The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas
Abstract
1. Introduction
2. Significance and Context
2.1. Innovation in Molecular Diagnostics Prompts Necessary Revisions
2.2. Overview of Updates
2.2.1. Rising Importance of Molecular Diagnostics
2.2.2. Update: Not Otherwise Specified and Not Elsewhere Classified
2.2.3. Update: Types and Subtypes
2.2.4. Update: Tumor Grading
2.2.5. Update: Continued Integration of Histological and Molecular Markers
2.3. Broad Categorization Overview
2.3.1. Gliomas, Glioneuronal Tumors and Neuronal Tumors
2.3.2. Update: Gliomas, Glioneuronal Tumors and Neuronal Tumors
2.3.3. Adult-Type Diffuse Gliomas
3. Astrocytoma, IDH-Mutant
3.1. Epidemiology and Localization
3.2. Brief Genetic Overview
Relevance
3.3. Update
Brief Genetic Overview of Relevance of CDKN2A/B Homozygous Deletion as a Marker
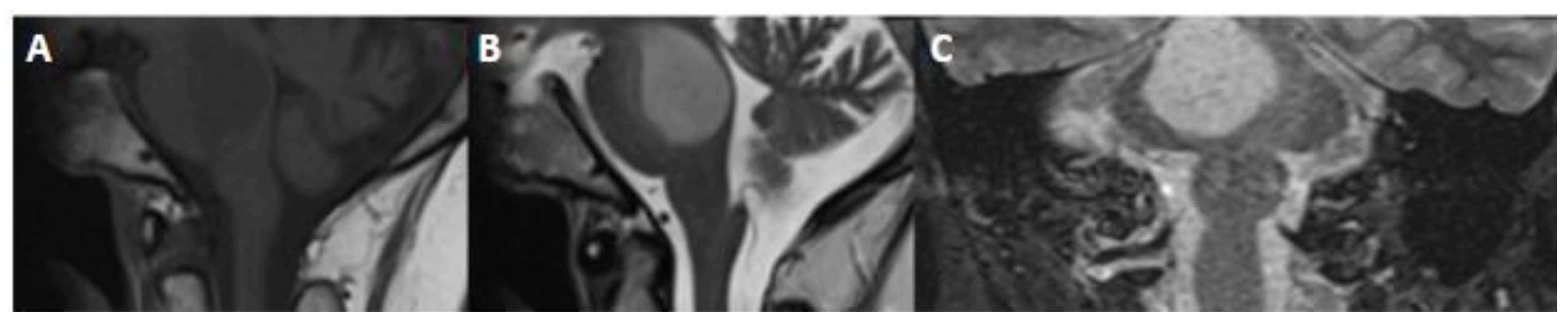
3.4. Imaging
4. Oligodendrioglioma, IDH-Mutant and 1p/19q-Codeleted
4.1. Epidemiology and Localization
4.2. Brief Genetic Overview
4.3. Clinical Features
4.4. Imaging
5. Glioblastoma, IDH-Wildtype
5.1. Update
Brief Genetic Overview of Relevance of Molecular Markers
5.2. Clinical Features and Localization
5.3. Imaging
6. Reiterated Update: Age Demographics in Glioma Classification
Pediatric-Type Diffuse High-Grade Glioma
7. Diffuse Midline Glioma, H3 K27-Altered
7.1. Epidemiology and Localization
7.2. Clinical Features
7.3. Brief Genetic Overview
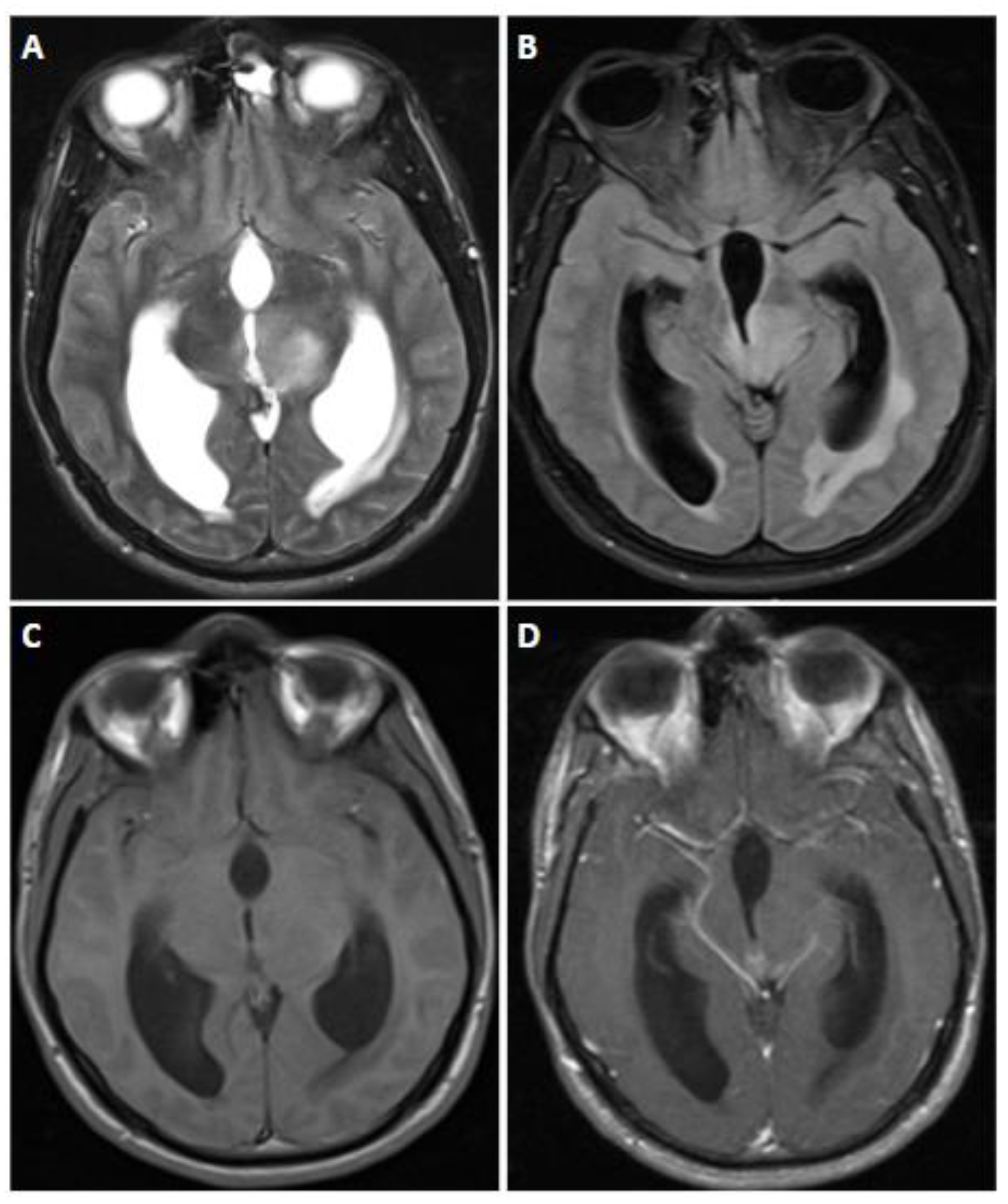
7.4. Imaging
8. Diffuse Hemispheric Glioma, H3 G34-Mutant
8.1. Clinical Features
8.2. Epidemiology and Localization
8.3. Brief Genetic Overview
8.4. Imaging
9. Diffuse Pediatric-Type High-Grade Glioma, H3-Wildtype and IDH-Wildtype
9.1. Brief Genetic Overview
Relevance
9.2. Imaging
10. High-Grade Astrocytoma with Piloid Features
Brief Genetic Overview
11. Discussion
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scheithauer, B.W. Development of the WHO classification of tumors of the central nervous system: A historical perspective. Brain Pathol. 2009, 19, 551–564. [Google Scholar] [CrossRef]
- Cree, I.A. The WHO Classification of Haematolymphoid Tumours. Leukemia 2022, 36, 1701–1702. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours [Internet]. In WHO Classification of Tumours Series, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, Available online: https://tumourclassification.iarc.who.int/chapters/45 (accessed on 4 May 2024).
