COVID-19: Unveiling the Neuropsychiatric Maze—From Acute to Long-Term Manifestations
Abstract
1. Introduction
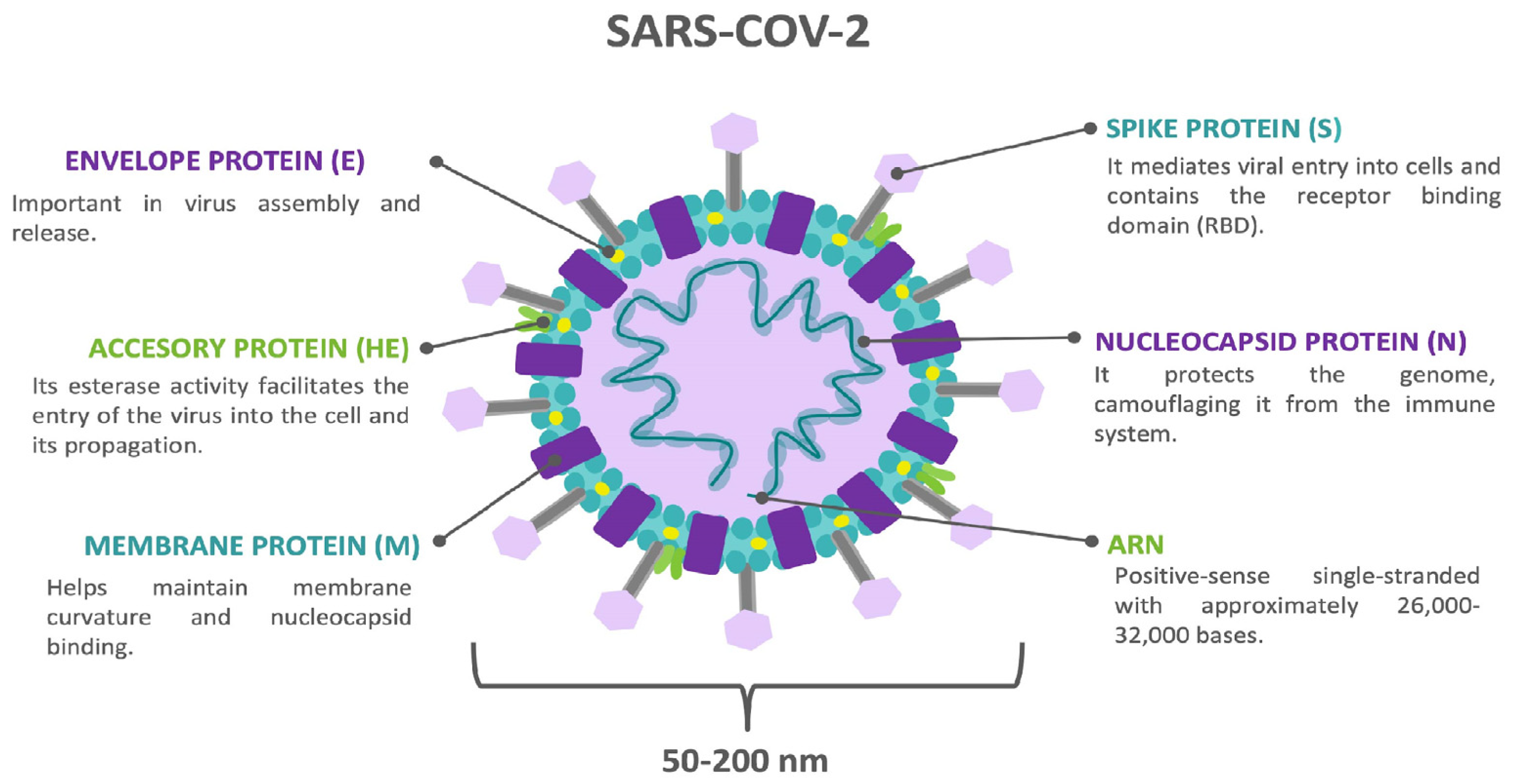
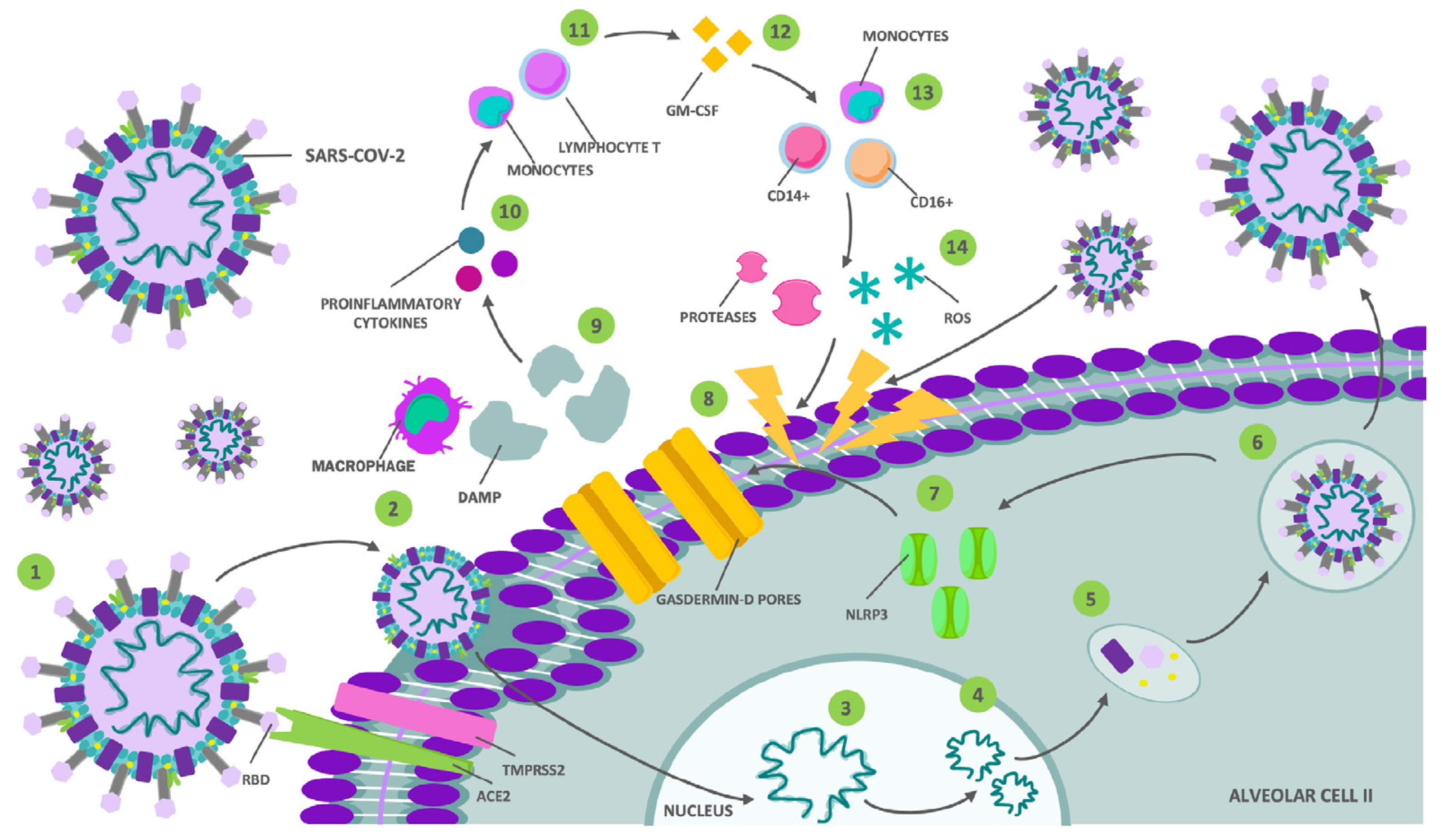
2. Central Nervous System (CNS) Invasion and Damage Mechanisms by COVID-19
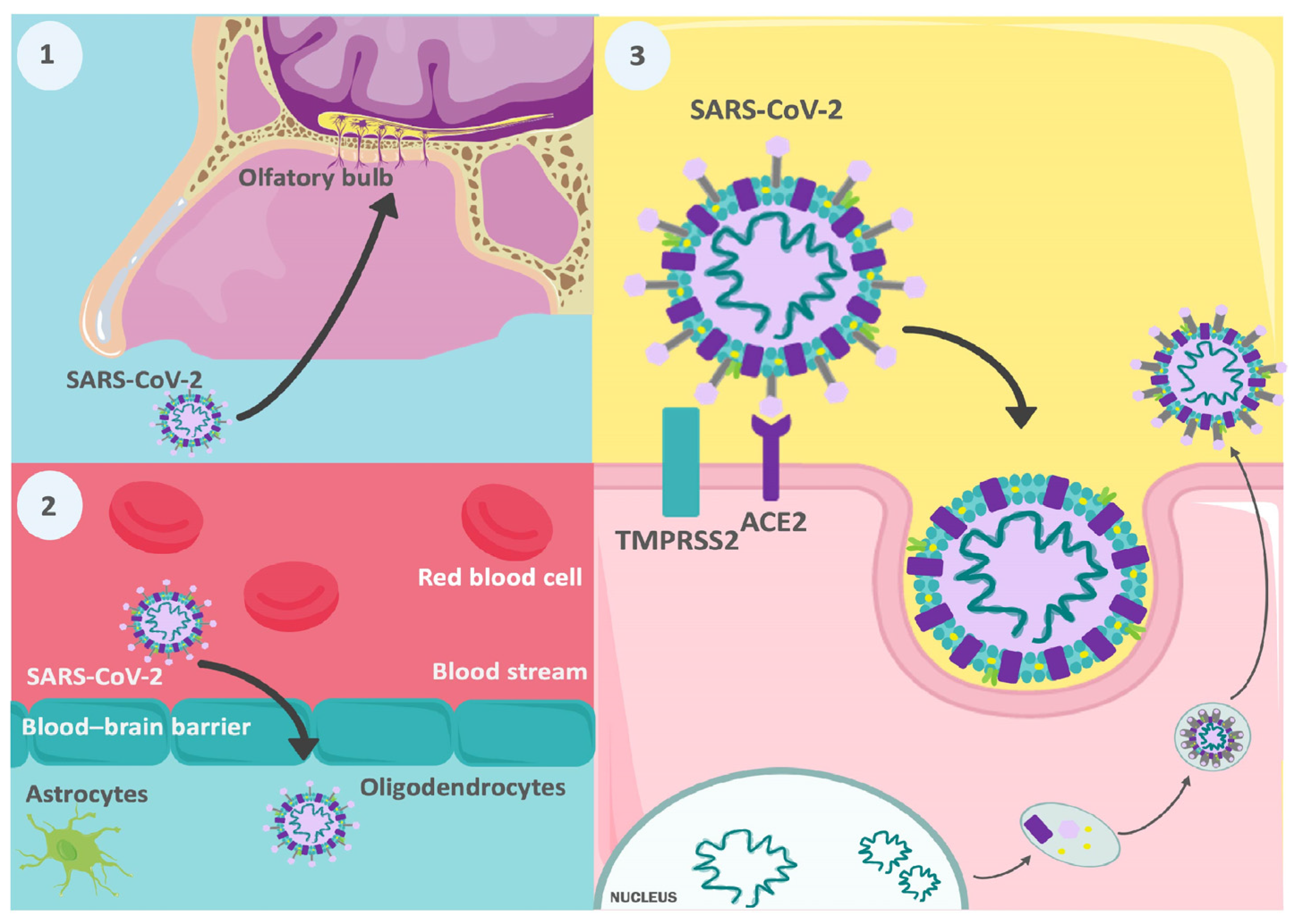
2.1. Direct Invasion by the SARS-CoV-2 Virus
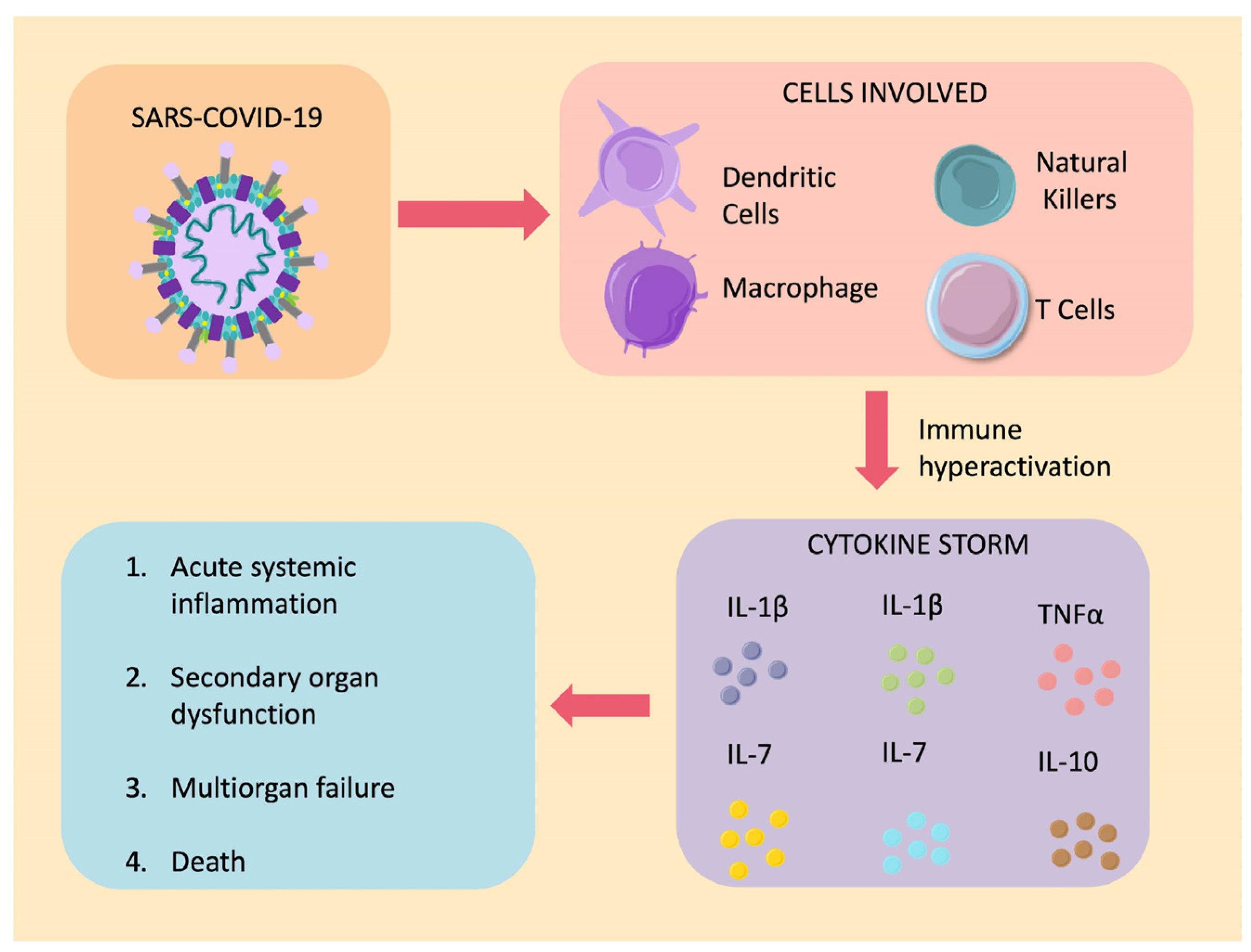
2.2. Aberrant Activation of the Immune System
3. Neurological Manifestations and Complications
3.1. Central Nervous System
3.1.1. Encephalitis
3.1.2. Acute Hemorrhagic Necrotizing Encephalopathy
3.1.3. Stroke
3.1.4. Seizures
3.1.5. Delirium and Other Neuropsychiatric Manifestations
3.1.6. Cephalea
3.1.7. Demyelinating Diseases of the Central Nervous System
3.1.8. Acute Myelitis
3.2. Peripheral Nervous System
3.2.1. Olfactory and Gustatory Disorders
3.2.2. Guillain–Barre Syndrome
4. Psychiatric Manifestations
4.1. Anxiety
4.2. Depression
4.3. Post-Traumatic Stress Disorder
4.4. Psychosis
4.5. Cognitive Impairments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. COVID-19 Deaths|WHO COVID-19; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Karim, S.S.A.; Wilkins, D.; Brechot, G.-D.A.C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J.Ž. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nature reviews. Microbiology 2023, 21, 133–146. [Google Scholar] [PubMed]
- Al-Husinat, L.; Nusir, M.; Al-Gharaibeh, H.; Alomari, A.A.; Smadi, M.M.; Battaglini, D.; Pelosi, P. Post-COVID-19 syndrome symptoms after mild and moderate SARS-CoV-2 infection. Front. Med. 2022, 9, 1017257. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Lopez-Echeverri, M.C.; Perez-Raga, M.F.; Quintero-Romero, V.; Valencia-Gallego, V.; Galindo-Herrera, N.; López-Alzate, S.; Sánchez-Vinasco, J.D.; Gutiérrez-Vargas, J.J.; Mayta-Tristan, P.; et al. The global challenges of the long COVID-19 in adults and children. Travel. Med. Infect. Dis. 2023, 54, 102606. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Ziebuh, J. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Kim, J.M.; Chung, Y.S.; Jo, H.J.; Lee, N.J.; Kim, M.S.; Woo, S.H.; Park, S.; Kim, J.W.; Kim, H.M.; Han, M.G. Identification of Coronavirus Isolated from a Patient in Korea with COVID-19. Osong Public Health Res. Perspect. 2020, 11, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Gandet, F.F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Kim, I.C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.S.; Wang, X.; Niu, Y.R.; Ye, L.L.; Peng, W.B.; Wang, Z.H.; Yang, W.B.; Yang, B.H.; Zhang, J.C.; Ma, W.L.; et al. Diarrhea Is Associated With Prolonged Symptoms and Viral Carriage in Corona Virus Disease 2019. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 1753–1759. [Google Scholar] [CrossRef]
- Rossi, G.M.; Delsante, M.; Pilato, F.P.; Gnetti, L.; Gabrielli, L.; Rossini, G.; Re, M.C.; Cenacchi, G.; Affanni, P.; Colucci, M.E.; et al. Kidney Biopsy Findings in a Critically Ill COVID-19 Patient with Dialysis-Dependent Acute Kidney Injury: A Case against "SARS-CoV-2 Nephropathy. Kidney Int. Rep. 2020, 5, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 74, 807–816. [Google Scholar] [CrossRef]
- Rong-Hui, D.; Li-Rong, L.; Cheng-Qing, Y.; Wen, W.; Tan-Ze, C.; Ming, L.; Guang-Yun, G.; Juan, D.; Chun-Lan, Z.; Qi, Z.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Horwitz, L.I. Factors associated with hospitalization and critical illness among 4103 patients with COVID-19 disease in New York City. MedRxiv 2020. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Zhao, R.L.J.; Hu, Y.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA Neurol. 2020, 323, 2089–2090. [Google Scholar] [CrossRef] [PubMed]
- Lechien, R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Berger, J.R. COVID-19 and the nervous system. J. Neurovirol. 2020, 26, 143–148. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Hu, B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.W.; Dieiev, V.; Kang, J.; Barbi, M.; Nia, S.F.M.; Gabr, M.; Eman, G.; Kajita, G.; Swedish, K. Rhabdomyolysis in COVID-19 Patients: A Retrospective Observational Study. Cureus 2021, 13, e12552. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Easton, S.M.A.; Breen, G.; Zandi, M.; Coles, J.P.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.O.; Ahmad, S.A.; Hassan, M.N.; Kakamad, F.H.; Salih, R.Q.; Abdulla, B.A.; Salih, A.M. Post COVID-19 neurological complications; a meta-analysis. Ann. Med. Surg. 2022, 76, 103440. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e928996. [Google Scholar] [CrossRef] [PubMed]
