Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome?
Abstract
1. Introduction
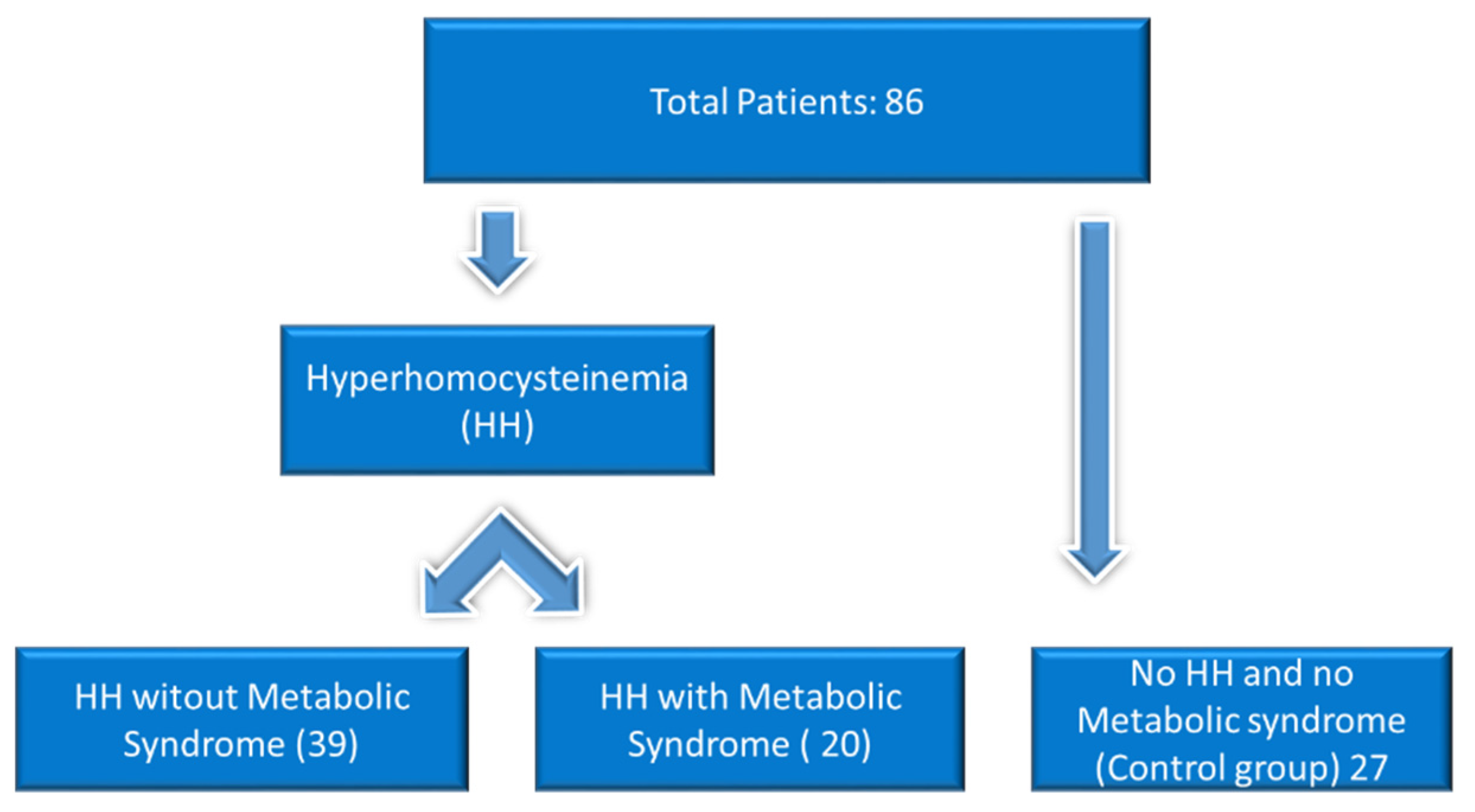
2. Materials and Methods
2.1. Anamnesis and Objective Examination
2.2. Nutrigenetic Testing
2.3. Nutrigenetic Management of Physically Active Individuals or Athletes
2.4. Statistical Analysis
3. Results
3.1. The Study of the Parameters According to the Research Cohort
3.2. The Study of the Parameters According to the Research Groups
3.3. Correlations
- -
- gender and the need for zinc, magnesium, vitamin B2, vitamin B3 and vitamin B6, respectively
- -
- age and folic acid
- -
- weight status and zinc requirement
- -
- risk of obesity and the need for zinc, magnesium, vitamin B2, vitamin B3
- -
- the risk of hypertension and the need for folic acid and vitamin B3
- -
- the risk of hypercholesterolemia and the need for folic acid
- -
- N6/N3 in relation to zinc, magnesium, vitamin B2, B3, and B6 requirements.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Ghitea, T.C.; Vlad, S.; Birle, D.; Tit, D.M.; Lazar, L.; Nistor-Cseppento, C.; Behl, T.; Bungau, S. The influence of diet therapeutic intervention on the sarcopenic index of patients with metabolic syndrome. Acta Endocrinol. 2020, 16, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Al Shehri, M.; Al Saleb, A.; Othman, F.; Al Hazmi, M.; Al Amri, F.; Ferwana, M.; Al Yousef, M.Z.; Al Turki, M. The association between plasma homocysteine level and metabolic syndrome. A record-based study of Saudi patients attending King Abdulaziz Medical City in Riyadh, Saudi Arabia. Saudi Med. J. 2020, 41, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Shih, C.C.; Huang, T.C.; Chen, J.Y. The Relationship between Elevated Homocysteine and Metabolic Syndrome in a Community-Dwelling Middle-Aged and Elderly Population in Taiwan. Biomedicines 2023, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Hajer, G.R.; van der Graaf, Y.; Olijhoek, J.K.; Verhaar, M.C.; Visseren, F.L. Levels of homocysteine are increased in metabolic syndrome patients but are not associated with an increased cardiovascular risk, in contrast to patients without the metabolic syndrome. Heart 2007, 93, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Singal, P.; Gill, V. The antihypertensive effect of cysteine. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc 2009, 18, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Schalinske, K.L.; Smazal, A.L. Homocysteine imbalance: A pathological metabolic marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520962230. [Google Scholar] [CrossRef]