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Saracci, R.; Wild, C.P. International Agency for Research on Cancer: The First 50 Years, 1965–2015; International Agency for Re-search on Cancer: Lyon, France, 2015. [Google Scholar]
- Santanam, L.; Hurkmans, C.; Mutic, S.; van Vliet-Vroegindeweij, C.; Brame, S.; Straube, W.; Galvin, J.; Tripuraneni, P.; Michalski, J.; Bosch, W. Standardizing naming conventions in radiation oncology. Int. J. Radiat. Oncol. 2012, 83, 1344–1349. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Castro, L.N.G. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Cree, I.A.; Ruiz, B.I.I.; Zavadil, J.; McKay, J.; Olivier, M.; Kozlakidis, Z.; Lazar, A.J.; Hyde, C.; Holdenrieder, S.; Hastings, R.; et al. The International Collaboration for Cancer Classification and Research. Int. J. Cancer 2021, 148, 560–571. [Google Scholar] [CrossRef]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef]
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Johnson, D.R.; Giannini, C.; Vaubel, R.A.; Morris, J.M.; Eckel, L.J.; Kaufmann, T.J.; Guerin, J.B. A Radiologist’s Guide to the 2021 WHO Central Nervous System Tumor Classification: Part I—Key Concepts and the Spectrum of Diffuse Gliomas. Radiology 2022, 304, 494–508. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Capper, D.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Dutt, M.; Suraweera, N.; Pfister, S.M.; von Deimling, A.; Brandner, S. Methylation array profiling of adult brain tumours: Diagnostic outcomes in a large, single centre. Acta Neuropathol. Commun. 2019, 7, 24. [Google Scholar] [CrossRef]
- Osborn, A.; Louis, D.; Poussaint, T.; Linscott, L.; Salzman, K. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Bent, M.J.v.D.; Weller, M.; Wen, P.Y.; Kros, J.M.; Aldape, K.; Chang, S. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro-Oncology 2017, 19, 614–624. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Figrarella-Branger, D.; Fuller, G.N.; Giannini, C.; Holland, E.C.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Komori, T.; et al. cIMPACT-NOW update 5: Rec-ommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020, 139, 603–608. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef]
- Louis, D.N.; Wesseling, P.; Paulus, W.; Giannini, C.; Batchelor, T.T.; Cairncross, J.G.; Capper, D.; Figarella-Branger, D.; Lopes, M.B.; Wick, W.; et al. cIMPACT-NOW update 1: Not Otherwise Specified (NOS) and Not Elsewhere Classified (NEC). Acta Neuropathol. 2018, 135, 481–484. [Google Scholar] [CrossRef]
- Louis, D.N.; Ellison, D.W.; Brat, D.J.; Aldape, K.; Capper, D.; Hawkins, C.; Paulus, W.; Perry, A.; Reifenberger, G.; Figarella-Branger, D.; et al. cIMPACT-NOW: A practical summary of diagnostic points from Round 1 updates. Brain Pathol. 2019, 29, 469–472. [Google Scholar] [CrossRef]
- Louis, D.N.; Giannini, C.; Capper, D.; Paulus, W.; Figarella-Branger, D.; Lopes, M.B.; Batchelor, T.T.; Cairncross, J.G.; van den Bent, M.; Wick, W.; et al. cIMPACT-NOW update 2: Diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018, 135, 639–642. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U.; et al. Genomic analysis of diffuse in-trinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef]
- Roux, A.; Pallud, J.; Saffroy, R.; Edjlali-Goujon, M.; Debily, M.-A.; Boddaert, N.; Sanson, M.; Puget, S.; Knafo, S.; Adam, C.; et al. High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro-Oncology 2020, 22, 1190–1202. [Google Scholar] [CrossRef]
- Balss, J.; Meyer, J.; Mueller, W.; Korshunov, A.; Hartmann, C.; von Deimling, A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008, 116, 597–602. [Google Scholar] [CrossRef]