- Mullaguri, N.; Sivakumar, S.; Battineni, A.; Anand, S.; Vanderwerf, J. COVID-19 Related Acute Hemorrhagic Necrotizing Encephalitis: A Report of Two Cases and Literature Review. Cureus 2021, 13, e14236. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Hernández, J.P.; Marin-Medina, D.S.; Sánchez-Duque, J.A. Neurological manifestations of SARS-CoV-2 infection. Semergen 2020, 46 (Suppl. S1), 106–108. [Google Scholar] [CrossRef] [PubMed]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jaunmuktane, Z.; Mahadeva, U.; Green, A.; Sekhawat, V.; Barrett, N.A.; Childs, L.; Shankar-Hari, M.; Thom, M.; Jäger, H.R.; Brandner, S. Microvascular injury and hypoxic damage: Emerging neuropathological signatures in COVID-19. Acta Neuropathol. 2020, 140, 397–400. [Google Scholar] [CrossRef]
- von Weyhern, C.H.; Kaufmann, I.; Neff, F.; Kremer, M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020, 395, e109. [Google Scholar] [CrossRef]
- Coolen, T.; Lolli, V.; Sadeghi, N.; Rovai, A.; Trotta, N.; Taccone, F.S.; Creteur, J.; Henrard, S.; Goffard, J.C.; Dewitte, O.; et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology 2020, 95, e2016–e2027. [Google Scholar] [CrossRef] [PubMed]
- Kanberg, N.; Ashton, N.J.; Andersson, L.M.; Yilmaz, A.; Lindh, M.; Nilsson, S.; Price, R.W.; Blennow, K.; Zetterberg, H.; Gisslén, M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020, 95, e1754–e1759. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Hanif, M.; Ali, M.J.; Haider, M.A.; Kherani, D.; Memon, G.M.; Sattar, A. Neurological manifestations of COVID-19 (SARS-CoV-2): A review. Front. Neurol. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.A.; Collins, A.; Cohen, M.E.; Duffner, P.K.; Faden, H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004, 113, e73–e76. [Google Scholar] [PubMed]
- Dubé, M.; Le Coupanec, A.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol. 2018, 92, e00404-18. [Google Scholar] [CrossRef]
- Chan, J.F.; Chan, K.H.; Choi, G.K.; To, K.K.; Tse, H.; Cai, J.P.; Yeung, M.L.; Cheng, V.C.; Chen, H.; Che, X.Y.; et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: Implications for disease pathogenesis and clinical manifestation. J. Infect. Dis. 2013, 207, 1743–1752. [Google Scholar] [CrossRef]
- Pamies, D.; Barreras, P.; Block, K.; Makri, G.; Kumar, A.; Wiersma, D.; Smirnova, L.; Zang, C.; Bressler, J.; Christian, K.M.; et al. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 2017, 34, 362–376. [Google Scholar] [CrossRef]
- Bullen, C.K.; Hogberg, H.T.; Bahadirli-Talbott, A.; Bishai, W.R.; Hartung, T.; Keuthan, C.; Looney, M.M.; Pekosz, A.; Romero, J.C.; Sillé, F.C.M.; et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX 2020, 37, 665–671. [Google Scholar]
- Mesci, P.; de Souza, J.S.; Martin-Sancho, L.; Macia, A.; Saleh, A.; Yin, X.; Snethlage, C.; Adams, J.W.; Avansini, S.H.; Herai, R.H.; et al. SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol. 2022, 20, e3001845. [Google Scholar] [CrossRef]
- Hou, Y.; Li, C.; Yoon, C.; Leung, O.W.; You, S.; Cui, X.; Chan, J.F.; Pei, D.; Cheung, H.H.; Chu, H. Enhanced replication of SARS-CoV-2 Omicron BA.2 in human forebrain and midbrain organoids. Signal Transduct. Target. Ther. 2022, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhou, Y.; Hua, J.; Zhang, L.; Bian, J.; Liu, B.; Zhao, Z.; Jin, S. The scRNA-seq Expression Profiling of the Receptor ACE2 and the Cellular Protease TMPRSS2 Reveals Human Organs Susceptible to SARS-CoV-2 Infection. Int. J. Environ. Res. Public Health 2021, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Antinone, S.E.; Smith, G.A. Retrograde axon transport of herpes simplex virus and pseudorabies virus: A live-cell comparative analysis. J. Virol. 2010, 84, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- MacGibeny, M.A.; Koyuncu, O.O.; Wirblich, C.; Schnell, M.J.; Enquist, L.W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLoS Pathog. 2018, 14, e1007188. [Google Scholar] [CrossRef]
