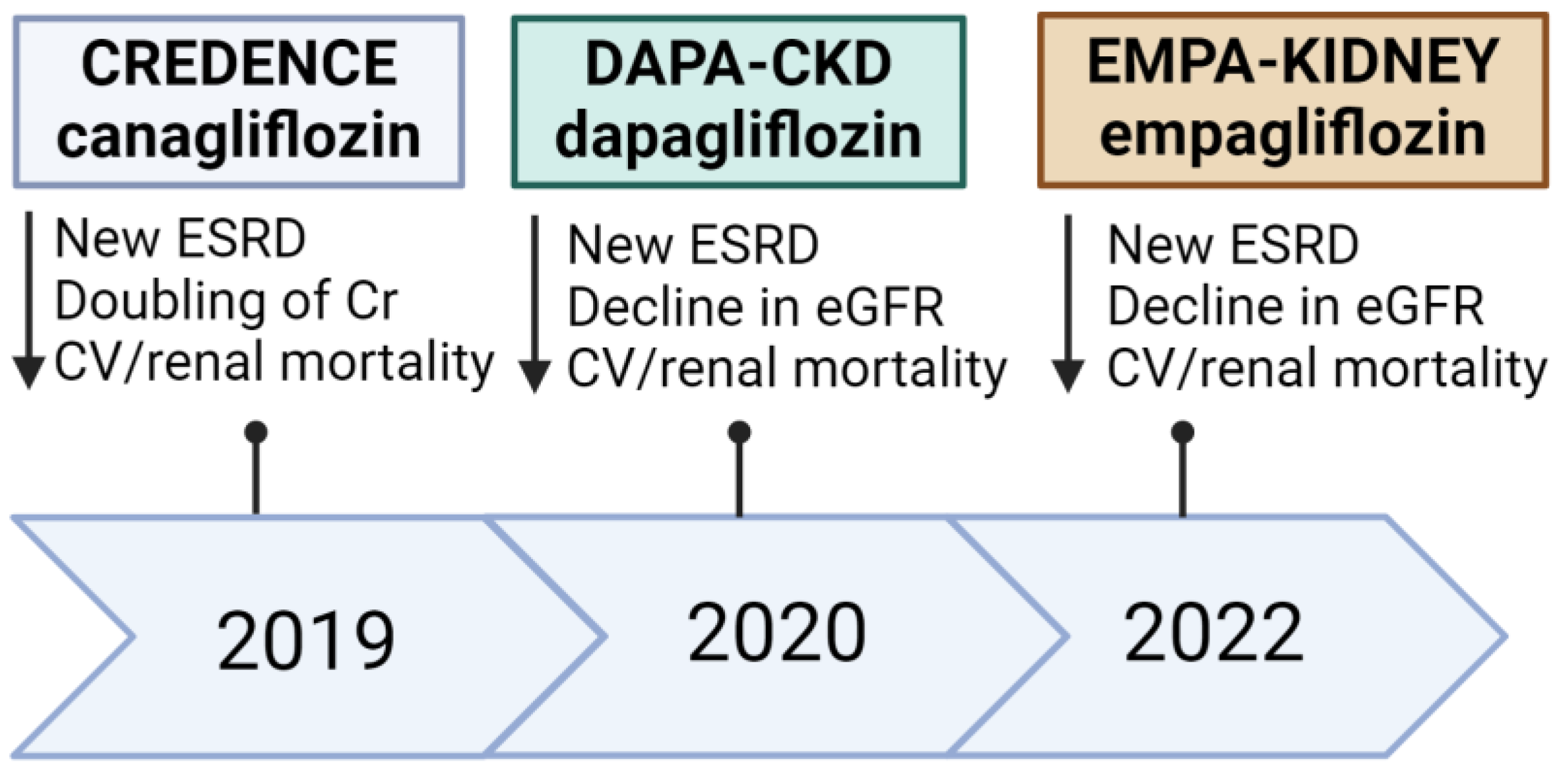
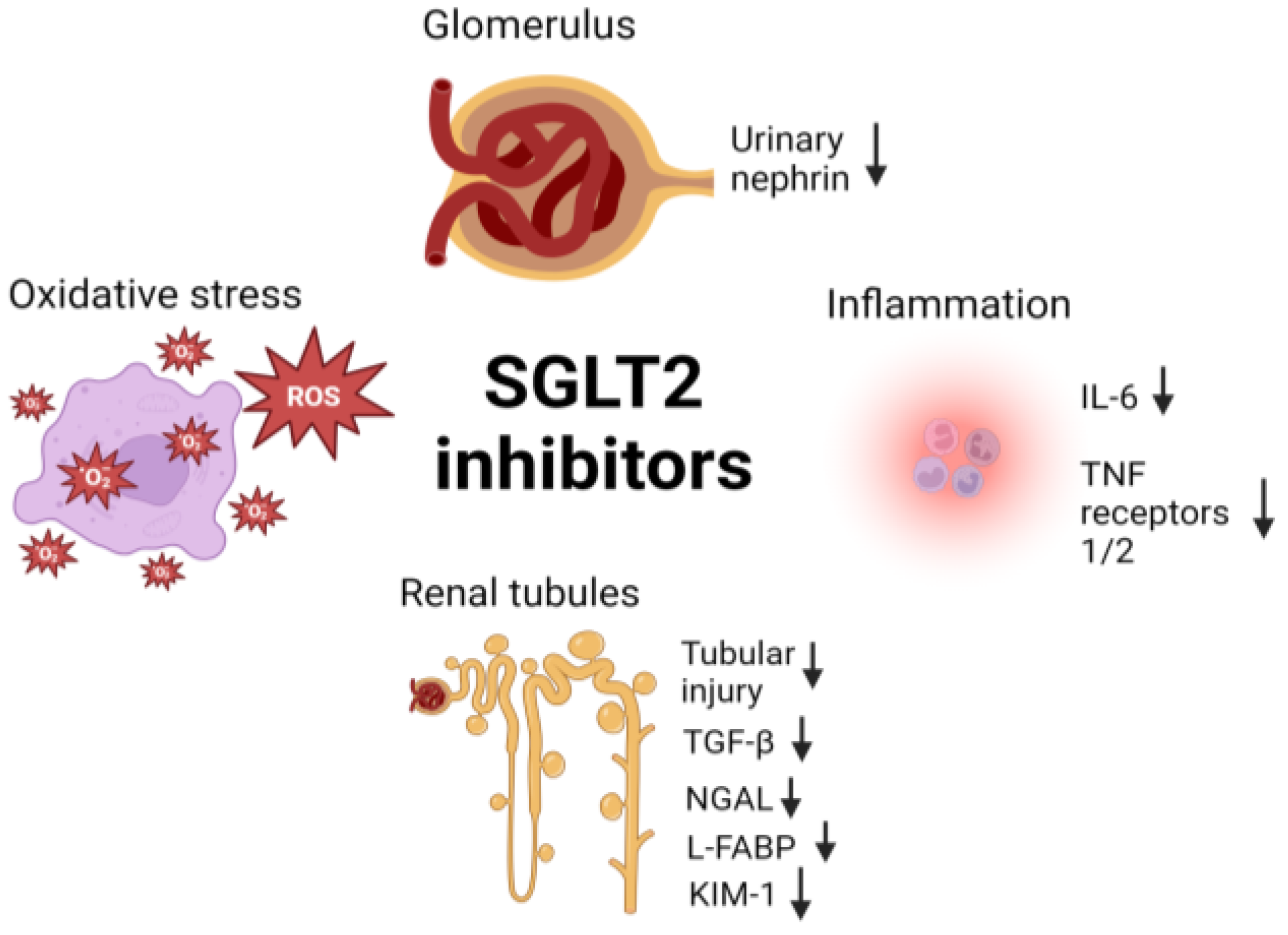
3.4. SGLT2 Inhibitors and the Kidneys
- Clinical trials focusing on renal outcomes
- 2.
- Mechanisms of SGLT2 inhibitors in renal protection.