- Capper, D.; Zentgraf, H.; Balss, J.; Hartmann, C.; von Deimling, A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009, 118, 599–601. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Du, X.; Hu, H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021, 9, 651317. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Noushmehr, H.; Weisenberger, D.J.; Diefes, K.; Phillips, H.S.; Pujara, K.; Berman, B.P.; Pan, F.; Pelloski, C.E.; Sulman, E.P.; Bhat, K.P.; et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010, 17, 510–522. [Google Scholar] [CrossRef]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.-M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.H.; Li, X.S.; Woon, E.C.Y.; Yang, M.; et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Ozair, A.; Bhat, V.; Alisch, R.S.; Khosla, A.A.; Kotecha, R.R.; Odia, Y.; McDermott, M.W.; Ahluwalia, M.S. DNA Methylation and Histone Modification in Low-Grade Gliomas: Current Understanding and Potential Clinical Targets. Cancers 2023, 15, 1342. [Google Scholar] [CrossRef]
- Ye, D.; Guan, K.-L.; Xiong, Y. Metabolism, Activity, and Targeting of D- and L-2-Hydroxyglutarates. Trends Cancer 2018, 4, 151–165. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010, 465, 966. [Google Scholar] [CrossRef]
- Zhang, L.; Sorensen, M.D.; Kristensen, B.W.; Reifenberger, G.; McIntyre, T.M.; Lin, F. D-2-Hydroxyglutarate Is an Intercellular Mediator in IDH-Mutant Gliomas Inhibiting Complement and T Cells. Clin. Cancer Res. 2018, 24, 5381–5391. [Google Scholar] [CrossRef]
- Modrek, A.S.; Golub, D.; Khan, T.; Bready, D.; Prado, J.; Bowman, C.; Deng, J.; Zhang, G.; Rocha, P.P.; Raviram, R.; et al. Low-Grade Astrocytoma Mutations in IDH1, P53, and ATRX Cooperate to Block Differentiation of Human Neural Stem Cells via Repression of SOX2. Cell Rep. 2017, 21, 1267–1280. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Kannan, K.; Inagaki, A.; Silber, J.; Gorovets, D.; Zhang, J.; Kastenhuber, E.R.; Heguy, A.; Petrini, J.H.; Chan, T.A.; Huse, J.T. Whole exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget 2012, 3, 1194–1203. [Google Scholar] [CrossRef]
- Appay, R.; Dehais, C.; Maurage, C.-A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncology 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Ming, Z.; Lim, S.Y.; Rizos, H. Genetic Alterations in the INK4a/ARF Locus: Effects on Melanoma Development and Progression. Biomolecules 2020, 10, 1447. [Google Scholar] [CrossRef]
- Malumbres, M.; Carnero, A. Cell cycle deregulation: A common motif in cancer. Prog. Cell Cycle Res. 2003, 5, 5–18. [Google Scholar]
- Ichimura, K.; Bolin, M.B.; Goike, H.M.; Schmidt, E.E.; Moshref, A.; Collins, V.P. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res. 2000, 60, 417–424. [Google Scholar]
- Xia, Y.; Liu, Y.; Yang, C.; Simeone, D.M.; Sun, T.-T.; DeGraff, D.J.; Tang, M.-S.; Zhang, Y.; Wu, X.-R. Dominant role of CDKN2B/p15INK4B of 9p21.3 tumor suppressor hub in inhibition of cell-cycle and glycolysis. Nat. Commun. 2021, 12, 2047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xi, Y.-F.; Zhao, Q.; Guo, J.-H.; Zhang, Z.; Zhang, M.-B.; Chang, J.; Wu, Y.-Q.; Su, W. CDKN2A promoter methylation enhances self-renewal of glioblastoma stem cells and confers resistance to carmustine. Mol. Biol. Rep. 2024, 51, 385. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.E.; Ichimura, K.; Messerle, K.R.; Goike, H.M.; Collins, V.P. Infrequent methylation of CDKN2A(MTS1/p16) and rare mutation of both CDKN2A and CDKN2B(MTS2/p15) in primary astrocytic tumours. Br. J. Cancer 1997, 75, 2–8. [Google Scholar] [CrossRef]
- Andersson, N.; Saba, K.H.; Magnusson, L.; Nilsson, J.; Karlsson, J.; Nord, K.H.; Gisselsson, D. Inactivation of RB1, CDKN2A, and TP53 have distinct effects on genomic stability at side-by-side comparison in karyotypically normal cells. Genes. Chromo-somes Cancer 2023, 62, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Bent, M.V.D.; Vogelbaum, M.A.; Wick, W.; Miller, C.R.; Taphoorn, M.; Pope, W.; Brown, P.D.; Platten, M.; Jalali, R.; et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro-Oncology 2019, 21, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Tans, J.; de Jongh, I. Computed tomography of supratentorial astrocytoma. Clin. Neurol. Neurosurg. 1978, 80, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Nakazawa, S. Analysis of peritumoral edema--with special reference to the value of contrast-enhanced CT scan and dynamic CT scan. No Shinkei 1984, 36, 1055–1062. [Google Scholar]