- van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.S.; Salsano, E.; Grimaldi, M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020, 77, 1028–1029. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- Nampoothiri, S.; Sauve, F.; Ternier, G.; Fernandois, D.; Coelho, C.; Imbernon, M.; Prevot, V. The hypothalamus as a hub for putative SARS-CoV-2 brain infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Proust, A.; Queval, C.J.; Harvey, R.; Adams, L.; Bennett, M.; Wilkinson, R.J. Differential effects of SARS-CoV-2 variants on central nervous system cells and blood-brain barrier functions. J. Neuroinflamm. 2023, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E.; et al. In-Silico Evidence for a Two Receptor Based Strategy of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 690655. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 2020, 177, 104759. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Lo, Y.; Chapagain, M.; Lum, S.; Kumar, M.; Gurjav, U.; Luo, H.; Nakatsuka, A.; Nerurkar, V.R. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology 2009, 385, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Tabor-Godwin, J.M.; Ruller, C.M.; Bagalso, N.; An, N.; Pagarigan, R.R.; Harkins, S.; Gilbert, P.E.; Kiosses, W.B.; Gude, N.A.; Cornell, C.T.; et al. A novel population of myeloid cells responding to coxsackievirus infection assists in the dissemination of virus within the neonatal CNS. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8676–8691. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, V.; Pasetto, L.; Lisi, I.; Carbonara, M.; Zangari, R.; Ferrari, E.; Punzi, V.; Luotti, S.; Bottino, N.; Biagianti, B.; et al. Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Front. Immunol. 2022, 13, 1070379. [Google Scholar] [CrossRef]
- Patil, S.; Patil, S.; Gondhali, G.; Toshniwal, S. Immune Dysregulation during and after COVID-19: “Tomorrow Never Dies” Situation. J. Transl. Crit. Care Med. 1793, 5, e00024. [Google Scholar] [CrossRef]
- Wright, H.; Alex, P.; Nguyen, T.; Bader, T.; Gurakar, A.; Sebastian, A.; Gonzales, L.; Wallis, G.; Naylor, M.; Dozmorov, I.; et al. Multiplex cytokine profiling of initial therapeutic response in patients with chronic hepatitis C virus infection. Dig. Dis. Sci. 2005, 50, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Ruan, O.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.L.; NLRP, T. COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Mader, S.; Valdés-Ferrer, S.I. Systemic inflammation and the brain: Novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, C.M. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e781. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, C.M. Pathogenesis of immune-mediated neuropathies. Biochim. Biophys. Acta 2015, 185, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Burr, T.; Barton, C.; Doll, E.; Lakhotia, A.; Sweeney, M. N-Methyl-d-Aspartate Receptor Encephalitis Associated with COVID-19 Infection in a Toddler. Pediatr. Neurol. 2021, 114, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Durovic, E.; Bien, C.; Bien, C.G.; Isenmann, S. MOG antibody-associated encephalitis secondary to COVID-19: Case report. BMC Neurol. 2021, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.; Vakrakou, A.G.; Anastasiou, A.; Sourdi, N.G.A.; Tzartos, J.S.; Kilidireas, C.; Dimitrakopoulos, A. Cerebrospinal Fluid Anti-Neuronal Autoantibodies in COVID-19-Associated Limbic Encephalitis with Acute Cerebellar Ataxia and Myoclonus Syndrome: Case Report and Literature Review. Diagnostics 2023, 13, 2055. [Google Scholar] [CrossRef]
- Flannery, P.; Yang, I.; Keyvani, M.; Sakoulas, G. Acute Psychosis Due to Anti-N-Methyl D-Aspartate Receptor Encephalitis Following COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 764197. [Google Scholar] [CrossRef]