3.5. Adverse Effects Associated with SGLT2 Inhibitors
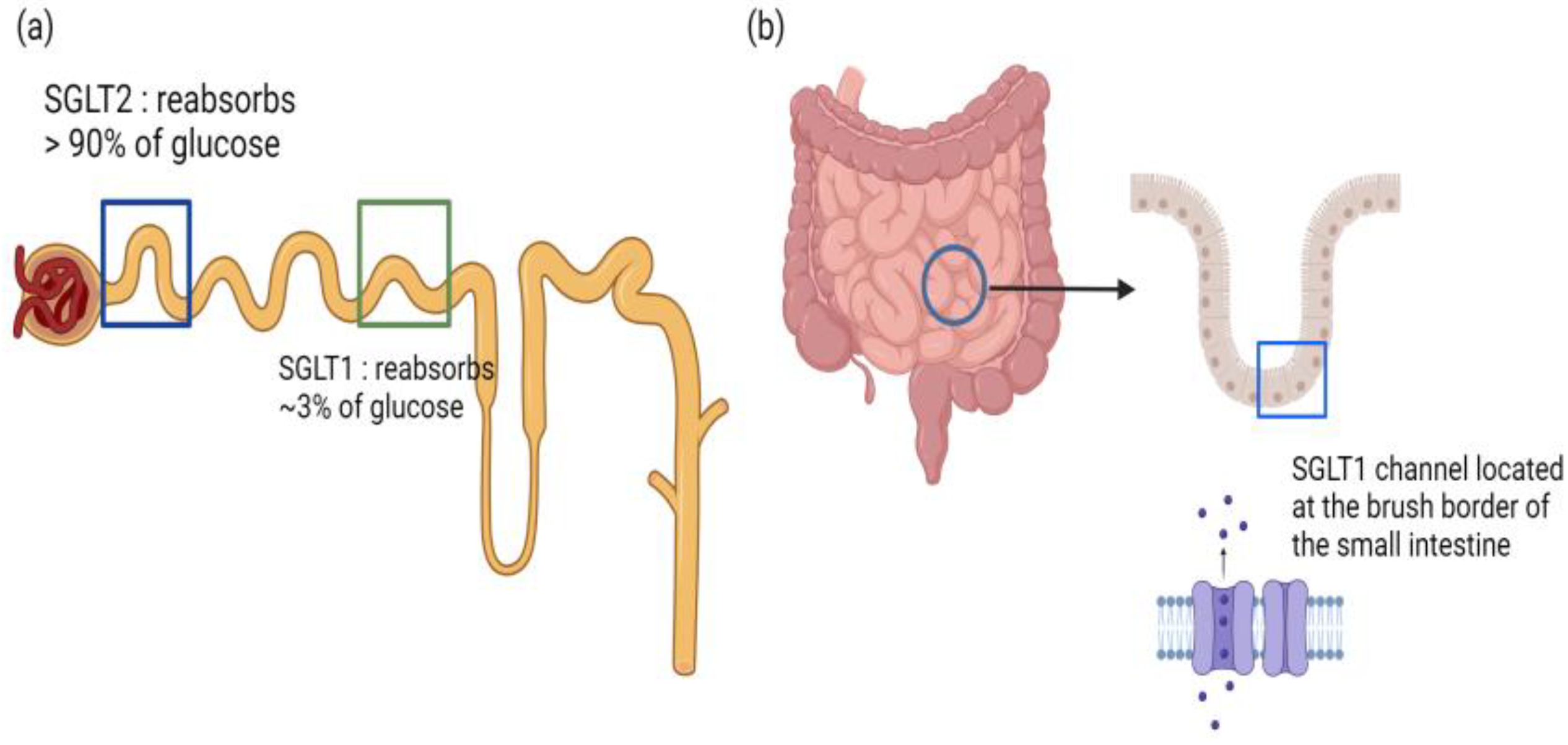
3.6. Dual SGLT1 and SGLT2 Inhibitors
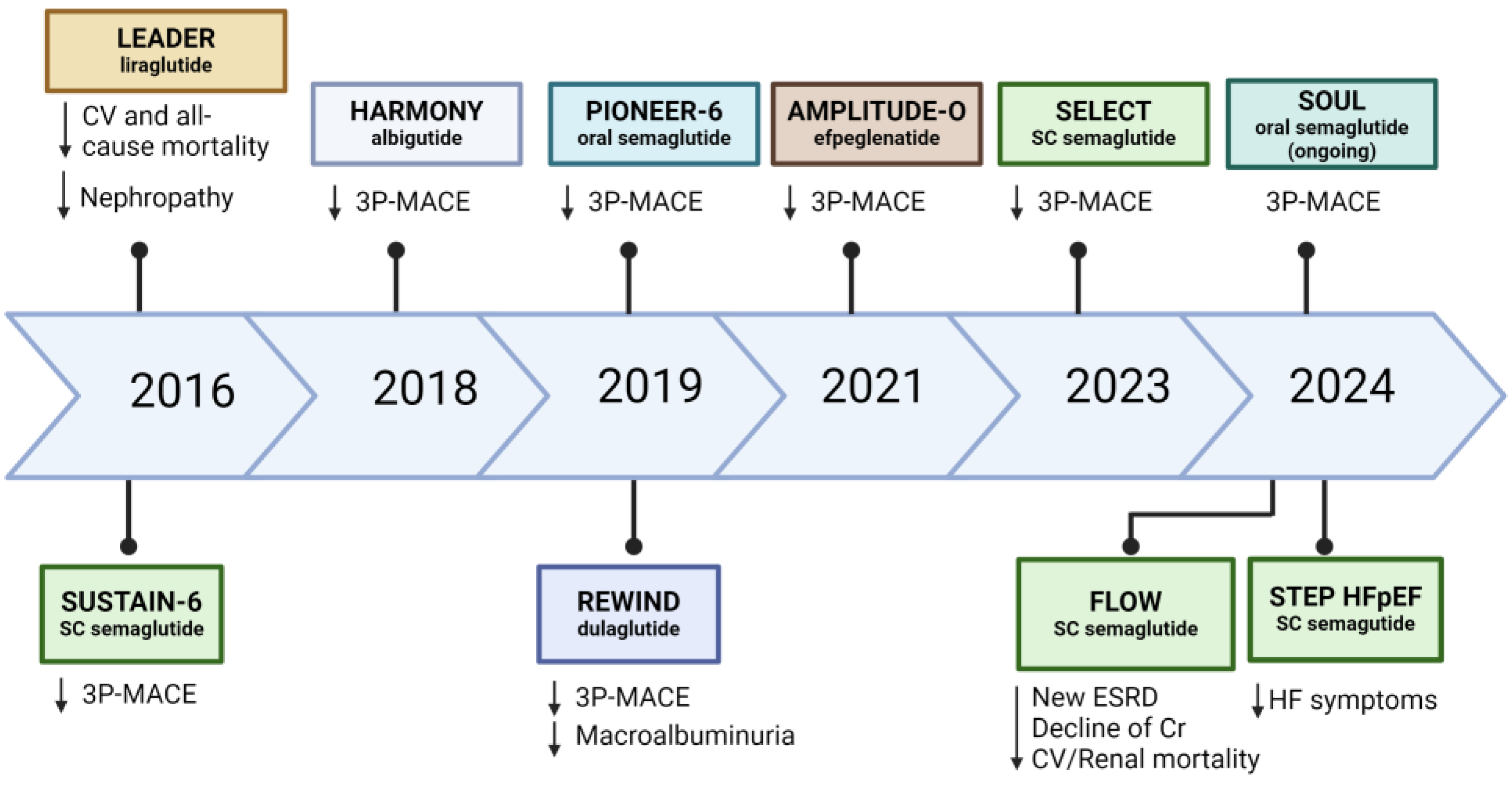
4. GLP-1 Receptor Agonist (GLP-1 RA)
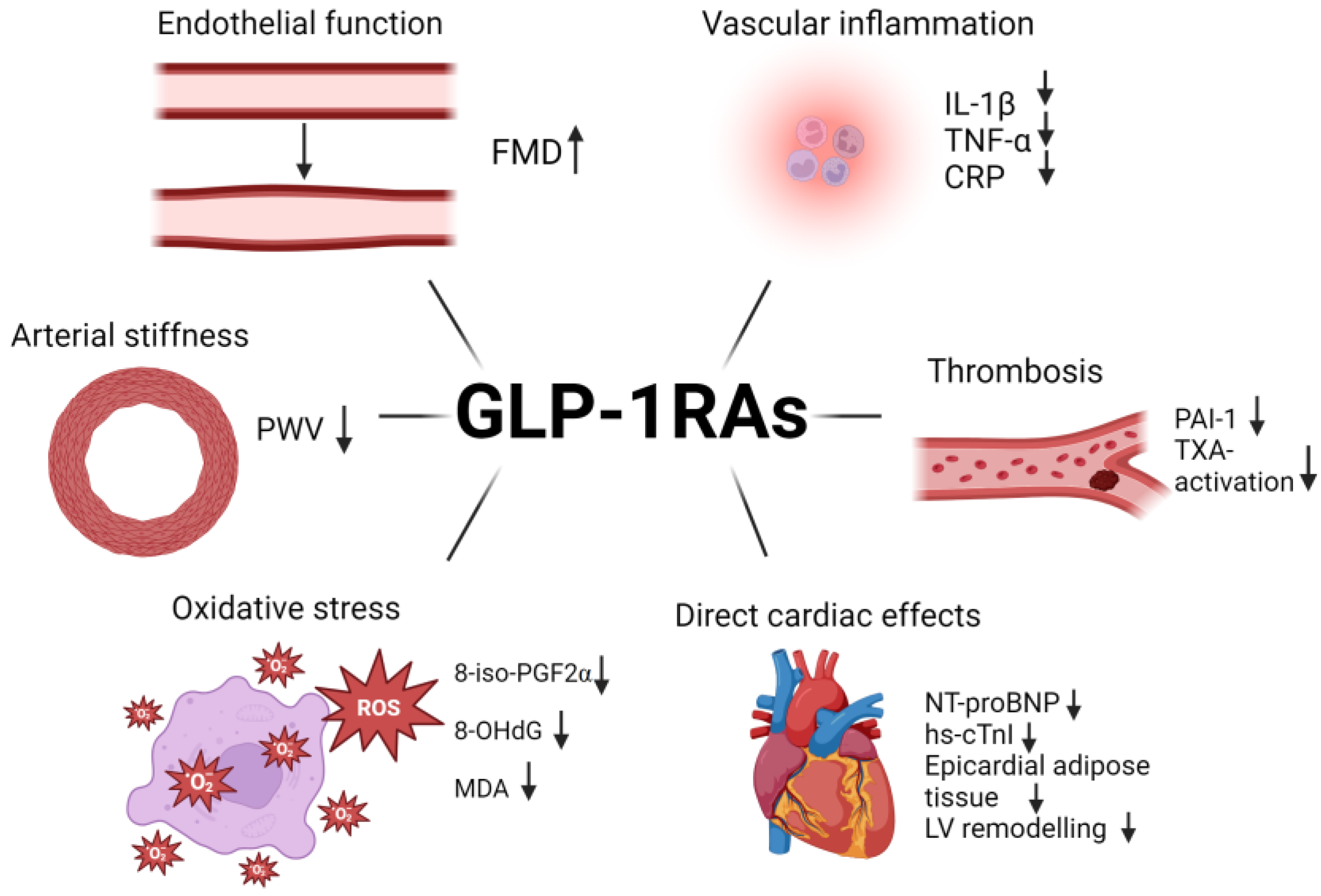
4.1. Effects of GLP-1 RA on Endothelial Function and Arterial Stiffness
4.2. Mechanisms of Cardiovascular Protection by GLP-1 RA
- Inflammation
- 2.
- Oxidative stress
- 3.
- Thrombosis
4.3. Effects of GLP-1 RA on Heart Failure
4.4. Effects of GLP-1 RA on Renal Function
4.5. Adverse Effects Associated with GLP-1 RA
4.6. Combination Agents with GLP-1
- GLP-1 + amylin analogue
- 2.
- GLP-1 + GIP receptor agonist (tirzepatide)
- 3.
- GLP-1 + glucagon receptor agonist
- 4.
- GLP-1-glucagon agonism and potential cardiorenal benefits
- 5.
- Triple GLP-1 + GIP + glucagon receptor agonist (retratrutide)
5. Novel Glucose-Lowering Agents in Early-Phase Trials
- Glimin
- 2.
- Dual peroxisome proliferator-activated receptor (PPARα/γ) agonist
- 3.
- Glucokinase activator
- 4.
- G-protein coupled receptor 40 (GPR40) agonist
6. Future Directions and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nathan, D.M.; Meigs, J.; Singer, D.E. The epidemiology of cardiovascular disease in type 2 diabetes mellitus: How sweet it is… or is it? Lancet 1997, 350 (Suppl. 1), SI4–SI9. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, J.V.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.; Cull, C.; Holman, R. United Kingdom Prospective Diabetes Study 17: A 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann. Intern. Med. 1996, 124 Pt 2, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.H.; Fei, Y.; Cheung, B.M.Y. Cardiovascular Outcomes in Trials of New Antidiabetic Drug Classes. Card. Fail. Rev. 2021, 7, e04. [Google Scholar] [CrossRef] [PubMed]
- Giaccari, A.; Pontremoli, R.; Filardi, P.P. SGLT-2 inhibitors for treatment of heart failure in patients with and without type 2 diabetes: A practical approach for routine clinical practice. Int. J. Cardiol. 2022, 351, 66–70. [Google Scholar] [CrossRef]
- Roddick, A.J.; Wonnacott, A.; Webb, D.; Watt, A.; Watson, M.A.; Staplin, N.; Riding, A.; Lioudaki, E.; Kuverji, A.; El Kossi, M.; et al. UK Kidney Association Clinical Practice Guideline: Sodium-Glucose Co-transporter-2 (SGLT-2) Inhibition in Adults with Kidney Disease 2023 UPDATE. BMC Nephrol 2023, 24, 310. [Google Scholar] [CrossRef]
- Arnold, S.V.; Tang, F.; Cooper, A.; Chen, H.; Gomes, M.B.; Rathmann, W.; Shimomura, I.; Vora, J.; Watada, H.; Khunti, K.; et al. Global use of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. Results from DISCOVER. BMC Endocr. Disord. 2022, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Stern, M.P.; Hazuda, H.P.; Mitchell, B.D.; Patterson, J.K. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 1990, 263, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.W.; Jardine, M.J.; Bompoint, S.; Cannon, C.P.; Neal, B.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; et al. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation 2019, 140, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Kassimis, G.; Vassilikos, V.; Karagiannis, A.; Doumas, M. Meta-Analysis Addressing the Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Flow-Mediated Dilation in Patients With Type 2 Diabetes Mellitus. Am. J. Cardiol. 2022, 165, 133–135. [Google Scholar] [CrossRef]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: A systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors-Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public. Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Gohari, S.; Reshadmanesh, T.; Khodabandehloo, H.; Karbalaee-Hasani, A.; Ahangar, H.; Arsang-Jang, S.; Ismail-Beigi, F.; Dadashi, M.; Ghanbari, S.; Taheri, H.; et al. The effect of EMPAgliflozin on markers of inflammation in patients with concomitant type 2 diabetes mellitus and Coronary ARtery Disease: The EMPA-CARD randomized controlled trial. Diabetol. Metab. Syndr. 2022, 14, 170. [Google Scholar] [CrossRef]
- Taheri, H.; Chiti, H.; Reshadmanesh, T.; Gohari, S.; Jalilvand, A.; Arsang-Jang, S.; Ismail-Beigi, F.; Ghanbari, S.; Dadashi, M.; Asgari, A.; et al. Empagliflozin improves high-sensitive cardiac troponin-I and high-density lipoprotein cholesterol in patients with type 2 diabetes mellitus and coronary artery disease: A post-hoc analysis of EMPA-CARD Trial. J. Diabetes Metab. Disord. 2023, 22, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Zamorano, J.L.; Domingo, M.; Morillas, H.; Nuñez, J.; Marcos, M.C.; Riquelme-Pérez, A.; Teis, A.; Santas, E.; Caro-Martinez, C.; et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: The DAPA-MODA study. Eur. J. Heart Fail. 2023, 25, 1352–1360. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Butler, J.; Jarolim, P.; Sattar, N.; Vijapurkar, U.; Desai, M.; Davies, M.J. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults With Type 2 Diabetes. J. Am. Coll. Cardiol. 2017, 70, 704–712. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, S.H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, W.; Pan, Q.; Guo, L. Effects of SGLT-2 Inhibitors on Vascular Endothelial Function and Arterial Stiffness in Subjects With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 826604. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, M.; Wang, J.; Liu, H.; Zhang, X.; Zhao, L.; Hu, X.; Guan, H.; Lyu, Z. Effects of Antidiabetic Drugs on Endothelial Function in Patients With Type 2 Diabetes Mellitus: A Bayesian Network Meta-Analysis. Front. Endocrinol. 2022, 13, 818537. [Google Scholar] [CrossRef]