- Zhao, K.; Sun, G.; Wang, Q.; Xue, Z.; Liu, G.; Xia, Y.; Yao, A.; Zhao, Y.; You, N.; Yang, C.; et al. The Diagnostic Value of Conventional MRI and CT Features in the Identification of the IDH1-Mutant and 1p/19q Co-Deletion in WHO Grade II Gliomas. Acad. Radiol. 2021, 28, e189–e198. [Google Scholar] [CrossRef]
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2–FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-grade Gliomas: A TCGA/TCIA Project. Clin. Cancer Res. 2017, 23, 6078–6085. [Google Scholar] [CrossRef]
- Mohammed, S.; Ravikumar, V.; Warner, E.; Patel, S.; Bakas, S.; Rao, A.; Jain, R. Quantifying T2-FLAIR Mismatch Using Geographically Weighted Regression and Predicting Molecular Status in Lower-Grade Gliomas. Am. J. Neuroradiol. 2022, 43, 33–39. [Google Scholar] [CrossRef]
- van Garderen, K.A.; Vallentgoed, W.R.; Lavrova, A.; Niers, J.M.; de Leng, W.W.; Hoogstrate, Y.; de Heer, I.; Ylstra, B.; van Dijk, E.; Klein, S.; et al. Longitudinal char-acteristics of T2-FLAIR mismatch in IDH-mutant astrocytomas: Relation to grade, histopathology, and overall survival in the GLASS-NL cohort. Neuro-Oncol. Adv. 2023, 5, vdad149. [Google Scholar] [CrossRef]
- Juratli, T.A.; Tummala, S.S.; Riedl, A.; Daubner, D.; Hennig, S.; Penson, T.; Zolal, A.; Thiede, C.; Schackert, G.; Krex, D.; et al. Radiographic assessment of contrast en-hancement and T2/FLAIR mismatch sign in lower grade gliomas: Correlation with molecular groups. J. Neuro-Oncol. 2019, 141, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Doig, D.; Kachramanoglou, C.; Dumba, M.; Tona, F.; Gontsarova, A.; Limbäck, C.; Jan, W. Characterisation of isocitrate de-hydrogenase gene mutant WHO grade 2 and 3 gliomas: MRI predictors of 1p/19q co-deletion and tumour grade. Clin. Radiol. 2021, 785.e9, 785.e16. [Google Scholar] [CrossRef] [PubMed]
- Lasocki, A.; Gaillard, F. Non-Contrast-Enhancing Tumor: A New Frontier in Glioblastoma Research. Am. J. Neuroradiol. 2019, 40, 758–765. [Google Scholar] [CrossRef]
- Bent, M.J.v.D.; Smits, M.; Kros, J.M.; Chang, S.M. Diffuse Infiltrating Oligodendroglioma and Astrocytoma. J. Clin. Oncol. 2017, 35, 2394–2401. [Google Scholar] [CrossRef]
- Smits, M. Imaging of oligodendroglioma. Br. J. Radiol. 2016, 89, 20150857. [Google Scholar] [CrossRef]
- Chen, R.; Nishimura, M.C.; Kharbanda, S.; Peale, F.; Deng, Y.; Daemen, A.; Forrest, W.F.; Kwong, M.; Hedehus, M.; Hatzivassiliou, G.; et al. Hominoid-specific enzyme GLUD2 promotes growth of IDH1 R132H glioma. Proc. Natl. Acad. Sci. USA 2014, 111, 14217–14222. [Google Scholar] [CrossRef] [PubMed]
- Ghisai, S.A.; van Hijfte, L.; Vallentgoed, W.R.; Tesileanu, C.M.S.; de Heer, I.; Kros, J.M.; Sanson, M.; Gorlia, T.; Wick, W.; Vogelbaum, M.A.; et al. Epigenetic landscape reorgan-ization and reactivation of embryonic development genes are associated with malignancy in IDH-mutant astrocytoma. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lai, A.; Kharbanda, S.; Pope, W.B.; Tran, A.; Solis, O.E.; Peale, F.; Forrest, W.F.; Pujara, K.; Carrillo, J.A.; Pandita, A.; et al. Evidence for Sequenced Molecular Evolution of IDH1 Mutant Glioblastoma From a Distinct Cell of Origin. J. Clin. Oncol. 2011, 29, 4482–4490. [Google Scholar] [CrossRef]
- Wesseling, P.; Bent, M.v.D.; Perry, A. Oligodendroglioma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 809–827. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Deng, L.; Bai, H.X.; Sun, J.; Neale, N.; Wu, J.; Wang, Y.; Chang, K.; Huang, R.Y.; Zhang, P.J.; et al. Reduced expression of DNA repair genes and chemosensitivity in 1p19q codeleted lower-grade gliomas. J. Neuro-Oncol. 2018, 139, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, J.G.; Wang, M.; Jenkins, R.B.; Shaw, E.G.; Giannini, C.; Brachman, D.G.; Buckner, J.C.; Fink, K.L.; Souhami, L.; Laperriere, N.J.; et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014, 32, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Agrawal, N.; Jiao, Y.; Sausen, M.; Wood, L.D.; Hruban, R.H.; Rodriguez, F.J.; Cahill, D.P.; McLendon, R.; Riggins, G.; et al. Mutations in CIC and FUBP1 Contribute to Human Oligodendroglioma. Science 2011, 333, 1453–1455. [Google Scholar] [CrossRef]