- Clarke, R.; Daly, L.; Robinson, K.; Naughten, E.; Cahalane, S.; Fowler, B.; Graham, I. Hyperhomocysteinemia: An independent risk factor for vascular disease. N. Engl. J. Med. 1991, 324, 1149–1155. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- McCaddon, A.; Miller, J.W. Homocysteine-a retrospective and prospective appraisal. Front. Nutr. 2023, 10, 1179807. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Tulchinsky, T.H. Marc Lalonde, the Health Field Concept and Health Promotion. Case Stud. Public Health 2018, 30, 523. [Google Scholar]
- Miller, A.L. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern. Med. Rev. 2003, 8, 7–19. [Google Scholar] [PubMed]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.B.; Steffen, B.T.; Nomura, S.O.; Guan, W.; Garg, P.K.; Szklo, M.; Budoff, M.J.; Tsai, M.Y. Association Between Homocysteine and Vascular Calcification Incidence, Prevalence, and Progression in the MESA Cohort. J. Am. Heart Assoc. 2020, 9, e013934. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, Cognitive Functions, and Degenerative Dementias: State of the Art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Ocal, P.; Ersoylu, B.; Cepni, I.; Guralp, O.; Atakul, N.; Irez, T.; Idil, M. The association between homocysteine in the follicular fluid with embryo quality and pregnancy rate in assisted reproductive techniques. J. Assist. Reprod. Genet. 2012, 29, 299–304. [Google Scholar] [CrossRef]
- Langman, L.J.; Cole, D.E. Homocysteine. Crit. Rev. Clin. Lab. Sci. 1999, 36, 365–406. [Google Scholar] [CrossRef]
- Brustolin, S.; Giugliani, R.; Félix, T.M. Genetics of homocysteine metabolism and associated disorders. Braz. J. Med. Biol. Res. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of Hyperhomocysteinemia in Neurological Disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Wiklund, O.; Fager, G.; Andersson, A.; Lundstam, U.; Masson, P.; Hultberg, B. N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 1996, 119, 99–106. [Google Scholar] [CrossRef]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Caruso, P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Prtina, A.; Rašeta Simović, N.; Milivojac, T.; Vujnić, M.; Grabež, M.; Djuric, D.; Stojiljković, M.P.; Soldat Stanković, V.; Čolić, M.J.; Škrbić, R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules 2021, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Trifan, D.F.; Tirla, A.G.; Mos, C.; Danciu, A.; Bodog, F.; Manole, F.; Ghitea, T.C. Involvement of Vitamin D3 in the Aging Process According to Sex. Cosmetics 2023, 10, 114. [Google Scholar] [CrossRef]