- Tochiya, M.; Makino, H.; Tamanaha, T.; Matsuo, M.; Hishida, A.; Koezuka, R.; Ohata, Y.; Tomita, T.; Son, C.; Miyamoto, Y.; et al. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: A pilot study. J. Diabetes Investig. 2020, 11, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Yun, H.M.; Sohn, M.; Lim, S. Vascular and metabolic effects of ipragliflozin versus sitagliptin (IVS) in type 2 diabetes treated with sulphonylurea and metformin: IVS study. Diabetes Obes. Metab. 2023, 25, 1922–1931. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. The SGLT2 Inhibitor Dapagliflozin Significantly Improves the Peripheral Microvascular Endothelial Function in Patients with Uncontrolled Type 2 Diabetes Mellitus. Intern. Med. 2018, 57, 2147–2156. [Google Scholar] [CrossRef]
- Salvatore, T.; Caturano, A.; Galiero, R.; Di Martino, A.; Albanese, G.; Vetrano, E.; Sardu, C.; Marfella, R.; Rinaldi, L.; Sasso, F.C. Cardiovascular Benefits from Gliflozins: Effects on Endothelial Function. Biomedicines 2021, 9, 1356. [Google Scholar] [CrossRef]
- Cai, C.; Guo, Z.; Chang, X.; Li, Z.; Wu, F.; He, J.; Cao, T.; Wang, K.; Shi, N.; Zhou, H.; et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022, 52, 102288. [Google Scholar] [CrossRef]
- Ma, L.; Zou, R.; Shi, W.; Zhou, N.; Chen, S.; Zhou, H.; Chen, X.; Wu, Y. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics 2022, 12, 5034–5050. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.; Benjamin, E.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, Y.; Li, Y.; Zhang, J.; Zhang, H.; Fu, X.; Guo, Z.; Yang, Y.; Kang, K.; et al. Effects of first-line antidiabetic drugs on the improvement of arterial stiffness: A Bayesian network meta-analysis. J. Diabetes 2023, 15, 685–698. [Google Scholar] [CrossRef]
- Katakami, N.; Mita, T.; Yoshii, H.; Shiraiwa, T.; Yasuda, T.; Okada, Y.; Kurozumi, A.; Hatazaki, M.; Kaneto, H.; Osonoi, T.; et al. Tofogliflozin long-term effects on atherosclerosis progression and major clinical parameters in patients with type 2 diabetes mellitus lacking a history of cardiovascular disease: A 2-year extension study of the UTOPIA trial. Cardiovasc. Diabetol. 2023, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Spizzo, I.; Liu, H.; Hu, Y.; Simpson, R.W.; Widdop, R.E.; Dear, A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diab Vasc. Dis. Res. 2018, 15, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Herrera, A.; Moraña-Fernández, S.; Otero-Santiago, M.; Anido-Varela, L.; Campos-Toimil, M.; García-Seara, J.; Román, A.; Seijas, J.; García-Caballero, L.; Rodríguez, J.; et al. The lipidomic and inflammatory profiles of visceral and subcutaneous adipose tissues are distinctly regulated by the SGLT2 inhibitor empagliflozin in Zucker diabetic fatty rats. Biomed. Pharmacother. 2023, 161, 114535. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, X.; Wang, Q. Effects and mechanisms of SGLT2 inhibitors on the NLRP3 inflammasome, with a focus on atherosclerosis. Front. Endocrinol. 2022, 13, 992937. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; Oh, C.-M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Wang, C.; Qin, Y.; Zhang, X.; Yang, Y.; Wu, X.; Liu, J.; Qin, S.; Chen, K.; Xiao, W. Effect of Dapagliflozin on Indicators of Myocardial Fibrosis and Levels of Inflammatory Factors in Heart Failure Patients. Dis. Markers 2022, 2022, 5834218. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Zhong, L.; Li, S.; Zhou, L.; Zhang, Q.; Li, M.; Xiao, X. The effect of sodium-glucose cotransporter 2 inhibitors on biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1045235. [Google Scholar] [CrossRef] [PubMed]
- Buttice, L.; Ghani, M.; Suthakar, J.; Gnanalingham, S.; Carande, E.; Kennedy, B.W.C.; Pitcher, A.; Gamble, J.H.P.; Ahmad, M.; Lewis, A.; et al. The effect of sodium-glucose cotransporter-2 inhibitors on inflammatory biomarkers: A meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024; ahead of print. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Deem, T.L.; Cook-Mills, J.M. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: Role of reactive oxygen species. Blood 2004, 104, 2385–2393. [Google Scholar] [CrossRef]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Nowak, W.N.; Deng, J.; Ruan, X.Z.; Xu, Q. Reactive Oxygen Species Generation and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2017, 37, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox Signal 2020, 32, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Iannantuoni, F.; de Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Coenen, D.M.; Mastenbroek, T.G.; Cosemans, J.M.E.M. Platelet interaction with activated endothelium: Mechanistic insights from microfluidics. Blood 2017, 130, 2819–2828. [Google Scholar] [CrossRef]
- Hamilos, M.; Petousis, S.; Parthenakis, F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018, 8, 568–580. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Langer, H.F. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020, 307, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Kohlmorgen, C.; Gerfer, S.; Feldmann, K.; Twarock, S.; Hartwig, S.; Lehr, S.; Klier, M.; Krüger, I.; Helten, C.; Keul, P.; et al. Dapagliflozin reduces thrombin generation and platelet activation: Implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia 2021, 64, 1834–1849. [Google Scholar] [CrossRef]
- Hegazy, S.; Elsabaawy, M.; Eltabakh, M.; Hammad, R.; Bedair, H. CD62P (P-selectin) expression as a platelet activation marker in patients with liver cirrhosis with and without cholestasis. Clin. Exp. Hepatol. 2021, 7, 231–240. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Xu, J.; Li, J.; Shaw, W.; Oh, R.; Pfeifer, M.; Butler, J.; Sattar, N.; Mahaffey, K.W.; Neal, B.; et al. Effects of Canagliflozin on Amino-Terminal Pro-B-Type Natriuretic Peptide: Implications for Cardiovascular Risk Reduction. J. Am. Coll. Cardiol. 2020, 76, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Singh, T.; Newby, D.E.; Singh, J. Sodium-glucose co-transporter 2 inhibitor therapy: Mechanisms of action in heart failure. Heart 2021, 107, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Gamaza-Chulián, S.; Díaz-Retamino, E.; González-Testón, F.; Gaitero, J.C.; Castillo, M.J.; Alfaro, R.; Rodríguez, E.; González-Caballero, E.; Martín-Santana, A. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on left ventricular remodelling and longitudinal strain: A prospective observational study. BMC Cardiovasc. Disord. 2021, 21, 456. [Google Scholar] [CrossRef] [PubMed]