- Abdelhady, M.; Husain, M.A.; Hawas, Y.; Elazb, M.A.; Mansour, L.S.; Mohamed, M.; Abdelwahab, M.M.; Aljabali, A.; Negida, A. Encephalitis following COVID-19 Vaccination: A Systematic Review. Vaccines 2023, 11, 576. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.D.; Rato, M.L.; Cruz, D.; Valadas, A.; Antunes, A.P.; Albuquerque, L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J. Neuroimmunol. 2021, 361, 577739. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Stoian, M.; Bajko, Z.; Maier, S.; Andone, S.; Cioflinc, R.A.; Motataianu, A.; Barcutean, L.; Balasa, R. Autoimmune Encephalitis in COVID-19 Infection: Our Experience and Systematic Review of the Literature. Biomedicines 2022, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.W.K.; Bassi, G.L.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis Underlying Neurological Manifestations of Long COVID Syndrome and Potential Therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 7265–7269. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.G.; Wilczynski, S.; Wenceslau, C.F.; Webb, R.C. A new storm on the horizon in COVID-19: Bradykinin-induced vascular complications. Vasc. Pharmacol. 2021, 137, 106826. [Google Scholar] [CrossRef] [PubMed]
- Sidarta-Oliveira, D.; Jara, C.P.; Ferruzzi, A.J.; Skaf, M.S.; Velander, W.H.; Araujo, E.P.; Velloso, L.A. SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cell. Sci. Rep. 2020, 10, 19522. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Iqbal, M.S.; Sultan, S.; Alhuthali, R.A.; Alshubaili, D.I.; Sayyam, R.S.; Abyad, L.M.; Qasem, A.H.; Arbaeen, A.F. Dysregulated Bradykinin: Mystery in the Pathogenesis of COVID-19. Mediat. Inflamm. 2022, 2022, 7423537. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head. Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Odolini, S.; Masciocchi, S.; Comelli, A.; Volonghi, I.; Gazzina, S.; Nocivelli, S.; Pezzini, A.; Focà, E.; Caruso, A.; et al. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. Ann. Neurol. 2020, 88, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Beyrouti, R.; Adams, M.E.; Benjamin, L.; Cohen, H.; Farmer, S.F.; Goh, Y.Y.; Humphries, F.; Jäger, H.R.; Losseff, N.A.; Perry, R.J.; et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 2020, 91, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Razavi, A.S.; Rouhani, N. Frequent Convulsive Seizures in an Adult Patient with COVID-19: A Case Report. Iran. Red. Crescent Med. J. 2020, 22, e102828. [Google Scholar] [CrossRef]
- Caamaño, D.S.J.; Beato, R.A. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 77, 230–232. [Google Scholar]
- Arabi, Y.M.; Harthi, A.; Hussein, J.; Bouchama, A.; Johani, S.; Hajeer, A.H.; Saeed, B.T.; Wahbi, A.; Saedy, A.; AlDabbagh, T.; et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 2015, 43, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Fan, R.; Wen, B.; Zhang, J.; Cao, X.; Wang, C.; Song, Z.; Li, S.; Li, X.; et al. Coronavirus Infections in the Central Nervous System and Respiratory Tract Show Distinct Features in Hospitalized Children. Intervirology 2016, 59, 163–169. [Google Scholar] [CrossRef]
- Siow, I.; Lee, K.S.; Zhang, J.J.Y.; Saffari, S.E.; Ng, A. Encephalitis as a neurological complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes, and predictors. Eur. J. Neurol. 2021, 28, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; He, Z.C.; Yao, X.H.; Tang, R.; Ma, J.; Luo, T.; Zhu, C.; Li, T.R.; Liu, X.; Zhang, D.; et al. COVID-19-associated monocytic encephalitis (CAME): Histological and proteomic evidence from autopsy. Signal Transduct. Target. Ther. 2023, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, F.; Molaee, H.; Nejad, J.H. COVID-19 vaccines and neurological complications: A systematic review. Zeitschrift fur Naturforschung. J. Biosci. 2022, 78, 1–8. [Google Scholar]
- Zhou, L.; Zhang, M.; Wang, J.; Gao, J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med. Infect. Dis. 2020, 36, 101642. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Valnet, R.; Pizzarotti, B.; Anichini, A.; Demars, Y.; Russo, E.; Schmidhauser, M.; Cerutti-Sola, J.; Rossetti, A.O.; Pasquier, R.D. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur. J. Neurol. 2020, 27, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology 2020, 296, E119–E120. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, N.; El Halabi, T.; Melhem, J.; Dib, G.; Ghosn, Y.; Hourani, M.; Nasreddine, W.; Beydoun, A. COVID-19-Associated Acute Asymmetric Hemorrhagic Necrotizing Encephalopathy: A Case Report. Neurohospitalist 2022, 12, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nandan, R.; Sharma, G.; Nandolia, K.; Saxena, S.; Verma, P.K. Acute Hemorrhagic Necrotizing Encephalopathy in Patients with COVID-19. Ann. Indian Acad. Neurol. 2022, 25, 511–513. [Google Scholar] [PubMed]