- Halani, S.H.; Yousefi, S.; Vega, J.V.; Rossi, M.R.; Zhao, Z.; Amrollahi, F.; Holder, C.A.; Baxter-Stoltzfus, A.; Eschbacher, J.; Griffith, B.; et al. Multi-faceted computational assessment of risk and progression in oligodendroglioma implicates NOTCH and PI3K pathways. NPJ Precis. Oncol. 2018, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kilic, O.; Deo, M.; Jimenez-Cowell, K.; Demirdizen, E.; Kim, H.; Turcan, S. CIC reduces xCT/SLC7A11 expression and glutamate release in glioma. Acta Neuropathol. Commun. 2023, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Brandner, S.; Bertero, L.; Capper, D.; French, P.J.; Figarella-Branger, D.; Giangaspero, F.; Haberler, C.; Hegi, M.E.; Kristensen, B.W.; et al. Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline. Neuro-Oncology 2023, 25, 1731–1749. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Khalid, L.; Carone, M.; Dumrongpisutikul, N.; Intrapiromkul, J.; Bonekamp, D.; Barker, P.B.; Yousem, D.M. Imaging Char-acteristics of Oligodendrogliomas That Predict Grade. Am. J. Neuroradiol. 2012, 33, 852–857. [Google Scholar] [CrossRef]
- Kumar, M.; Nanga, R.P.R.; Verma, G.; Wilson, N.; Brisset, J.C.; Nath, K.; Chawla, S. Emerging MR Imaging and Spectroscopic Methods to Study Brain Tumor Metabolism. Front. Neurol. 2022, 13, 789355. [Google Scholar] [CrossRef]
- de Godoy, L.L.; Lim, K.C.; Rajan, A.; Verma, G.; Hanaoka, M.; O’rourke, D.M.; Lee, J.Y.K.; Desai, A.; Chawla, S.; Mohan, S. Non-Invasive Assessment of Isocitrate Dehydrogenase-Mutant Gliomas Using Optimized Proton Magnetic Resonance Spectroscopy on a Routine Clinical 3-Tesla MRI. Cancers 2023, 15, 4453. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.J.; Brown, M.S. Quantitative analysis in magnetic resonance spectroscopy: From metabolic profiling to in vivo biomarkers. Bioanalysis 2012, 4, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclas-sification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Rohle, D.; Versele, M.; Iwanami, A.; Kuga, D.; Oldrini, B.; Tanaka, K.; Dang, J.; Kubek, S.; Palaskas, N.; et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc. Natl. Acad. Sci. USA 2010, 107, 6459–6464. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Desai, S.M.; Cooper, A.N.; Steinbach, M.A.; Gosselin, K.; Wanebo, J.E. The clinical progression of patients with glioblastoma. Interdiscip. Neurosurg. 2023, 32, 101756. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849. [Google Scholar] [CrossRef]
- Carrillo, J.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.M.; et al. Relationship between Tumor En-hancement, Edema, IDH1 Mutational Status, MGMT Promoter Methylation, and Survival in Glioblastoma. Am. J. Neuroradiol. 2012, 33, 1349–1355. [Google Scholar] [CrossRef]
- Fan, H.; Yu, Y.; Du, J.; Liu, L.; Luo, Y.; Yu, H.; Liao, X. Computed Tomography, Magnetic Resonance Imaging, and Pathological Features of Gliosarcoma. Neuropsychiatr. Dis. Treat. 2022, 18, 2577–2589. [Google Scholar] [CrossRef] [PubMed]
- Melhem, J.M.; Detsky, J.; Lim-Fat, M.J.; Perry, J.R. Updates in IDH-Wildtype Glioblastoma. Neurotherapeutics 2022, 19, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Drumm, M.R.; Dixit, K.S.; Grimm, S.; Kumthekar, P.; Lukas, R.V.; Raizer, J.J.; Stupp, R.; Chheda, M.G.; Kam, K.-L.; McCord, M.; et al. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro-Oncology 2020, 22, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Matute-González, M.; Mosteiro-Cadaval, A.; Vidal-Robau, N.; Páez-Carpio, A.; Valduvieco, I.; Pineda, E.; González, J.J.; Aldecoa, I.; Oleaga, L. Clinico-pathological and Neuroimaging Features of Primary Gliosarcoma: A Case Series and Review of Literature. World Neurosurg. 2023, 178, e480–e488. [Google Scholar] [CrossRef]
- Peckham, M.E.; Osborn, A.G.; Palmer, C.A.; Tsai, A.; Salzman, K.L. Gliosarcoma: Neuroimaging and Immunohistochemical Findings. J. Neuroimaging 2019, 29, 126–132. [Google Scholar] [CrossRef]
- Weis, S.; Sonnberger, M.; Dunzinger, A.; Voglmayr, E.; Aichholzer, M.; Kleiser, R.; Strasser, P. Gliosarcoma WHO Grade IV-Giant Cell Glioblastoma WHO Grade IV. In Imaging Brain Diseases; Springer: Vienna, Austria, 2019. [Google Scholar]
- Zipp, L.; Schwartz, K.M.; Hewer, E.; Yu, Y.; Stippich, C.; Slopis, J.M. Magnetic Resonance Imaging and Computed Tomography Findings in Pediatric Giant Cell Glioblastoma. Clin. Neuroradiol. 2012, 22, 359–363. [Google Scholar] [CrossRef][Green Version]