- Tang, O.; Matsushita, K.; Coresh, J.; Ndumele, C.; McEvoy, J.W.; Sharrett, A.R.; Hoogeveen, R.; Ballantyne, C.M.; Selvin, E. High-Sensitivity Cardiac Troponin I and T for Cardiovascular Risk Stratification in Adults With Diabetes. Diabetes Care 2020, 43, e144–e146. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y. SGLT2 Inhibitors Play a Salutary Role in Heart Failure via Modulation of the Mitochondrial Function. Front. Cardiovasc. Med. 2020, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Leccisotti, L.; Cinti, F.; Sorice, G.P.; D’amario, D.; Lorusso, M.; Guzzardi, M.A.; Mezza, T.; Gugliandolo, S.; Cocchi, C.; Capece, U.; et al. Dapagliflozin improves myocardial flow reserve in patients with type 2 diabetes: The DAPAHEART Trial: A preliminary report. Cardiovasc. Diabetol. 2022, 21, 173. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- EMPA-KIDNEY Collaborative Group. Impact of primary kidney disease on the effects of empagliflozin in patients with chronic kidney disease: Secondary analyses of the EMPA-KIDNEY trial. Lancet Diabetes Endocrinol. 2024, 12, 51–60. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, X.M.; Liang, X.M.; Wu, X.B.; Yao, C.M. SGLT2 inhibitors attenuate nephrin loss and enhance TGF-β1 secretion in type 2 diabetes patients with albuminuria: A randomized clinical trial. Sci. Rep. 2022, 12, 15695. [Google Scholar] [CrossRef] [PubMed]
- Mesfine, B.B.; Vojisavljevic, D.; Kapoor, R.; Watson, D.; Kandasamy, Y.; Rudd, D. Urinary nephrin-a potential marker of early glomerular injury: A systematic review and meta-analysis. J. Nephrol. 2024, 37, 39–51. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Inoue, H.; Izutsu, T.; Matsumoto, S.; Antoku, S.; Yamasaki, T.; Mori, T.; Togane, M. Changes in the estimated glomerular filtration rate and predictors of the renal prognosis in Japanese patients with type 2 diabetes: A retrospective study during the 12 months after the initiation of tofogliflozin. PLoS ONE 2023, 18, e0292014. [Google Scholar] [CrossRef] [PubMed]
- Noiri, E.; Doi, K.; Negishi, K.; Tanaka, T.; Hamasaki, Y.; Fujita, T.; Portilla, D.; Sugaya, T. Urinary fatty acid-binding protein 1: An early predictive biomarker of kidney injury. Am. J. Physiol. Ren. Physiol. 2009, 296, F669–F679. [Google Scholar] [CrossRef]
- Takashima, H.; Yoshida, Y.; Nagura, C.; Furukawa, T.; Tei, R.; Maruyama, T.; Maruyama, N.; Abe, M. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: A randomized open-label prospective trial. Diab Vasc. Dis. Res. 2018, 15, 469–472. [Google Scholar] [CrossRef]
- Sen, T.; Li, J.; Neuen, B.L.; Neal, B.; Arnott, C.; Parikh, C.R.; Coca, S.G.; Perkovic, V.; Mahaffey, K.W.; Yavin, Y.; et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 2021, 64, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar] [PubMed]
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z.I. Markers of Kidney Injury, Inflammation, and Fibrosis Associated With Ertugliflozin in Patients With CKD and Diabetes. Kidney Int. Rep. 2021, 6, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes. Metab. 2018, 20, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Nelinson, D.S.; Sosa, J.M.; Chilton, R.J. SGLT2 inhibitors: A narrative review of efficacy and safety. J. Osteopath. Med. 2021, 121, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Heo, K.N.; Ah, Y.M.; Yang, B.R.; Lee, J.Y. Age- and sex-specific risk of urogenital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A population-based self-controlled case-series study. Maturitas 2021, 150, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Neuen, B.L.; Jun, M.; Ohkuma, T.; Neal, B.; Jardine, M.J.; Heerspink, H.L.; Wong, M.G.; Ninomiya, T.; Wada, T.; et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; A Marra, C.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef] [PubMed]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Ren. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef]
- Cefalo, C.M.A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the first dual SGLT inhibitor: Current outlook and perspectives. Cardiovasc. Diabetol. 2019, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Bhatt, D.; Szarek, M.; Davies, M.; Banks, P.; Pitt, B.; Steg, P.G. Sotagliflozin Reduces Stroke Outcomes in Patients with Diabetes and Chronic Kidney Disease. J. Am. Coll. Cardiol. 2024, 83 (Suppl. 13), 1733. [Google Scholar] [CrossRef]
- Pitt, B.; Steg, G.; Leiter, L.A.; Bhatt, D.L. The Role of Combined SGLT1/SGLT2 Inhibition in Reducing the Incidence of Stroke and Myocardial Infarction in Patients with Type 2 Diabetes Mellitus. Cardiovasc. Drugs Ther. 2022, 36, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Bhatt, D.L.; Metra, M. Does SGLT1 inhibition add to the benefits of SGLT2 inhibition in the prevention and treatment of heart failure? Eur. Heart J. 2022, 43, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Mariam, Z.; Niazi, S.K. Glucagon-like peptide agonists: A prospective review. Endocrinol. Diabetes Metab. 2024, 7, e462. [Google Scholar] [CrossRef] [PubMed]
- Bulum, T. Nephroprotective Properties of the Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) Receptor Agonists. Biomedicines 2022, 10, 2586. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Kulve, J.S.T.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Bhavsar, S.; Mudaliar, S.; Cherrington, A. Evolution of exenatide as a diabetes therapeutic. Curr. Diabetes Rev. 2013, 9, 161–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef]