- Dixon, L.; Varley, J.; Gontsarova, A.; Mallon, D.; Tona, F.; Muir, D.; Luqmani, A.; Jenkins, I.H.; Nicholas, R.; Jones, B.; et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e789. [Google Scholar] [CrossRef] [PubMed]
- Lazarte-Rantes, C.; Guevara-Castañón, J.; Romero, L.; Guillén-Pinto, D. Acute Necrotizing Encephalopathy Associated with SARS-CoV-2 Exposure in a Pediatric Patient. Cureus 2021, 13, e15018. [Google Scholar] [CrossRef] [PubMed]
- Ciolac, D.; Crivorucica, I.; Zota, E.; Gorincioi, N.; Efremova, D.; Manea, D.; Crivorucica, V.; Ciocanu, M.; Groppa, S.A. Extensive cerebellar involvement and cognitive impairment in COVID-19-associated acute necrotizing encephalopathy. Ther. Adv. Neurol. Disord. 2021, 14, 1756286420985175. [Google Scholar] [CrossRef] [PubMed]
- Delamarre, L.; Gollion, C.; Grouteau, G.; Rousset, D.; Jimena, G.; Roustan, J.; Gaussiat, F.; Aldigé, E.; Gaffard, C.; Duplantier, J.; et al. COVID-19-associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Pongpitakmetha, T.; Hemachudha, P.; Rattanawong, W.; Thanapornsangsuth, P.; Viswanathan, A.; Hemachudha, T. COVID-19 related acute necrotizing encephalopathy with extremely high interleukin-6 and RANBP2 mutation in a patient with recently immunized inactivated virus vaccine and no pulmonary involvement. BMC Infect. Dis. 2022, 22, 640. [Google Scholar] [CrossRef]
- Yu, H.H.; Qin, C.; Chen, M.; Wang, W.; Tian, D.S. D-dimer level is associated with the severity of COVID-19. Thromb. Res. 2020, 195, 219–225. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Bao, K.; Huang, C. Ischemic stroke associated with COVID-19: A systematic review and meta-analysis. J. Neurol. 2022, 269, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Troccoli, H.D.M. Tratamiento trombolítico del ictus isquémico agudo. Gac. Méd. Caracas 2020, 121, 183–198. [Google Scholar]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Medetalibeyoglu, A.; Kaytaz, M.; Senkal, N.; Genc, S.; Aktar, I.; Omer, B.; Oncul, O.; Tukek, T. Would Tracking Coagulation Together with Inflamation Markers be a Prospect for COVID-19 Disease Prognosis? Clin. Lab. 2022, 68. [Google Scholar] [CrossRef]
- Elkind, M. Why now? Moving from stroke risk factors to stroke triggers. Curr. Opin. Neurol. 2007, 20, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef] [PubMed]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. ypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. JTH 2020, 18, 1738–1742. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef]
- Boss, G.R.; Seegmiller, J.E. Age-related physiological changes and their clinical significance. West. J. Med. 1981, 135, 434–440. [Google Scholar] [PubMed]
- Trejo-Gabriel-Galán, J.M. Stroke as a complication and prognostic factor of COVID-19. Ictus como complicación y como factor pronóstico de COVID-19. Neurologia 2020, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, H.; Rogers, S.; Kataria, S.; Uddin, K.; Mohamed, K.H.; Mohamed, A.S.; Tariq, F.; Ahmad, S.; Awais, A.; Ahmed, Z.; et al. COVID-19-Induced Seizures: A Meta-Analysis of Case Series and Retrospective Cohorts. Cureus 2022, 14, e28633. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.E.; Bueno, A.C.; Zeas, M.V.; Klinger, L.G.; Núñez, A.H.T.; García Alvarado, L.; Dalgo Pozo, A. Estatus epiléptico en niños: Aspectos generales diagnósticos y terapéuticos. Arch. Venez. Farmacol. Ter. 2019, 38, 377–381. [Google Scholar]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M. Update on neurological manifestations of COVID-19. Life Sci. 2020, 257, 118063. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Singhi, P. Infectious causes of seizures and epilepsy in the developing world. Dev. Med. Child Neurol. 2011, 53, 600–609. [Google Scholar] [CrossRef]