- Giese, A.; Bjerkvig, R.; Berens, M.; Westphal, M. Cost of Migration: Invasion of Malignant Gliomas and Implications for Treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.O.; et al. ESTRO-ACROP guideline “target de-lineation of glioblastomas”. Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.P.; Xu, L.W.; Lober, R.M.; Li, G.; Nagpal, S. The incidence and significance of multiple lesions in glioblastoma. J. Neuro-Oncol. 2013, 112, 91–97. [Google Scholar] [CrossRef]
- Farhat, M.; Fuller, G.N.; Wintermark, M.; Chung, C.; Kumar, V.A.; Chen, M. Multifocal and multicentric glioblastoma: Imaging signature, molecular characterization, patterns of spread, and treatment. Neuroradiol. J. 2023. [Google Scholar] [CrossRef]
- Paulsson, A.K.; Holmes, J.A.; Peiffer, A.M.; Miller, L.D.; Liu, W.; Xu, J.; Hinson, W.H.; Lesser, G.J.; Laxton, A.W.; Tatter, S.B.; et al. Comparison of clinical outcomes and genomic characteristics of single focus and multifocal glioblastoma. J. Neuro-Oncol. 2014, 119, 429–435. [Google Scholar] [CrossRef]
- Korshunov, A.; Ryzhova, M.; Hovestadt, V.; Bender, S.; Sturm, D.; Capper, D.; Meyer, J.; Schrimpf, D.; Kool, M.; Northcott, P.A.; et al. Integrated analysis of pediatric glio-blastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015, 129, 669–678. [Google Scholar] [CrossRef]
- Meyronet, D.; Esteban-Mader, M.; Bonnet, C.; Joly, M.-O.; Uro-Coste, E.; Amiel-Benouaich, A.; Forest, F.; Rousselot-Denis, C.; Burel-Vandenbos, F.; Bourg, V.; et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro-Oncology 2017, 19, 1127–1134. [Google Scholar] [CrossRef]
- Damodharan, S.; Lara-Velazquez, M.; Williamsen, B.C.; Helgager, J.; Dey, M. Diffuse Intrinsic Pontine Glioma: Molecular Landscape, Evolving Treatment Strategies and Emerging Clinical Trials. J. Pers. Med. 2022, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; van Zanten, S.E.V.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Steinbok, P.; Gopalakrishnan, C.V.; Hengel, A.R.; Vitali, A.M.; Poskitt, K.; Hawkins, C.; Drake, J.; Lamberti-Pasculli, M.; Ajani, O.; Hader, W.; et al. Pediatric thalamic tumors in the MRI era: A Canadian perspective. Child's Nerv. Syst. 2015, 32, 269–280. [Google Scholar] [CrossRef]
- Cao, L.; Li, C.; Zhang, Y.; Gui, S. Surgical resection of unilateral thalamic tumors in adults: Approaches and outcomes. BMC Neurol. 2015, 15, 229. [Google Scholar] [CrossRef]
- Lowe, B.R.; Maxham, L.A.; Hamey, J.J.; Wilkins, M.R.; Partridge, J.F. Histone H3 Mutations: An Updated View of Their Role in Chromatin Deregulation and Cancer. Cancers 2019, 11, 660. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Erratum: Corrigendum: Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Kfoury-Beaumont, N.; Prakasam, R.; Pondugula, S.; Lagas, J.S.; Matkovich, S.; Gontarz, P.; Yang, L.; Yano, H.; Kim, A.H.; Rubin, J.B.; et al. The H3K27M mutation alters stem cell growth, epigenetic regulation, and differentiation potential. BMC Biol. 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.S.; Krug, B.; Chen, H.; Papillon-Cavanagh, S.; Zeinieh, M.; De Jay, N.; Deshmukh, S.; Chen, C.C.; Belle, J.; Mikael, L.G.; et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 2019, 10, 1262. [Google Scholar] [CrossRef]
- Lewis, P.W.; Müller, M.M.; Koletsky, M.S.; Cordero, F.; Lin, S.; Banaszynski, L.A.; Garcia, B.A.; Muir, T.W.; Becher, O.J.; Allis, C.D. Inhibition of PRC2 Activity by a Gain-of-Function H3 Mutation Found in Pediatric Glioblastoma. Science 2013, 340, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.; Monje, M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 2019, 25, 367–376. [Google Scholar] [CrossRef]
- Auffret, L.; Ajlil, Y.; Tauziède-Espariat, A.; Kergrohen, T.; Puiseux, C.; Riffaud, L.; Blouin, P.; Bertozzi, A.-I.; Leblond, P.; Blomgren, K.; et al. A new subtype of diffuse midline glioma, H3 K27 and BRAF/FGFR1 co-altered: A clinico-radiological and histomolecular characterisation. Acta Neuropathol. 2023, 147, 2. [Google Scholar] [CrossRef]