- Barnett, A.H. Lixisenatide: Evidence for its potential use in the treatment of type 2 diabetes. Core Evid. 2011, 6, 67–79. [Google Scholar] [CrossRef][Green Version]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients With Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Beckman, J.A.; Nian, H.; Garner, E.M.; Mayfield, D.; Devin, J.K.; Koethe, J.R.; Brown, J.D.; Cahill, K.N.; Yu, C.; et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: A randomized controlled trial. Diabetes Obes. Metab. 2023, 25, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiao, C.; Zhao, Y.; Yin, H.; Yu, M. Liraglutide Improves Endothelial Function via the mTOR Signaling Pathway. J. Diabetes Res. 2021, 2021, 2936667. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, Y.; Xu, M.; Zhao, X.; Chen, M. Effects of dulaglutide on endothelial progenitor cells and arterial elasticity in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chen, S.; Ren, Q.; Niu, S.; Pan, X.; Chen, X.; Li, Z.; Chen, X. Effects of semaglutide on vascular structure and proteomics in high-fat diet-induced obese mice. Front. Endocrinol. 2022, 13, 995007. [Google Scholar] [CrossRef] [PubMed]
- Hachuła, M.; Kosowski, M.; Ryl, S.; Basiak, M.; Okopień, B. Impact of Glucagon-Like Peptide 1 Receptor Agonists on Biochemical Markers of the Initiation of Atherosclerotic Process. Int. J. Mol. Sci. 2024, 25, 1854. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.R. The role of MCP-1 in atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Pickett, J.R.; Wu, Y.; Zacchi, L.F.; Ta, H.T. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: Drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc. Res. 2023, 119, 2278–2293. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhang, W.Q.; Tian, Y.; Wang, L.F.; Chen, C.C.; Qiu, C.M. Liraglutide suppresses non-esterified free fatty acids and soluble vascular cell adhesion molecule-1 compared with metformin in patients with recent-onset type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Tian, Y.; Chen, X.M.; Wang, L.F.; Chen, C.C.; Qiu, C.M. Liraglutide ameliorates beta-cell function, alleviates oxidative stress and inhibits low grade inflammation in young patients with new-onset type 2 diabetes. Diabetol. Metab. Syndr. 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Malondialdehyde as an Important Key Factor of Molecular Mechanisms of Vascular Wall Damage under Heart Diseases Development. Int. J. Mol. Sci. 2022, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Coughlan, M.T.; Cooper, M.E. The postprandial actions of GLP-1 receptor agonists: The missing link for cardiovascular and kidney protection in type 2 diabetes. Cell Metab. 2023, 35, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Liani, R.; Tripaldi, R.; Di Castelnuovo, A.; Guagnano, M.T.; Tartaro, A.; Bonadonna, R.C.; Federico, V.; Cipollone, F.; Consoli, A.; et al. Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: Effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss. Nutrients 2018, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiang, Q.; Li, N.; Feng, P.; Wei, W.; Hölscher, C. Neuroprotective Mechanisms of Glucagon-Like Peptide-1-Based Therapies in Ischemic Stroke: An Update Based on Preclinical Research. Front. Neurol. 2022, 13, 844697. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Lee, S.F.; Paré, G.; Bethel, M.A.; Colhoun, H.M.; Hoover, A.; Lakshmanan, M.; Lin, Y.; Pirro, V.; Qian, H.R.; et al. Biomarker Changes Associated With Both Dulaglutide and Cardiovascular Events in the REWIND Randomized Controlled Trial: A Nested Case-Control Post Hoc Analysis. Diabetes Care 2023, 46, 1046–1051. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef]
- Butler, J.; Abildstrøm, S.Z.; Borlaug, B.A.; Davies, M.J.; Kitzman, D.W.; Petrie, M.C.; Shah, S.J.; Verma, S.; Abhayaratna, W.P.; Chopra, V.; et al. Semaglutide in Patients With Obesity and Heart Failure Across Mildly Reduced or Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2023, 82, 2087–2096. [Google Scholar] [CrossRef]
- Jalil, J.E.; Gabrielli, L.; Ocaranza, M.P.; MacNab, P.; Fernández, R.; Grassi, B.; Jofré, P.; Verdejo, H.; Acevedo, M.; Cordova, S.; et al. New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review. Int. J. Mol. Sci. 2024, 25, 4407. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Backhaus, S.J.; Lange, T.; Evertz, R.; Kutty, S.; Kowallick, J.T.; Hasenfuß, G.; Schuster, A. Impact of epicardial adipose tissue on cardiac function and morphology in patients with diastolic dysfunction. ESC Heart Fail. 2024; ahead of print. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obes 2017, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Bakris, G.L.; Rodbard, H.W.; Tuttle, K.R. Glucagon-like peptide-1 receptor agonists in diabetic kidney disease: A review of their kidney and heart protection. Am. J. Prev. Cardiol. 2023, 14, 100502. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, R.; Lin, T.; Chaudhari, S.; Shotorbani, P.Y.; Yang, L.; Wu, P. Glucagon-like peptide-1 receptor pathway inhibits extracellular matrix production by mesangial cells through store-operated Ca2+ channel. Exp. Biol. Med. 2019, 244, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Mima, A.; Nomura, A.; Fujii, T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: A narrative review. Biomed. Pharmacother. 2023, 165, 115032. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; De Sio, V.; Calabrò, P. FLOW trial stopped early due to evidence of renal protection with semaglutide. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024; ahead of print. [Google Scholar] [CrossRef]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- A Research Study to Find Out How Semaglutide Works in the Kidneys Compared to Placebo in People with Type 2 Diabetes Chronic Kidney Disease (the REMODELTrial) Identifier NCT04865770, U.S. National Library of Medicine. 2021. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04865770 (accessed on 3 April 2024).