- Aydin, S.; Özdemir, C.; Gündüz, A.; Kiziltan, M.E. Seizures in patients with respiratory disease—A retrospective single center study. Arq. Neuro-Psiquiatr. 2020, 78, 247–254. [Google Scholar] [CrossRef]
- Niazkar, H.R.; Zibaee, B.; Nasimi, A.; Bahri, N. The neurological manifestations of COVID-19: A review article. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 1667–1671. [Google Scholar] [CrossRef]
- Ying, W.; Qian, Y.; Kun, Z. Drugs supply and pharmaceutical care management practices at a designated hospital during the COVID-19 epidemic. Res. Soc. Adm. Pharm. RSAP 2021, 17, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.J.; Klepacz, L.; Lynch, S.; Tavakkoli, M.; Dornbush, R.; Baharani, R.; Smolin, Y.; Bartell, A. COVID-19 Psychosis: A Potential New Neuropsychiatric Condition Triggered by Novel Coronavirus Infection and the Inflammatory Response? Psychosomatics 2020, 61, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Fukushima, H.; Matsuishi, K. COVID-19 delirium and encephalopathy: Pathophysiology assumed in the first 3 years of the ongoing pandemic. Brain Disord. 2023, 10, 100074. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Ö.; Ertaş, M.; Ekizoğlu, E.; Bolay, H.; Özge, A.; Orhan, E.K.; Çağatay, A.A.; Baykan, B. Headache characteristics in COVID-19 pandemic-a survey study. J. Headache Pain 2020, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.N.; Dias, A.R.N.; Paranhos, A.C.M.; Silva, C.C.; Bastos, T.D.R.; de Brito, B.B.; da Silva, N.M.; de Sousa, E.J.S.; Quaresma, J.A.S.; Falcão, L.F.M. Headache in long COVID as disabling condition: A clinical approach. Front. Neurol. 2023, 14, 1149294. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.; Grill, M.F.; Singh, R.B.H. Post-COVID Headache: A Literature Review. Curr. Pain Headache Rep. 2022, 26, 835–842. [Google Scholar] [CrossRef]
- Panariello, F.; Cellini, L.; Speciani, M.; De Ronchi, D.; Atti, A.R. How Does SARS-CoV-2 Affect the Central Nervous System? A Working Hypothesis. Front. Psychiatry 2020, 11, 582345. [Google Scholar] [CrossRef]
- Ismail, I.I.; Salama, S. Association of CNS demyelination and COVID-19 infection: An updated systematic review. J. Neurol. 2022, 269, 541–576. [Google Scholar] [CrossRef]
- Lotan, I.; Nishiyama, S.; Manzano, G.S.; Lydston, M.; Levy, M. COVID-19 and the risk of CNS demyelinating diseases: A systematic review. Front. Neurol. 2022, 13, 970383. [Google Scholar] [CrossRef] [PubMed]
- Gombolay, G.; Hallman-Cooper, J. COVID-19 and the Pandemic-Related Aspects in Pediatric Demyelinating Disorders. Semin. Pediatr. Neurol. 2023, 46, 101055. [Google Scholar] [CrossRef] [PubMed]
- Khair, A.M.; Nikam, R.; Husain, S.; Ortiz, M.; Kaur, G. Para and Post-COVID-19 CNS Acute Demyelinating Disorders in Children: A Case Series on Expanding the Spectrum of Clinical and Radiological Characteristics. Cureus 2022, 14, e23405. [Google Scholar] [CrossRef]
- Garg, R.K.; Paliwal, V.K.; Gupta, A. Spinal cord involvement in COVID-19: A review. J. Spinal Cord Med. 2023, 46, 390–404. [Google Scholar] [CrossRef]
- Schulte, E.C.; Hauer, L.; Kunz, A.B.; Sellner, J. Systematic review of cases of acute myelitis in individuals with COVID-19. Eur. J. Neurol. 2021, 28, 3230–3244. [Google Scholar] [CrossRef] [PubMed]
- AlKetbi, R.; AlNuaimi, D.; AlMulla, M.; AlTalai, N.; Samir, M.; Kumar, N.; AlBastaki, U. Acute myelitis as a neurological complication of Covid-19: A case report and MRI findings. Radiol. Case Rep. 2020, 15, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Wessendorf, S.; Koretsis, G.; Tewald, F.; Baegi, R.; Krämer, S.; Geissler, M.; Reinhard, M. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020, 267, 2196–2197. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Mao, L.W.M.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Hu, B. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef]
- Tsai, L.K.; Hsieh, S.T.; Chang, Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwanica 2005, 14, 113–119. [Google Scholar]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Tsuruoka, S.; Wakaumi, M.; Ioka, T.; Yamamoto, H.; Ando, H.; Sugimoto, K.; Fujimura, A. Angiotensin II receptor blocker-induces blunted taste sensitivity: Comparison of candesartan and valsartan. Br. J. Clin. Pharmacol. 2005, 60, 204–207. [Google Scholar] [CrossRef]