- Castel, D.; Philippe, C.; Calmon, R.; Le Dret, L.; Truffaux, N.; Boddaert, N.; Pagès, M.; Taylor, K.R.; Saulnier, P.; Lacroix, L.; et al. Histone H3F3A and HIST1H3B K27M mu-tations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015, 130, 815–827. [Google Scholar] [CrossRef]
- Gianno, F.; Giovannoni, I.; Cafferata, B.; Diomedi-Camassei, F.; Minasi, S.; Barresi, S.; Buttarelli, F.R.; Alesi, V.; Cardoni, A.; Antonelli, M.; et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica 2022, 114, 422–435. [Google Scholar] [CrossRef]
- Elsässer, S.J.; Allis, C.D.; Lewis, P.W. New Epigenetic Drivers of Cancers. Science 2011, 331, 1145–1146. [Google Scholar] [CrossRef]
- Castel, D.; Kergrohen, T.; Tauziede-Espariat, A.; Mackay, A.; Ghermaoui, S.; Lechapt, E.; Pfister, S.M.; Kramm, C.M.; Boddaert, N.; Blauwblomme, T.; et al. Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3-K27M mutation. Acta Neuropathol. 2020, 139, 1109–1113. [Google Scholar] [CrossRef]
- Vallero, S.G.; Bertero, L.; Morana, G.; Sciortino, P.; Bertin, D.; Mussano, A.; Ricci, F.S.; Peretta, P.; Fagioli, F. Pediatric diffuse midline glioma H3K27- altered: A complex clinical and biological landscape behind a neatly defined tumor type. Front. Oncol. 2022, 12, 1082062. [Google Scholar] [CrossRef]
- Mondal, G.; Lee, J.C.; Ravindranathan, A.; Villanueva-Meyer, J.E.; Tran, Q.T.; Allen, S.J.; Barreto, J.; Gupta, R.; Doo, P.; Van Ziffle, J.; et al. Pediatric bithalamic gliomas have a distinct epigenetic signature and frequent EGFR exon 20 insertions resulting in potential sensitivity to targeted kinase inhibition. Acta Neuropathol. 2020, 139, 1071–1088. [Google Scholar] [CrossRef]
- Aboian, M.; Solomon, D.; Felton, E.; Mabray, M.; Villanueva-Meyer, J.; Mueller, S.; Cha, S. Imaging Characteristics of Pediatric Diffuse Midline Gliomas with Histone H3 K27M Mutation. Am. J. Neuroradiol. 2017, 38, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef]
- Korshunov, A.; Capper, D.; Reuss, D.; Schrimpf, D.; Ryzhova, M.; Hovestadt, V.; Sturm, D.; Meyer, J.; Jones, C.; Zheludkova, O.; et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 2016, 131, 137–146. [Google Scholar] [CrossRef]
- Kurokawa, R.; Baba, A.; Kurokawa, M.; Pinarbasi, E.S.; Makise, N.; Ota, Y.; Kim, J.; Srinivasan, A.; Moritani, T. Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: A case series and systematic review. J. Neuroimaging 2022, 32, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soto, J.M.; Gonzalez, S.-M.; Murillo, J.; Trumble, E.R.; Shan, F.Y.; Huang, J.H. H3G34-Mutant Gliomas—A Review of Molecular Pathogenesis and Therapeutic Options. Biomedicines 2023, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Vettermann, F.J.; Felsberg, J.; Reifenberger, G.; Hasselblatt, M.; Forbrig, R.; Berding, G.; la Fougère, C.; Galldiks, N.; Schittenhelm, J.; Weis, J.; et al. Characterization of Diffuse Gliomas With Histone H3-G34 Mutation by MRI and Dynamic 18F-FET PET. Clin. Nucl. Med. 2018, 43, 895–898. [Google Scholar] [CrossRef]
- Korshunov, A.; Schrimpf, D.; Ryzhova, M.; Sturm, D.; Chavez, L.; Hovestadt, V.; Sharma, T.; Habel, A.; Burford, A.; Jones, C.; et al. H3-/IDH-wild type pediatric glio-blastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017, 134, 507–516. [Google Scholar] [CrossRef]
- Tauziède-Espariat, A.; Debily, M.-A.; Castel, D.; Grill, J.; Puget, S.; Sabel, M.; Blomgren, K.; Gareton, A.; Dangouloff-Ros, V.; Lechapt, E.; et al. An integrative radiological, histopathological and molecular analysis of pediatric pontine histone-wildtype glioma with MYCN amplification (HGG-MYCN). Acta Neuropathol. Commun. 2019, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.-J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-Oncology 2017, 19, 153–161. [Google Scholar] [CrossRef] [PubMed]