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef]
- Aldhaleei, W.A.; Abegaz, T.M.; Bhagavathula, A.S. Glucagon-like Peptide-1 Receptor Agonists Associated Gastrointestinal Adverse Events: A Cross-Sectional Analysis of the National Institutes of Health All of Us Cohort. Pharmaceuticals 2024, 17, 199. [Google Scholar] [CrossRef]
- Bezin, J.; Gouverneur, A.; Pénichon, M.; Mathieu, C.; Garrel, R.; Hillaire-Buys, D.; Pariente, A.; Faillie, J.L. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care 2023, 46, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Wintzell, V.; Hviid, A.; Eliasson, B.; Gudbjörnsdottir, S.; Jonasson, C.; Hveem, K.; Svanström, H.; Melbye, M.; Ueda, P. Glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer: Scandinavian cohort study. BMJ 2024, 385, e078225. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, R.; Zhou, J.; Doyle, M.E.; Egan, J.M. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000, 141, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Murad, H.; Agay, N.; Olmer, L.; Freedman, L.S. Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients With Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2350408. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: A multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 2023, 40, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S. Tirzepatide: A Novel, Once-weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. Touchrev Endocrinol. 2022, 18, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2012, 33, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ojima, A.; Matsui, T.; Maeda, S.; Takeuchi, M.; Yamagishi, S. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm. Metab. Res. 2012, 44, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Tsihlis, N.D.; Oustwani, C.S.; Vavra, A.K.; Jiang, Q.; Keefer, L.K.; Kibbe, M.R. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10. Cell Biochem. Biophys. 2011, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Matsui, T.; Hirano, T.; Yamagishi, S.I. GIP as a Potential Therapeutic Target for Atherosclerotic Cardiovascular Disease-A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1509. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Shen, S.; Zhang, J.; Wang, Q. Effect of the Dual Glucose-Dependent Insulinotropic Peptide/Gulcagon-like Peptide 1 Receptor Agonist Tirzepatide on Lipid Profile and Waist Circumference: A Systematic Review and Meta-analysis. Clin. Ther. 2023, 45, 787–796. [Google Scholar] [CrossRef]
- Nogi, Y.; Nagashima, M.; Terasaki, M.; Nohtomi, K.; Watanabe, T.; Hirano, T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE 2012, 7, e35683. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef]
- Hankosky, E.R.; Wang, H.; Neff, L.M.; Kan, H.; Wang, F.; Ahmad, N.N.; Griffin, R.; Stefanski, A.; Garvey, W.T. Tirzepatide reduces the predicted risk of atherosclerotic cardiovascular disease and improves cardiometabolic risk factors in adults with obesity or overweight: SURMOUNT-1 post hoc analysis. Diabetes Obes. Metab. 2024, 26, 319–328. [Google Scholar] [CrossRef]
- Lingvay, I.; Mosenzon, O.; Brown, K.; Cui, X.; O’Neill, C.; Fernández Landó, L.; Patel, H. Systolic blood pressure reduction with tirzepatide in patients with type 2 diabetes: Insights from SURPASS clinical program. Cardiovasc. Diabetol. 2023, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Bhatt, D.L.; Buse, J.B.; Prato, S.D.; Kahn, S.E.; Lincoff, A.M.; McGuire, D.K.; Nauck, M.A.; Nissen, S.E.; Sattar, N.; et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am. Heart J. 2024, 267, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Caruso, I.; Giorgino, F. Renal effects of GLP-1 receptor agonists and tirzepatide in individuals with type 2 diabetes: Seeds of a promising future. Endocrine, 2024; ahead of print. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Tamayo, A.; Hara, M.; Caicedo, A. The Local Paracrine Actions of the Pancreatic α-Cell. Diabetes 2020, 69, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Zeigerer, A.; Sekar, R.; Kleinert, M.; Nason, S.; Habegger, K.M.; Müller, T.D. Glucagon’s Metabolic Action in Health and Disease. Compr. Physiol. 2021, 11, 1759–1783. [Google Scholar] [CrossRef] [PubMed]
- Esler, W.P.; Bence, K.K. Metabolic Targets in Nonalcoholic Fatty Liver Disease. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Wewer Albrechtsen, N.J.; Holst, J.J.; Cherrington, A.D.; Finan, B.; Gluud, L.L.; Dean, E.D.; Campbell, J.E.; Bloom, S.R.; Tan, T.M.; Knop, F.K.; et al. 100 years of glucagon and 100 more. Diabetologia 2023, 66, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Hope, D.C.D.; Vincent, M.L.; Tan, T.M.M. Striking the Balance: GLP-1/Glucagon Co-Agonism as a Treatment Strategy for Obesity. Front. Endocrinol. 2021, 12, 735019. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.E.R.; Robertson, D.; Wang, T.; Hornigold, D.C.; Petrone, M.; Cooper, A.T.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Schlichthaar, H.; et al. Efficacy, Safety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J. Clin. Endocrinol. Metab. 2020, 105, dgz047. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Lawitz, E.; Shankar, R.R.; Chaudhri, E.; Liu, J.; Lam, R.L.H.; Kaufman, K.D.; Engel, S.S.; MK-6024 P001 Study Group. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J. Hepatol. 2023, 79, 888–897. [Google Scholar] [CrossRef]
- Alba, M.; Yee, J.; Frustaci, M.E.; Samtani, M.N.; Fleck, P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor co-agonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: A randomized dose-ranging study. Clin. Obes. 2021, 11, e12432. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Steen, O.; Lucas, K.J.; Startseva, E.; Unseld, A.; Hennige, A.M. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: A randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 2024, 12, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M.; Rosenstock, J.; Hoefler, J.; Manuel, R.; Hennige, A.M. Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: A randomised clinical trial. Diabetologia 2024, 67, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Sélley, E.; Kun, S.; Szijártó, I.A.; Kertész, M.; Wittmann, I.; Molnár, G.A. Vasodilator Effect of Glucagon: Receptorial Crosstalk Among Glucagon, GLP-1, and Receptor for Glucagon and GLP-1. Horm. Metab. Res. 2016, 48, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.M.; Bøgevig, S.; Holst, J.J.; Knop, F.K.; Christensen, M.B. Hemodynamic Effects of Glucagon: A Literature Review. J. Clin. Endocrinol. Metab. 2018, 103, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. The renal glucagon receptor is essential to kidney metabolic and homeostatic functions. Nat. Rev. Nephrol. 2024, 20, 203. [Google Scholar] [CrossRef]
- Parker, V.E.R.; Hoang, T.; Schlichthaar, H.; Gibb, F.W.; Wenzel, B.; Posch, M.G.; Rose, L.; Chang, Y.T.; Petrone, M.; Hansen, L.; et al. Efficacy and safety of cotadutide, a dual glucagon-like peptide-1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metab. 2022, 24, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Urva, S.; Roell, W.C.; Qu, H.; Loghin, C.; Moyers, J.S.; O’Farrell, L.S.; Briere, D.A.; Sloop, K.W.; Thomas, M.K.; et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 2022, 34, 1234–1247.e9. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Retatrutide Phase 2 Obesity Trial Investigators. Triple-Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]
- A Study of Retatrutide (LY3437943) in Participants with Obesity and Cardiovascular Disease (TRIUMPH-3). Identifier NCT05882045. U.S National Library of Medicine. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05882045 (accessed on 9 April 2024).