- Dietsch, A.M.; Solomon, N.P.; Steele, C.M.; Pelletier, C.A. The effect of barium on perceptions of taste intensity and palatability. Dysphagia 2014, 29, 96–108. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Taylor, R.S. Guillain-Barre Syndrome. I; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Scheidl, E.; Canseco, D.D.; Hadji-Naumov, A.; Bereznai, B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J. Peripher. Nerv. Syst. JPNS 2020, 25, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Marenco, A.; Lozada, J.M. Unusual Clinical Presentation of Guillain Barre Syndrome: A Case Report; Ciencia e Innovación en Salud: Barranquilla, Colombia, 2018. [Google Scholar]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef]
- Farzi, M.A.; Ayromlou, H.; Jahanbakhsh, N.; Bavil, P.H.; Janzadeh, A.; Shayan, F.K. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J. Neuroimmunol. 2020, 346, 577294. [Google Scholar] [CrossRef] [PubMed]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Chan, J.L.; Ebadi, H.; Sarna, J.R. Guillain-Barré Syndrome with Facial Diplegia Related to SARS-CoV-2 Infection. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 47, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, S.; Budowski, C.; Tin, S.N.W.; Degos, B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin. Neurophysiol. 2020, 131, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, Z.; Karimi, N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 76, 233–235. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, C.; Méndez-Guerrero, A.; Rodrigo-Rey, S.; Pedro-Murillo, E.S.; Bermejo-Guerrero, L.; Gordo-Mañas, R.; de Aragón-Gómez, F.; Benito-León, J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020, 95, e601–e605. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, T. Does COVID-19 cause axonal GBS? J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2020, 78, 448. [Google Scholar] [CrossRef] [PubMed]
- Virani, A.R.E.; Hanson, T.; Haag, A.; Elrufay, R.; Cheema, T.; Bhanot, N. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases 2020, 20, e00771. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, D.; Boso, F.; Tranquillini, E.; Gapeni, I.; Pedrotti, G.; Cozzio, S.; Guarrera, G.M.; Giometto, B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): A case report from an Italian COVID-hospital. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- El Otmani, H.; El Moutawakil, B.; Rafai, M.A.; El Benna, N.; El Kettani, C.; Soussi, M.; El Mdaghri, N.; Barrou, H.; Afif, H. Covid-19 and Guillain-Barré syndrome: More than a coincidence! Rev. Neurol. 2020, 176, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P.; Beretta, S.; Piatti, M.; Karantzoulis, A.; Piatti, M.L.; Santoro, P.; Viganò, M.; Giovannelli, G.; Pirro, F.; Montisano, D.A.; et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e741. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Salud Mental y COVID-19: Datos Iniciales Sobre las Repercusiones de la Pandemia; Organización Mundial de la Salud: Geneva, Switzerland, 2022. [Google Scholar]
- Nicolini, H. Depression and anxiety during COVID-19 pandemic. Cir. Cir. 2020, 88, 542–547. [Google Scholar] [CrossRef]
- Braga, J.; Lepra, M.; Kish, S.J.; Rusjan, P.M.; Nasser, Z.; Verhoeff, N.; Vasdev, N.; Bagby, M.; Boileau, I.; Husain, M.I.; et al. Neuroinflammation After COVID-19 with Persistent Depressive and Cognitive Symptoms. JAMA Psychiatry 2023, 80, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Sadock, B.; Sadock, V.; Ruiz, P. Kaplan y Sadock. Manual de Psiquiatría Clínica; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2018. [Google Scholar]
- González-González, C. Actualidades en la fisiopatología del trastorno por estrés postraumático (TEPT). Salud Jalisco 2018, 1, 128–134. [Google Scholar]
- Organización Panamericana de la Salud, La Pandemia por COVID-19 Provoca un Aumento del 25% en la Prevalencia de la Ansiedad y la Depresión en todo el Mundo. 6 Marzo 2022. Available online: https://www.paho.org/es/noticias/2-3-2022-pandemia-por-covid-19-provoca-aumento-25-prevalencia-ansiedad-depresion-todo (accessed on 15 March 2024).