- López, G.Y.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Clarke, J.; Bush, N.A.O.; Taylor, J.; Chang, S.; et al. The genetic landscape of gliomas arising after therapeutic radiation. Acta Neuropathol. 2019, 137, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Trkova, K.; Sumerauer, D.; Bubenikova, A.; Krskova, L.; Vicha, A.; Koblizek, M.; Zamecnik, J.; Jurasek, B.; Kyncl, M.; Malinova, B.; et al. Clinical and molecular study of radiation-induced gliomas. Sci. Rep. 2024, 14, 3118. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.; Perez, E.; Chirica, M.; Onken, J.; Kahn, J.; Brenner, W.; Ehret, F.; Euskirchen, P.; Koch, A.; Capper, D.; et al. High-grade astrocytoma with piloid features (HGAP): The Charité experience with a new central nervous system tumor entity. J. Neuro-Oncol. 2021, 153, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Stichel, D.; Schrimpf, D.; Sahm, F.; Korshunov, A.; Reuss, D.E.; Koelsche, C.; Huang, K.; Wefers, A.K.; Hovestadt, V.; et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018, 136, 273–291. [Google Scholar] [CrossRef]
- Soni, N.; Agarwal, A.; Ajmera, P.; Mehta, P.; Gupta, V.; Vibhute, M.; Gubbiotti, M.; Mark, I.T.; Messina, S.A.; Mohan, S.; et al. High-Grade Astrocytoma with Piloid Features: A Dual Institutional Review of Imaging Findings of a Novel Entity. Am. J. Neuroradiol. 2024, 45, 468–474. [Google Scholar] [CrossRef]
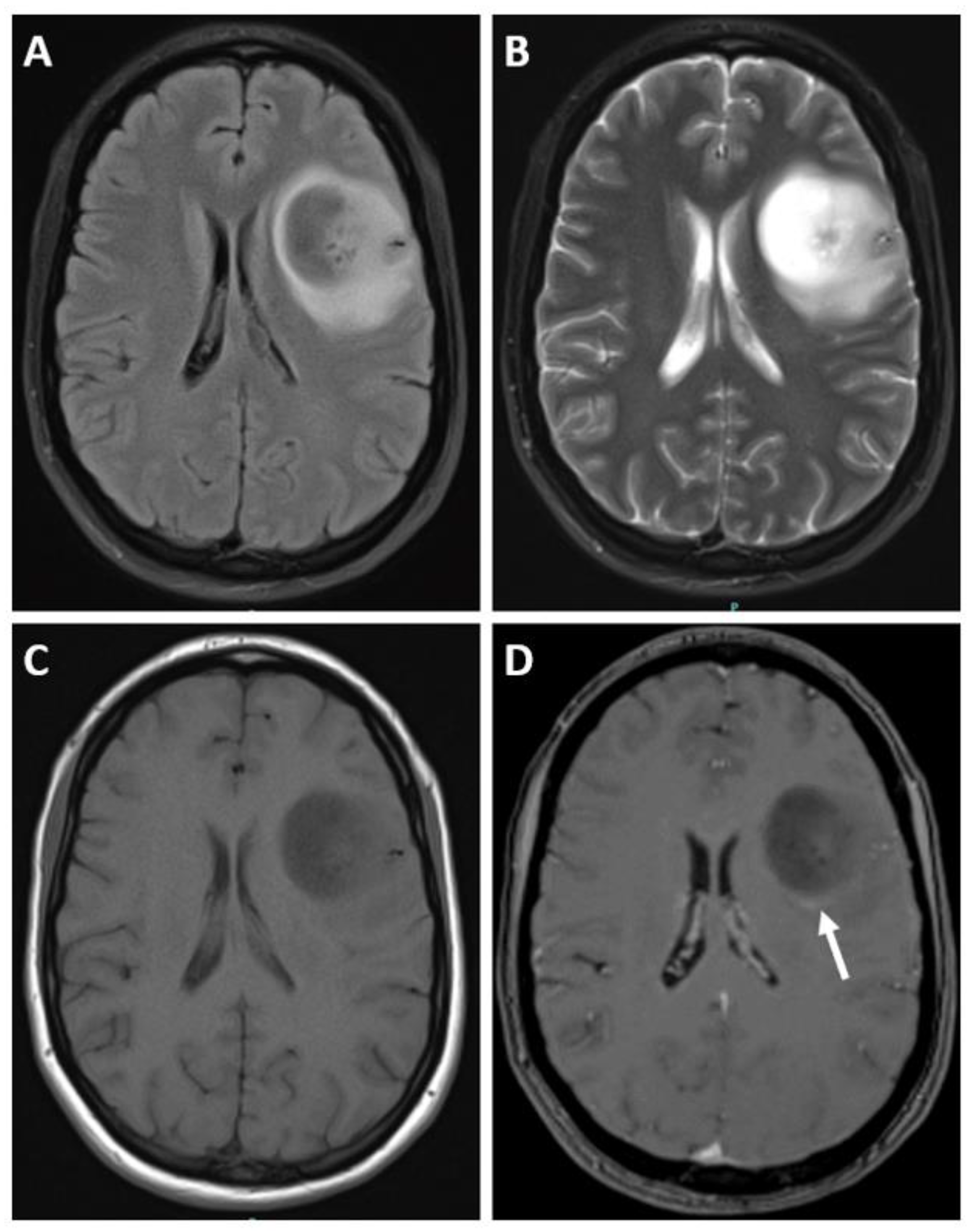
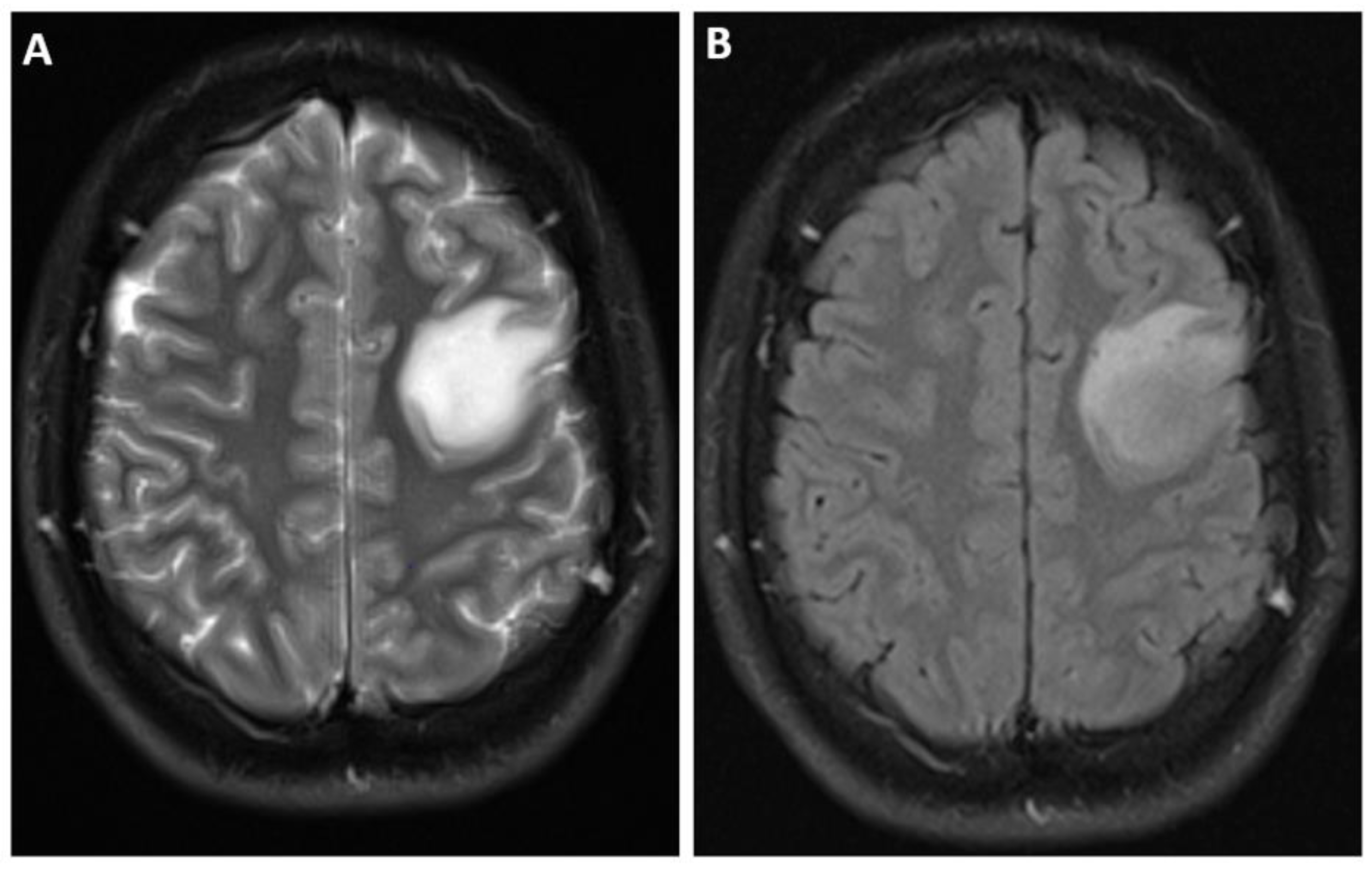
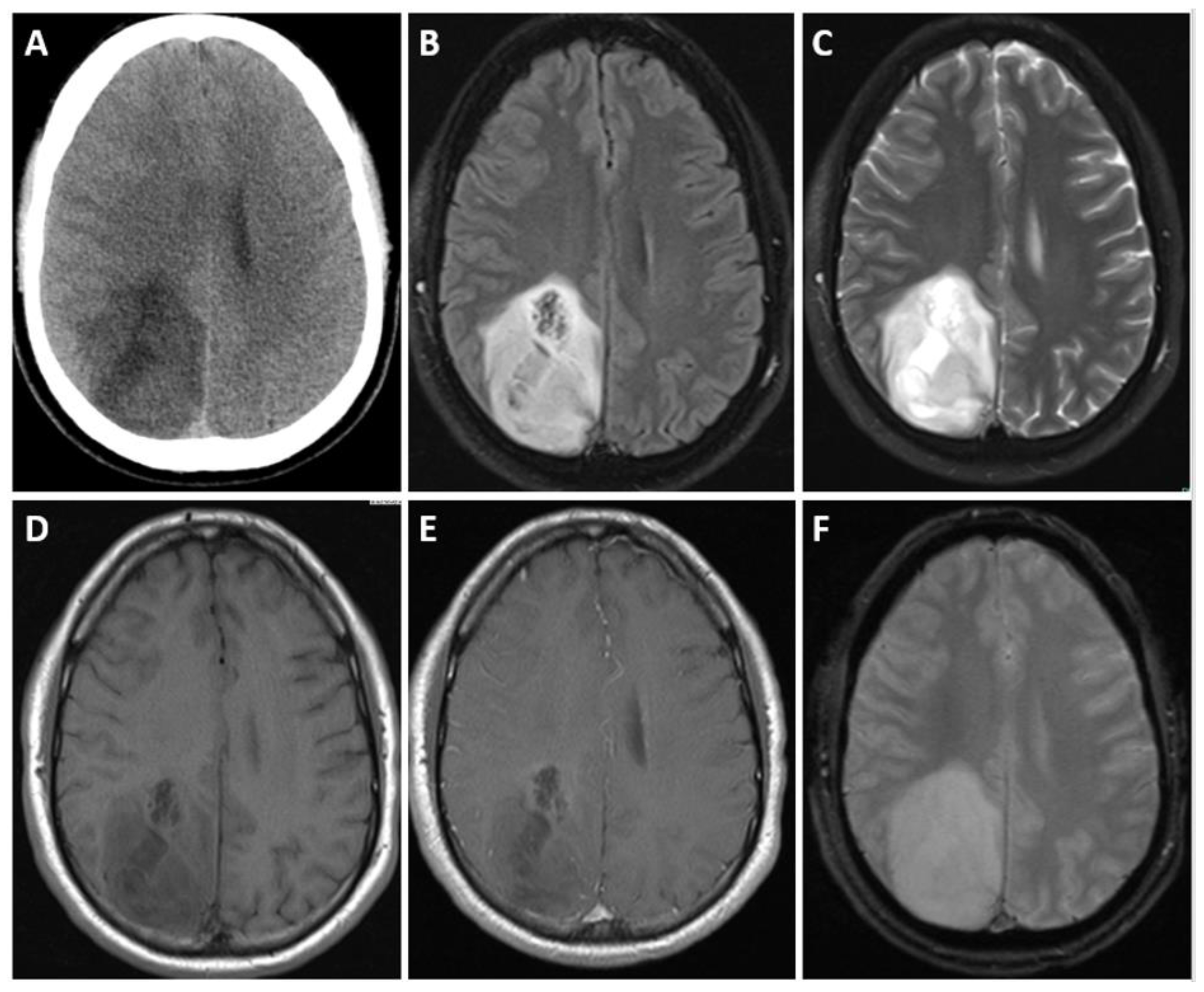
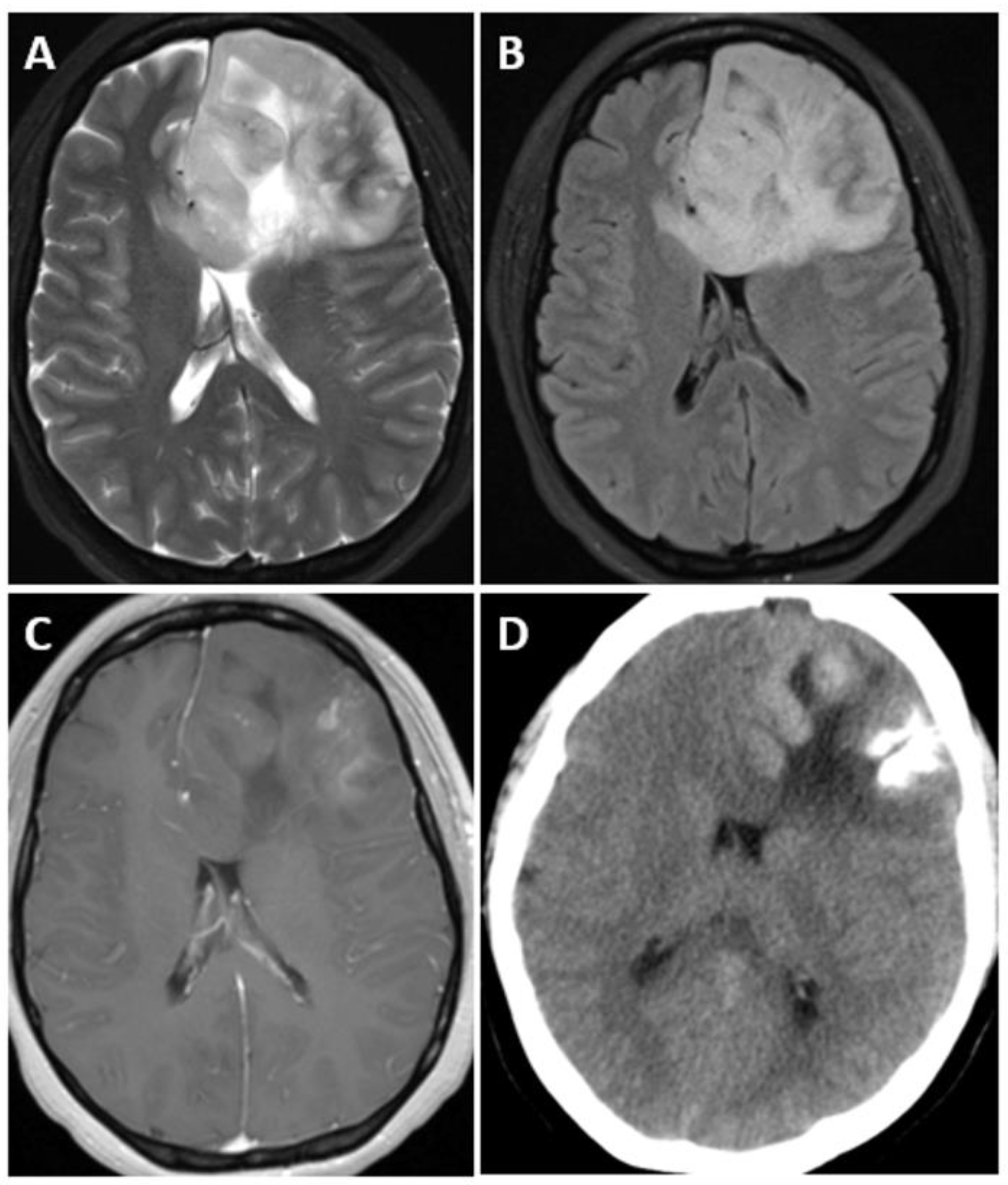
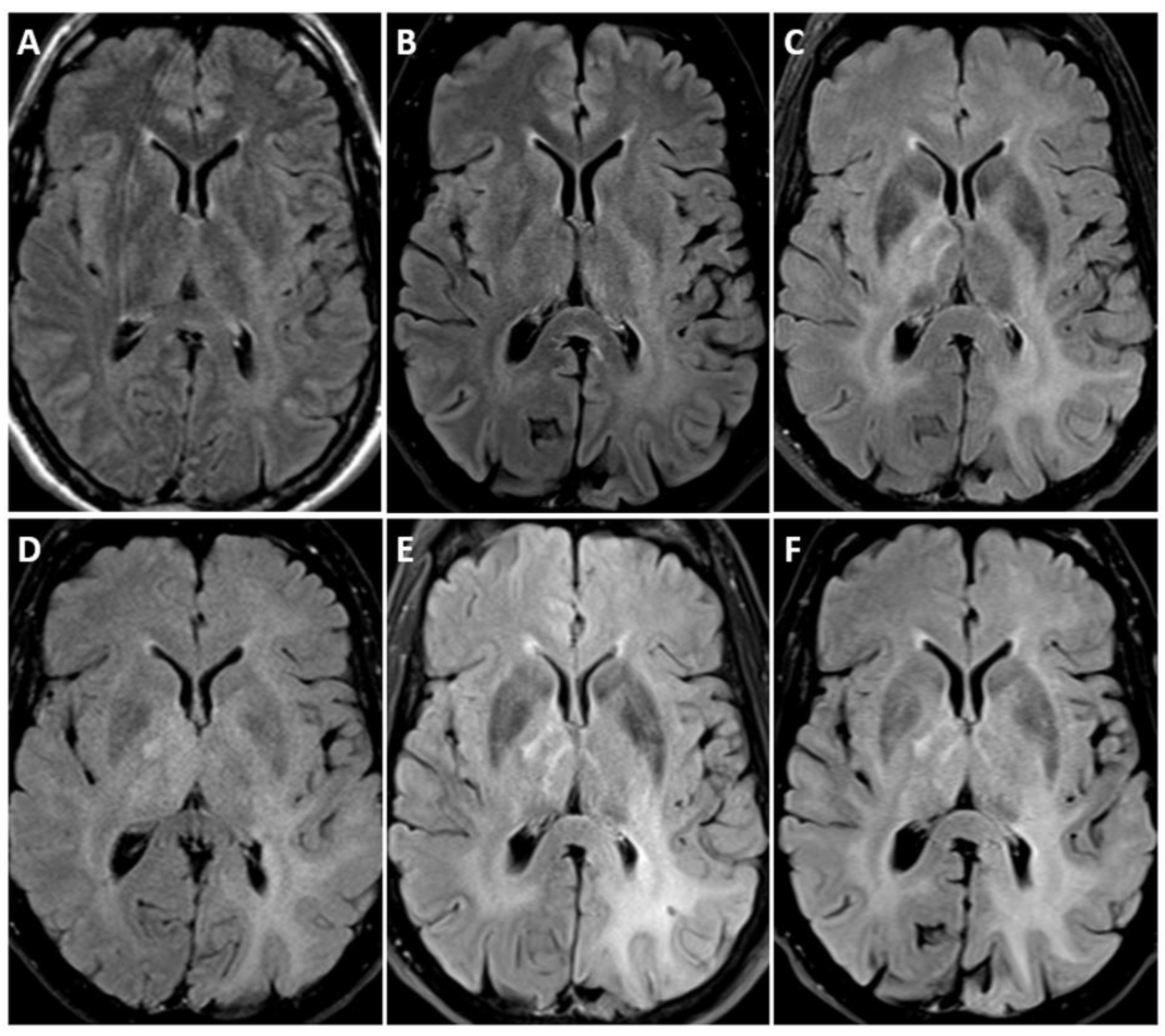
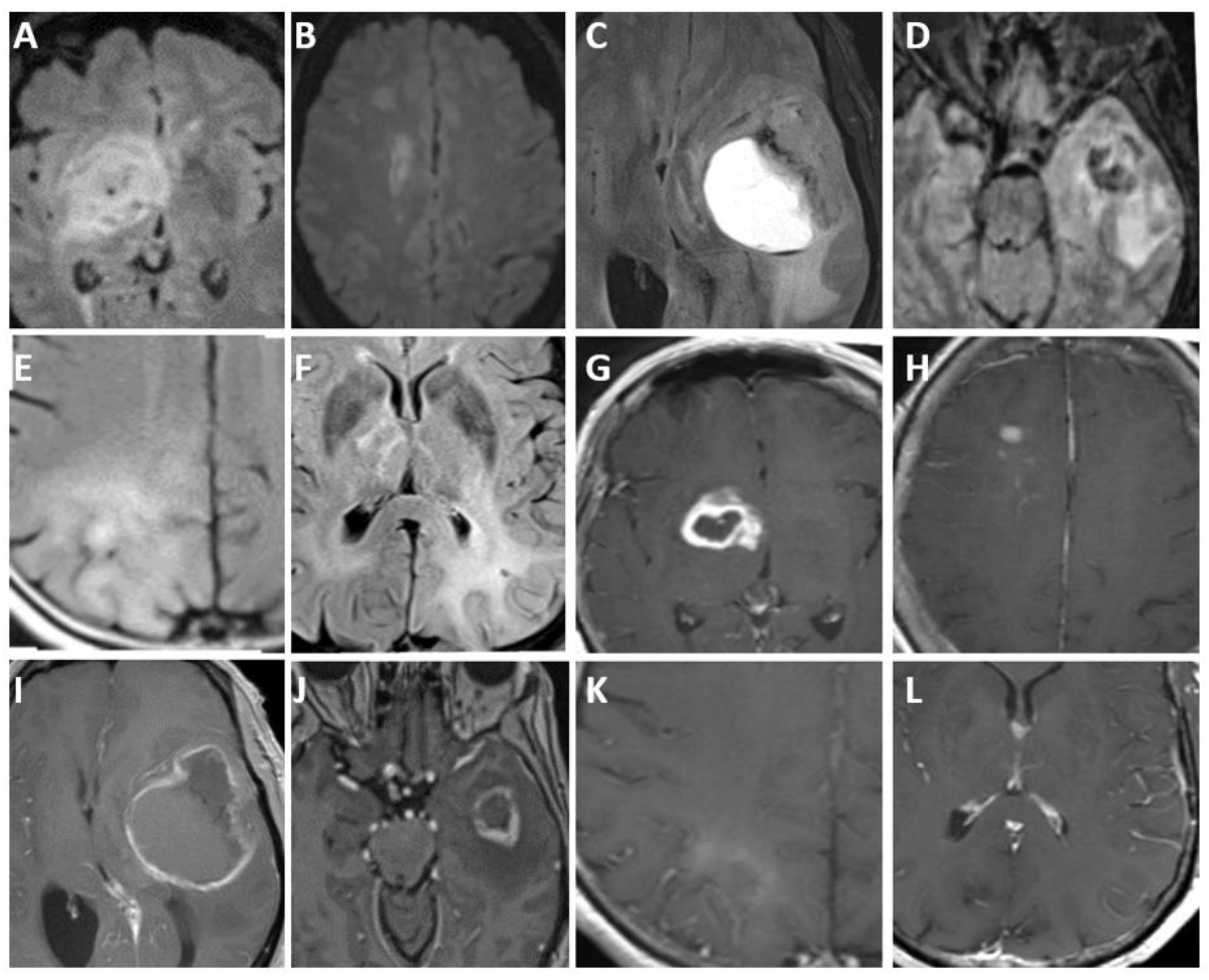
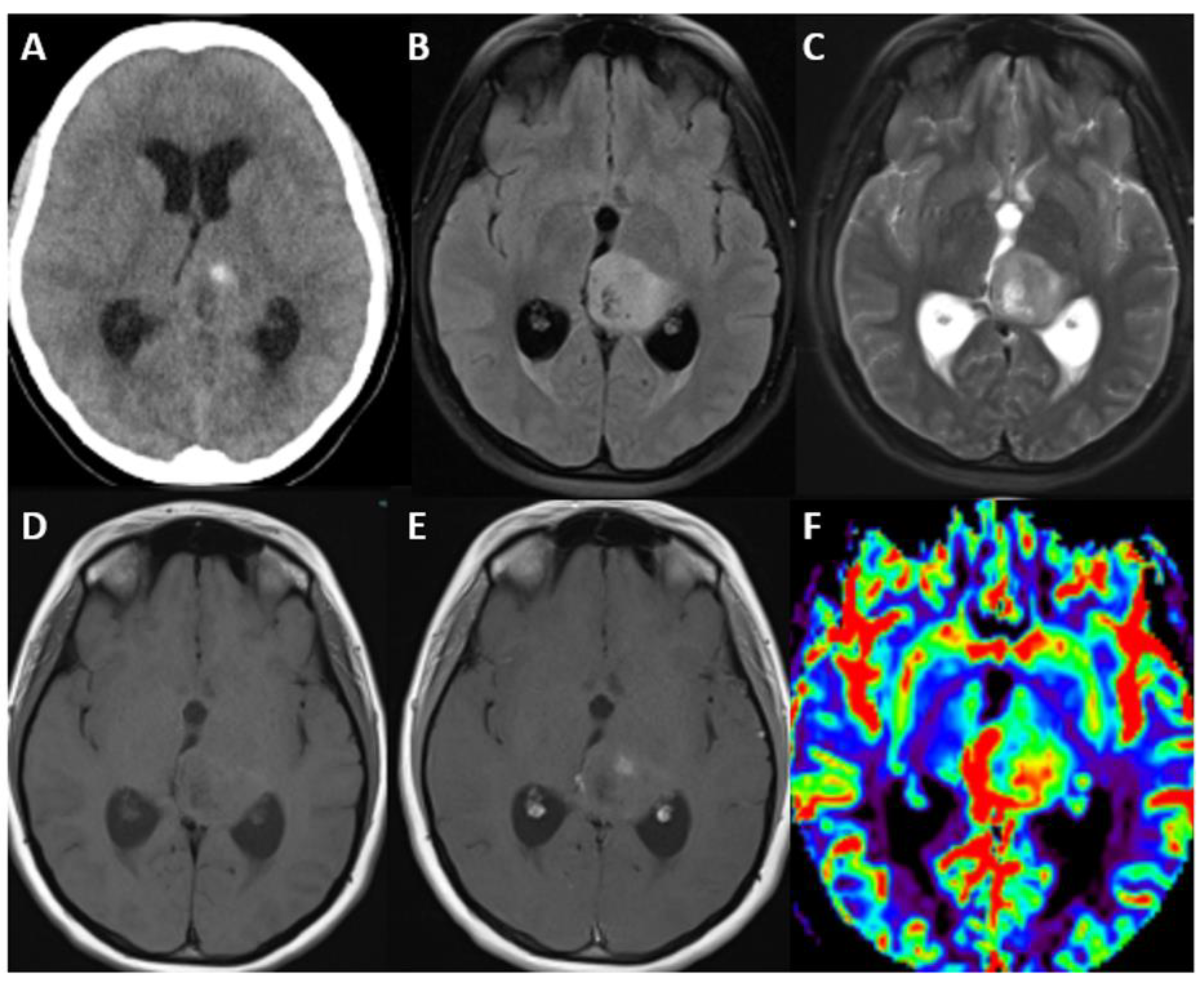
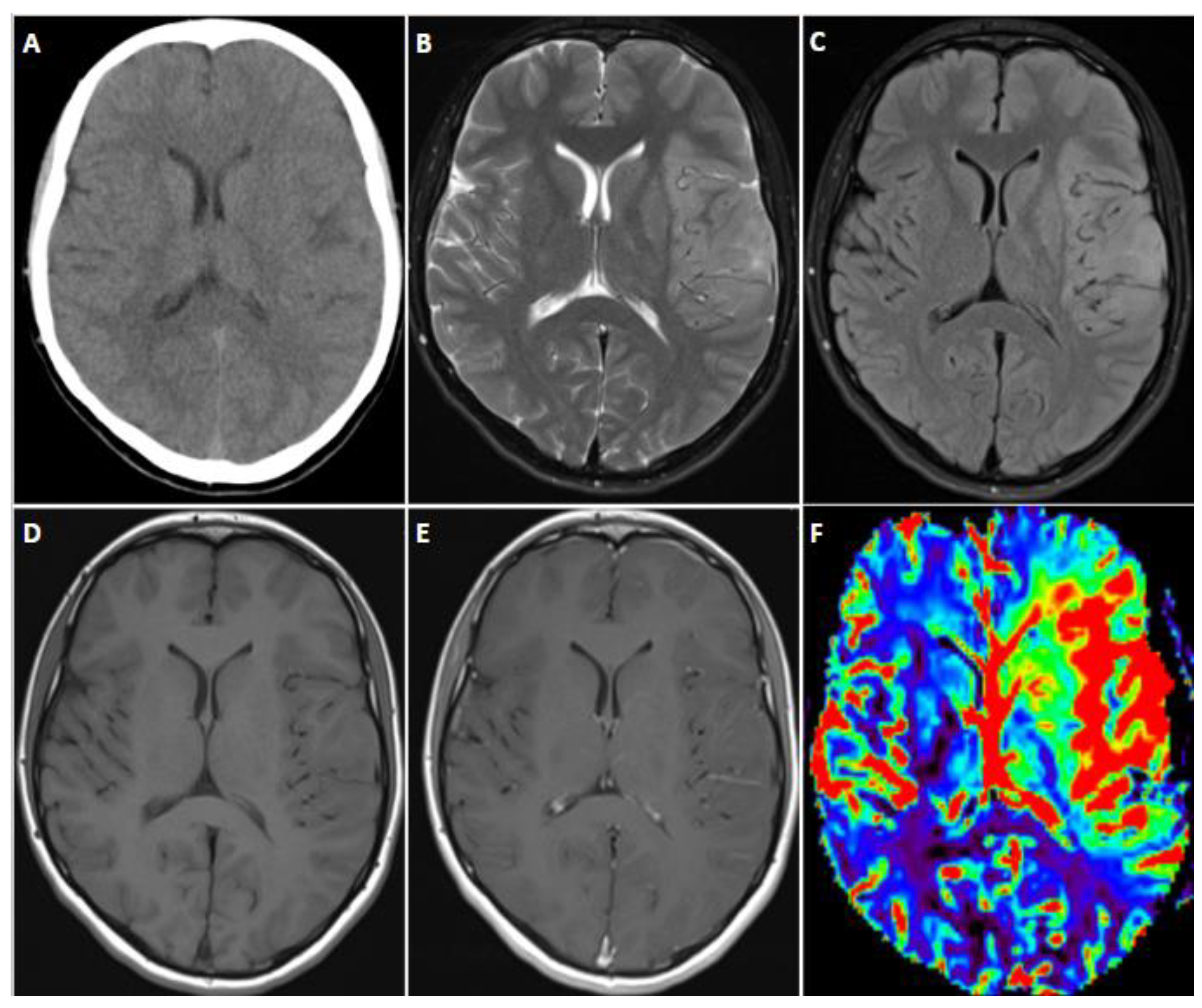
| Adult-Type Diffuse Glioma | Some Common Genetic/Molecular Alterations | Interval Update | Typical Imaging Characteristics |
|---|---|---|---|
| Astrocytoma, IDH-mutant | IDH1, IDH2, ATRX, TP53 | CDKN2A/B homozygous deletion designated grade 4, no IDH-wildtype | Homogenous, circumscribed, T2-FLAIR mismatch sign |
| Oligodendroglioma, IDH-mutant and 1p/19q-codeleted | IDH1, IDH2, 1p/19q, TERT Promotor, CIC, FUBP1 | CDKN2A/B homozygous deletion indicates a CNS WHO grade 3 | Heterogeneity, calcification, variable enhancement, possible increased rCBV |
| Glioblastoma, IDH-wildtype | IDH-wildtype, TERT promotor, +7/−10 chromosome copy number changes, EGFR | Only IDH-wildtype no IDH-mutant, | Inherent heterogeneity, heterogeneously enhancing, sometimes ring- enhancing around central necrosis, surrounding T2/FLAIR signal, edema and mass effect, increased rCBV, surrounding infiltration. |
| IDH-wildtype astrocytic tumors with 1 of following: Microvascular proliferation or necrosis, TERT promotor mutation, or EGFR gene amplification, +7/−10 chromosome copy number changes. |
| Pediatric-Type Diffuse High-grade Gliomas | Some Common Alterations | Interval Update | Imaging Characteristics |
|---|---|---|---|
| Diffuse midline glioma, H3 K27-altered | H3 K27, TP53, ACVR, PDGFRA, EGFR, EZHIP | Name changed from H3K27-mutant, | Variable, ill-defined, infiltrative masses, in brainstem, thalamus, or spinal cord, frequently no enhancement, H3.1 subgroup-necrosis and edema likely. |
| Subgroups recognized | |||
| Diffuse hemispheric glioma, H3 G34-mutant | H3 G34, TP53, ATRX | Newly recognized | Unifocal, cortex, ependymal and/or leptomeningeal contact. |
| Diffuse pediatric-type high-grade glioma, H3-wildtype, and IDH-wildtype | No IDH or H3 mutations, PDGFRA, MYCN, EGFR | Newly recognized | Heterogeneous, akin to other high-grade gliomas. |
| Infant-type hemispheric glioma (not discussed in this review) | NRTK family, ALK, ROS, MET | Newly recognized | Large masses with frequent superficial involvement including leptomeninges. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gue, R.; Lakhani, D.A. The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas. Biomedicines 2024, 12, 1349. https://doi.org/10.3390/biomedicines12061349
Gue R, Lakhani DA. The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas. Biomedicines. 2024; 12(6):1349. https://doi.org/10.3390/biomedicines12061349
Chicago/Turabian StyleGue, Racine, and Dhairya A. Lakhani. 2024. "The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas" Biomedicines 12, no. 6: 1349. https://doi.org/10.3390/biomedicines12061349
APA StyleGue, R., & Lakhani, D. A. (2024). The 2021 World Health Organization Central Nervous System Tumor Classification: The Spectrum of Diffuse Gliomas. Biomedicines, 12(6), 1349. https://doi.org/10.3390/biomedicines12061349