- Pirags, V.; Lebovitz, H.; Fouqueray, P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes. Metab. 2012, 14, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Sugawara, K.; Ishihara, T.; Ishihara, N.; Ogawa, W. Effects of imeglimin on mitochondrial function, AMPK activity, and gene expression in hepatocytes. Sci. Rep. 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Vial, G.; Chauvin, M.A.; Bendridi, N.; Durand, A.; Meugnier, E.; Madec, A.M.; Bernoud-Hubac, N.; Pais de Barros, J.P.; Fontaine, É.; Acquaviva, C.; et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes 2015, 64, 2254–2264. [Google Scholar] [CrossRef]
- Sanada, J.; Obata, A.; Fushimi, Y.; Kimura, T.; Shimoda, M.; Ikeda, T.; Nogami, Y.; Obata, Y.; Yamasaki, Y.; Nakanishi, S.; et al. Imeglimin exerts favorable effects on pancreatic β-cells by improving morphology in mitochondria and increasing the number of insulin granules. Sci. Rep. 2022, 12, 13220. [Google Scholar] [CrossRef]
- Vinayagam, P.; Senathipathi, V.; Shivam, V.; Velraju, N. The role of Imeglimin in glycemic control, beta cell function and safety outcomes in patients with type 2 diabetes mellitus: A comprehensive meta-analysis. Diabetes Epidemiol. Manag. 2023, 12, 100164. [Google Scholar] [CrossRef]
- Giruzzi, M. Imeglimin. Clin. Diabetes 2021, 39, 439–440. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Efficacy and safety of imeglimin in type 2 diabetes: A systematic review and meta-analysis of randomized placebo-controlled trials. Diabetes Metab. Syndr. 2023, 17, 102710. [Google Scholar] [CrossRef]
- Konkwo, C.; Perry, R.J. Imeglimin: Current Development and Future Potential in Type 2 Diabetes. Drugs 2021, 81, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Vial, G.; Borel, A.L.; Cottet-Rouselle, C.; Hallakou-Bozec, S.; Bolze, S.; Fouqueray, P.; Fontaine, E. Imeglimin prevents human endothelial cell death by inhibiting mitochondrial permeability transition without inhibiting mitochondrial respiration. Cell Death Discov. 2016, 2, 15072. [Google Scholar] [CrossRef]
- Uchida, T.; Ueno, H.; Konagata, A.; Taniguchi, N.; Kogo, F.; Nagatomo, Y.; Shimizu, K.; Yamaguchi, H.; Shimoda, K. Improving the Effects of Imeglimin on Endothelial Function: A Prospective, Single-Center, Observational Study. Diabetes Ther. 2023, 14, 569–579. [Google Scholar] [CrossRef]
- Kitakata, H.; Endo, J.; Hashimoto, S.; Mizuno, E.; Moriyama, H.; Shirakawa, K.; Goto, S.; Katsumata, Y.; Fukuda, K.; Sano, M. Imeglimin prevents heart failure with preserved ejection fraction by recovering the impaired unfolded protein response in mice subjected to cardiometabolic stress. Biochem. Biophys. Res. Commun. 2021, 572, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, M.; Soulié, M.; Hamzaoui, M.; Bailly, A.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Vendeville, C.; Gluais-Dagorn, P.; Hallakou-Bozec, S.; et al. Short-and long-term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol. Diabetes Metab. 2020, 3, e00128. [Google Scholar] [CrossRef] [PubMed]
- Chigurupati, S.; Dhanaraj, S.A.; Balakumar, P. A step ahead of PPARγ full agonists to PPARγ partial agonists: Therapeutic perspectives in the management of diabetic insulin resistance. Eur. J. Pharmacol. 2015, 755, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.R.; Buse, J.B.; Wu, H.; Durrwell, L.; Mingrino, R.; Jaekel, K.; El Azzouzi, B.; Andjelkovic, M.; Herz, M. Efficacy, safety and tolerability of aleglitazar in patients with type 2 diabetes: Pooled findings from three randomized phase III trials. Diabetes Obes. Metab. 2015, 17, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Tardif, J.C.; Schwartz, G.G.; Nicholls, S.J.; Rydén, L.; Neal, B.; Malmberg, K.; Wedel, H.; Buse, J.B.; Henry, R.R.; et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: The AleCardio randomized clinical trial. JAMA 2014, 311, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R. The first approved agent in the Glitazar’s Class: Saroglitazar. Curr. Drug Targets 2014, 15, 151–155. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Samajdar, S.S.; Das, S. Effects of saroglitazar in the treatment of non-alcoholic fatty liver disease or non-alcoholic steatohepatitis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102174. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.; Paneerselvam, A.; Mukhopadhyay, S.; Bhansali, A.; Kamath, D.; Shankar, V.; Gambhire, D.; Jani, R.H.; Joshi, S.; Patel, P. A Multicenter, Prospective, Randomized, Double-blind Study to Evaluate the Safety and Efficacy of Saroglitazar 2 and 4 mg Compared to Pioglitazone 45 mg in Diabetic Dyslipidemia (PRESS V). J. Diabetes Sci. Technol. 2014, 8, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.H.; Pai, V.; Jha, P.; Jariwala, G.; Mukhopadhyay, S.; Bhansali, A.; Joshi, S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol. Ther. 2014, 16, 63–71. [Google Scholar] [CrossRef]
- Shetty, S.R.; Kumar, S.; Mathur, R.P.; Sharma, K.H.; Jaiswal, A.D. Observational study to evaluate the safety and efficacy of saroglitazar in Indian diabetic dyslipidemia patients. Indian. Heart J. 2015, 67, 23–26. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Parmar, D.; Sheikh, F.; Sarin, S.K.; Cisneros, L.; Gawrieh, S.; Momin, T.; Duseja, A.; Sanyal, A.J. Saroglitazar, a Dual PPAR α/γ Agonist, Improves Atherogenic Dyslipidemia in Patients With Non-Cirrhotic Nonalcoholic Fatty Liver Disease: A Pooled Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2597–2605.e2. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate the Safety Effectiveness of Saroglitazar 4mg in Patients with NAFLD with Comorbidities Identifier NCT05872269, U.S. National Library of Medicine. 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05872269 (accessed on 10 April 2024).
- Kalliora, C.; Drosatos, K. The Glitazars Paradox: Cardiotoxicity of the Metabolically Beneficial Dual PPARα and PPARγ Activation. J. Cardiovasc. Pharmacol. 2020, 76, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Hou, J.; Liu, H.; Zeng, R.; Li, X.; Han, M.; Li, Q.; Ji, L.; Pan, D.; et al. Plasma proteome profiling reveals the therapeutic effects of the PPAR pan-agonist chiglitazar on insulin sensitivity, lipid metabolism, and inflammation in type 2 diabetes. Sci. Rep. 2024, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Wang, Y.; Xu, X.; Li, F.; Zhou, J.; Shan, T.; Huang, R.; Cai, T.; Liu, X.; et al. Comparative Evaluation of Chiglitazar and Sitagliptin on the Levels of Retinol-Binding Protein 4 and Its Correlation With Insulin Resistance in Patients With Type 2 Diabetes. Front. Endocrinol. 2022, 13, 801271. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Ma, J.; Miao, H.; Wang, C.; Wang, X.; Li, Q.; Lu, W.; Yang, J.; Zhang, L.; Yang, J.; et al. Chiglitazar monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: A randomized, double-blind, phase 3 trial (CMAS). Sci. Bull. 2021, 66, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- A Randomised Double-Blind Placebo Parallel Controlled Phase III Clinical Study to Evaluate the Efficacy and Safety of Chiglitazar Added to Metformin in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy (RECAM). Identifier NCT04807348. ICH Good Clinical Practice Network. 2021. Available online: https://ichgcp.net/clinical-trials-registry/NCT04807348 (accessed on 12 April 2024).