- González-González, C.; Arvilla-Arce, H.E. Alteraciones neuropsiquiátricas de la enfermedad por COVID-19. Salud Jalisco 2021, 8, 59–64. [Google Scholar]
- American Psychiatric Association [APA]. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Arlington: Westport, CT, USA, 2013. [Google Scholar]
- McWhirter, L.; Smyth, H.; Hoeritzauer, I.; Couturier, A.; Stone, J.; Carson, A.J. What is brain fog? J. Neurol. Neurosurg. Psychiatry 2023, 94, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Rosen, C.J. Long Covid and Impaired Cognition—More Evidence and More Work to Do. N. Engl. J. Med. 2024, 390, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Azor, A.; Atchison, C.; Trender, W.; Hellyer, P.J.; Giunchiglia, V.; Husain, M.; Cooke, G.S.; Cooper, E.; Lound, A.; et al. Cognition and Memory after Covid-19 in a Large Community Sample. N. Engl. J. Med. 2024, 390, 806–818. [Google Scholar] [CrossRef]
- Hugon, J. Long-COVID: Cognitive deficits (brain fog) and brain lesions in non-hospitalized patients. Presse Medicale 2022, 51, 104090. [Google Scholar] [CrossRef]
- Tavassoly, O.; Safavi, F.; Tavassoly, I. Seeding Brain Protein Aggregation by SARS-CoV-2 as a Possible Long-Term Complication of COVID-19 Infection. ACS Chem. Neurosci. 2020, 11, 3704–3706. [Google Scholar] [CrossRef]
| Manifestation/Complication | Authors | Study Type | Description of the Study/Case | Results |
|---|---|---|---|---|
| Olfactory and gustatory disorders | Tong et al. (2020) [80] | Systematic review and meta-analysis | The literature search included articles on the prevalence of olfactory or gustatory dysfunction in patients with COVID-19. | Ten studies were analyzed for olfactory dysfunction, while nine were examined to determine gustatory dysfunction. Subgroup analyses were performed for studies assessing olfactory dysfunction using validated and non-validated instruments. It was found that olfactory dysfunction had a prevalence of 52.73% and gustatory dysfunction of 43.93%. In general, studies reported a higher prevalence of olfactory dysfunction. |
| Lechien et al. (2020) [20] | Multicenter epidemiological study | Clinical data from patients with laboratory-confirmed COVID-19 infection have been collected from four Belgian hospitals. The clinical data was prospectively gathered. A total of 417 patients completed the study. | Among the general symptoms, cough, myalgia, loss of appetite, diarrhoea, fever, headache, and asthenia were the most prevalent, representing more than 45% of the patients. A total of 85.6% of the patients had olfactory dysfunction related to the infection. Phantosmia and parosmia affected 12.6% and 32.4% of the patients, respectively. Likewise, 88.8% reported taste disorders. | |
| Neuropsychiatric manifestations | Helms et al. (2020) [81] | Case series | An observational series of 58 out of 64 consecutive patients admitted to the hospital for COVID-19. | Neurological findings were recorded in 8 out of 58 patients (14%) upon admission to the ICU (prior to treatment) and in 39 patients (67%) when sedation and neuromuscular blockade were discontinued. Among the observed neuropsychiatric manifestations are delirium (26/40), agitation (40/58), signs of the corticospinal tract (39/58), and dysexecutive syndrome (14/39). |
| Myalgias | Yang et al. (2020) [82] | Retrospective observational study | Observational, retrospective, was performed at Wuhan Jin Yin-tan Hospital, where the clinical courses and outcomes of 52 critically ill patients were evaluated. | The most frequent symptoms were fever (98%), cough (77%) and dyspnea (63.5%). Myalgia was present in 11.5% of patients. |
| Encephalitis | Pilotto et al. (2020) [83] | Case Report | A 60-year-old man presented with severe alteration of consciousness, followed by fever, cough and cognitive fluctuations. CR of nasopharyngeal swab confirmed SARS-CoV-2 infection. He was treated with methylprednisolone 1 g/day for five days. | The cerebrospinal fluid PCR was negative for SARS-CoV-2, although inflammatory findings were present, with mild lymphocytic pleocytosis (18/μL) and a moderate protein increase. Additionally, the EEG showed generalized slowing, with decreased reactivity to auditory stimuli. After completing the 5-day therapy, the patient showed significant improvement. Upon discharge, on the 11th day of admission, the patient presented a normal neurological examination. |
| Moriguchi et al. (2020) [44] | Case Report | A 24-year-old man with altered consciousness, convulsions, and neck stiffness, followed by a headache, generalized fatigue, fever, and a sore throat. PCR detected SARS-CoV-2 in CSF. | CMR indicated right lateral ventriculitis and encephalitis, mainly in the right mesial lobe and hippocampus. After admission to the ICU, empirical treatment with EV ceftriaxone, vancomycin, acyclovir, steroids and favipiravir was started. However, his evolution was not reported. | |
| Stroke | Mao et al. (2020) [22] | Retrospective observational study | Multicenter, observational, retrospective study. In this study, 214 patients with COVID-19 were evaluated for severe (88) and non-severe (126) conditions. | A total of 78 patients (36.4%) had neurological complications. Patients with severe infection were more prone to these complications, were also older and had more underlying disorders. The prevalence of stroke was higher in patients with severe pneumonia (5.7%) compared to those with mild pneumonia (0.8%). |
| Beyrouti et al. (2020) [84] | Case series | The cases of six patients from the National Hospital for Neurology and Neurosurgery, Queen Square, London, UK, with acute ischemic stroke and COVID-19 are described. | All six patients had large vessel occlusion with markedly elevated D-dimer levels (≥1000 μg/L). Likewise, stroke occurred in two patients despite therapeutic anticoagulation. The findings suggest that ischemic stroke related to COVID-19 infection may occur in the context of a highly prothrombotic systemic state. | |
| Seizure | Karimi et al. (2020) [85] | Case Report | A 30-year-old woman previously healthy with tonic-clonic seizure whose respiratory specimen was positive for COVID-19 using PCR. | CMR was normal, and a chest CT revealed focal ground-glass opacities. The patient’s symptoms improved with anticonvulsants and antiviral medications. |
| Guillain–Barré Syndrome | Juliao Caamaño and Alonso Beato (2020) [86] | Case Report | A 61-year-old patient diagnosed and treated as SARS-CoV-2 infection with pneumonia (hydroxychloroquine and lopinavir/Ritonavir for 14 days). After symptoms disappeared, he presented bilateral facial nerve palsy. | No other neurological findings on examination were present. A chest x-ray showed significant improvement in pneumonia. He was diagnosed with variant GBS and treated with low-dose oral prednisone; at two weeks, he started with barely noticeable improvement on both sides. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza, D.; Castellar-Visbal, L.; Marquina, M.; Rivera-Porras, D.; Galbán, N.; Santeliz, R.; Gutiérrez-Rey, M.; Parra, H.; Vargas-Manotas, J.; Torres, W.; et al. COVID-19: Unveiling the Neuropsychiatric Maze—From Acute to Long-Term Manifestations. Biomedicines 2024, 12, 1147. https://doi.org/10.3390/biomedicines12061147
Ariza D, Castellar-Visbal L, Marquina M, Rivera-Porras D, Galbán N, Santeliz R, Gutiérrez-Rey M, Parra H, Vargas-Manotas J, Torres W, et al. COVID-19: Unveiling the Neuropsychiatric Maze—From Acute to Long-Term Manifestations. Biomedicines. 2024; 12(6):1147. https://doi.org/10.3390/biomedicines12061147
Chicago/Turabian StyleAriza, Daniela, Lily Castellar-Visbal, Maria Marquina, Diego Rivera-Porras, Nestor Galbán, Raquel Santeliz, Melissa Gutiérrez-Rey, Heliana Parra, José Vargas-Manotas, Wheeler Torres, and et al. 2024. "COVID-19: Unveiling the Neuropsychiatric Maze—From Acute to Long-Term Manifestations" Biomedicines 12, no. 6: 1147. https://doi.org/10.3390/biomedicines12061147
APA StyleAriza, D., Castellar-Visbal, L., Marquina, M., Rivera-Porras, D., Galbán, N., Santeliz, R., Gutiérrez-Rey, M., Parra, H., Vargas-Manotas, J., Torres, W., Quintana-Espinosa, L., Manzano, A., Cudris-Torres, L., & Bermúdez, V. (2024). COVID-19: Unveiling the Neuropsychiatric Maze—From Acute to Long-Term Manifestations. Biomedicines, 12(6), 1147. https://doi.org/10.3390/biomedicines12061147