- Matschinsky, F.M.; Wilson, D.F. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; Camastra, S.; Astiarraga, B.; Nannipieri, M.; Anselmino, M.; Ferrannini, E. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol. Metab. 2014, 4, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Haddad, D.; Dsouza, V.S.; Al-Mulla, F.; Al Madhoun, A. New-Generation Glucokinase Activators: Potential Game-Changers in Type 2 Diabetes Treatment. Int. J. Mol. Sci. 2024, 25, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Gan, S.; Liu, Y.; Ma, J.; Dong, X.; Song, W.; Zeng, J.; Wang, G.; Zhao, W.; Zhang, Q.; et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: A dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018, 6, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, Y.; Chen, L. 182-OR: A Novel Dual-Acting Glucokinase Activator (GKA) Dorzagliatin (HMS5552) Achieved Primary Efficacy Endpoint with Good Safety Profiles in T2DM Patients after 24 Weeks of Treatment in a Phase III Monotherapy Trial. Diabetes 2020, 69 (Suppl. 1), 182-OR. [Google Scholar] [CrossRef]
- Dutta, D.; Khandelwal, D.; Kumar, M.; Sharma, M. Efficacy and safety of novel dual glucokinase activator dorzagliatin in type-2 diabetes A meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102695. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, L.; Xiao, X.; Hou, X.; Ji, L. A multicentre, randomized, double-blind, parallel, active- and placebo-controlled Phase 3 clinical study of the glucokinase activator PB-201 in treatment-naive patients with type 2 diabetes mellitus: A study protocol. Diabetes Obes. Metab. 2023, 25, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, Y.; Yao, X.; Wei, Y.; Zhu, J.; Cui, C.; Zhou, H.; Xu, M.; Li, H.; Ji, L. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the glucokinase activator PB-201 and its effects on the glucose excursion profile in drug-naïve Chinese patients with type 2 diabetes: A randomised controlled, crossover, single-centre phase 1 trial. EClinicalMedicine 2021, 42, 101185. [Google Scholar] [CrossRef] [PubMed]
- Usui, R.; Yabe, D.; Fauzi, M.; Goto, H.; Botagarova, A.; Tokumoto, S.; Tatsuoka, H.; Tahara, Y.; Kobayashi, S.; Manabe, T.; et al. GPR40 activation initiates store-operated Ca2+ entry and potentiates insulin secretion via the IP3R1/STIM1/Orai1 pathway in pancreatic β-cells. Sci. Rep. 2019, 9, 15562. [Google Scholar] [CrossRef] [PubMed]
- Burant, C.F. Activation of GPR40 as a therapeutic target for the treatment of type 2 diabetes. Diabetes Care 2013, 36 (Suppl. 2), S175–S179. [Google Scholar] [CrossRef] [PubMed]
- Otieno, M.A.; Snoeys, J.; Lam, W.; Ghosh, A.; Player, M.R.; Pocai, A.; Salter, R.; Simic, D.; Skaggs, H.; Singh, B.; et al. Fasiglifam (TAK-875): Mechanistic Investigation and Retrospective Identification of Hazards for Drug Induced Liver Injury. Toxicol. Sci. 2018, 163, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Bazydlo-Guzenda, K.; Buda, P.; Mach, M.; Pieczykolan, J.; Kozlowska, I.; Janiszewski, M.; Drzazga, E.; Dominowski, J.; Ziolkowski, H.; Wieczorek, M.; et al. Evaluation of the hepatotoxicity of the novel GPR40 (FFAR1) agonist CPL207280 in the rat and monkey. PLoS ONE 2021, 16, e0257477. [Google Scholar] [CrossRef] [PubMed]
- Bazydło-Guzenda, K.; Jarus-Dziedzic, K.; Gierczak-Pachulska, A.; Buda, P.; Rudzki, P.J.; Buś-Kwaśnik, K.; Juszczyk, E.; Tratkiewicz, E.; Rabczenko, D.; Segiet-Święcicka, A.; et al. First-in-human study of CPL207280, a novel G-protein-coupled receptor 40/free fatty acid receptor 1 agonist, in healthy volunteers after single and multiple administration. Diabetes Obes. Metab. 2024, 26, 1376–1385. [Google Scholar] [CrossRef]
- Vella, A.; Freeman, J.L.R.; Dunn, I.; Keller, K.; Buse, J.B.; Valcarce, C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci. Transl. Med. 2019, 11, eaau3441. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Y.; Zheng, Q.; Adegbite, E.; Ross, S.; Telusca, L.; Jones, C.L.; Fenaux, M.; Xu, S.; Junaidi, M.K. An Open-Label, Active-Controlled Phase 2 Evaluation of Novel GLP-1 Analog Ecnoglutide in Adults with Obesity. Diabetes 2023, 72 (Suppl. 1), 79-LB. [Google Scholar] [CrossRef]
- Anwar, D.; Bao, Y.; Zhou, Y.; Chen, S.; Zhou, J.; Li, L.; Cheng, Z.; Dong, X.; Shi, X.; Su, X.; et al. Efficacy and Safety of Supaglutide as Add-on to Metformin in Patients with Type 2 Diabetes. Diabetes 2023, 72 (Suppl. 1), 778-P. [Google Scholar] [CrossRef]
- Chakravarthy, M.; Argüelles-Tello, F.A.; Sun, A.L.A.; Elliott, M.; Acosta, L.; Rankin, J.E.; Hansen, S.K. CT-388, A novel once-weekly dual GLP-1 and GIP receptor modulator, is safe, well-tolerated, and produces more than 8% weight loss in four weeks in overweight and obese adults. Diabetes 2023, 72, 75-LB. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, H.; An, P.; Deng, H.; Liu, M.; Li, L.; Feng, L.; Song, B.; Han-Zhang, H.; Ma, Q.; et al. IBI362 (LY3305677), a weekly-dose GLP-1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity: A randomised, placebo-controlled, multiple ascending dose phase 1b study. EClinicalMedicine 2021, 39, 101088. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Song, W.; Fang, H.; Li, W.; Geng, J.; Wang, Y.; Guo, L.; Cai, H.; Yang, T.; Li, H.; et al. Efficacy and safety of chiglitazar, a novel peroxisome proliferator-activated receptor pan-agonist, in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, phase 3 trial (CMAP). Sci. Bull. 2021, 66, 1571–1580. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frías, J.P.; Rodbard, H.W.; Tofé, S.; Sears, E.; Huh, R.; Fernández Landó, L.; Patel, H. Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631–1640. [Google Scholar] [CrossRef]
- Gao, L.; Lee, B.W.; Chawla, M.; Kim, J.; Huo, L.; Du, L.; Huang, Y.; Ji, L. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: The SURPASS-AP-Combo trial. Nat. Med. 2023, 29, 1500–1510. [Google Scholar] [CrossRef]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.-Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chee, Y.J.; Dalan, R. Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future. Biomedicines 2024, 12, 1386. https://doi.org/10.3390/biomedicines12071386
Chee YJ, Dalan R. Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future. Biomedicines. 2024; 12(7):1386. https://doi.org/10.3390/biomedicines12071386
Chicago/Turabian StyleChee, Ying Jie, and Rinkoo Dalan. 2024. "Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future" Biomedicines 12, no. 7: 1386. https://doi.org/10.3390/biomedicines12071386
APA StyleChee, Y. J., & Dalan, R. (2024). Novel Therapeutics for Type 2 Diabetes Mellitus—A Look at the Past Decade and a Glimpse into the Future. Biomedicines, 12(7), 1386. https://doi.org/10.3390/biomedicines12071386