- Yang, Q.; He, G.W. Imbalance of Homocysteine and H2S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury. Oxid. Med. Cell. Longev. 2019, 2019, 7629673. [Google Scholar] [CrossRef]
- Danciu, A.M.; Ghitea, T.C.; Bungau, A.F.; Vesa, C.M. The Relationship Between Oxidative Stress, Selenium, and Cumulative Risk in Metabolic Syndrome. Vivo 2023, 37, 2877–2887. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Wilborn, C.; Beckham, J.; Campbell, B.; Harvey, T.; Galbreath, M.; La Bounty, P.; Nassar, E.; Wismann, J.; Kreider, R. Obesity: Prevalence, theories, medical consequences, management, and research directions. J. Int. Soc. Sports Nutr. 2005, 2, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Tershakovec, A.M.; Jawad, A.F.; Stouffer, N.O.; Elkasabany, A.; Srinivasan, S.R.; Berenson, G.S. Persistent hypercholesterolemia is associated with the development of obesity among girls: The Bogalusa Heart Study123. Am. J. Clin. Nutr. 2002, 76, 730–735. [Google Scholar] [CrossRef]
- Steed, M.M.; Tyagi, S.C. Mechanisms of Cardiovascular Remodeling in Hyperhomocysteinemia. Antioxid. Redox Signal. 2010, 15, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyperhomocysteinemia as a Risk Factor for Vascular Contributions to Cognitive Impairment and Dementia. Front. Aging Neurosci. 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Gustafson, L.; Hultberg, B. Elevated plasma homocysteine level in vascular dementia reflects the vascular disease process. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ota, K.; Takahashi, T.; Yoshida, H. Impact of Homocysteine as a Preconceptional Screening Factor for In Vitro Fertilization and Prevention of Miscarriage with Folic Acid Supplementation Following Frozen-Thawed Embryo Transfer: A Hospital-Based Retrospective Cohort Study. Nutrients 2023, 15, 3730. [Google Scholar] [CrossRef] [PubMed]
- Olaso-Gonzalez, G.; Inzitari, M.; Bellelli, G.; Morandi, A.; Barcons, N.; Viña, J. Impact of supplementation with vitamins B6, B12, and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: A systematic review. IUBMB Life 2022, 74, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Yusuf, S.; Arnold, M.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Herrmann, M. The Controversial Role of HCY and Vitamin B Deficiency in Cardiovascular Diseases. Nutrients 2022, 14, 1412. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Salanti, G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2009, 4. [Google Scholar] [CrossRef]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef] [PubMed]

| Parameters | N | Mean | SD | Skewness | Kurtosis | Minim | Maxim | Percentiles | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
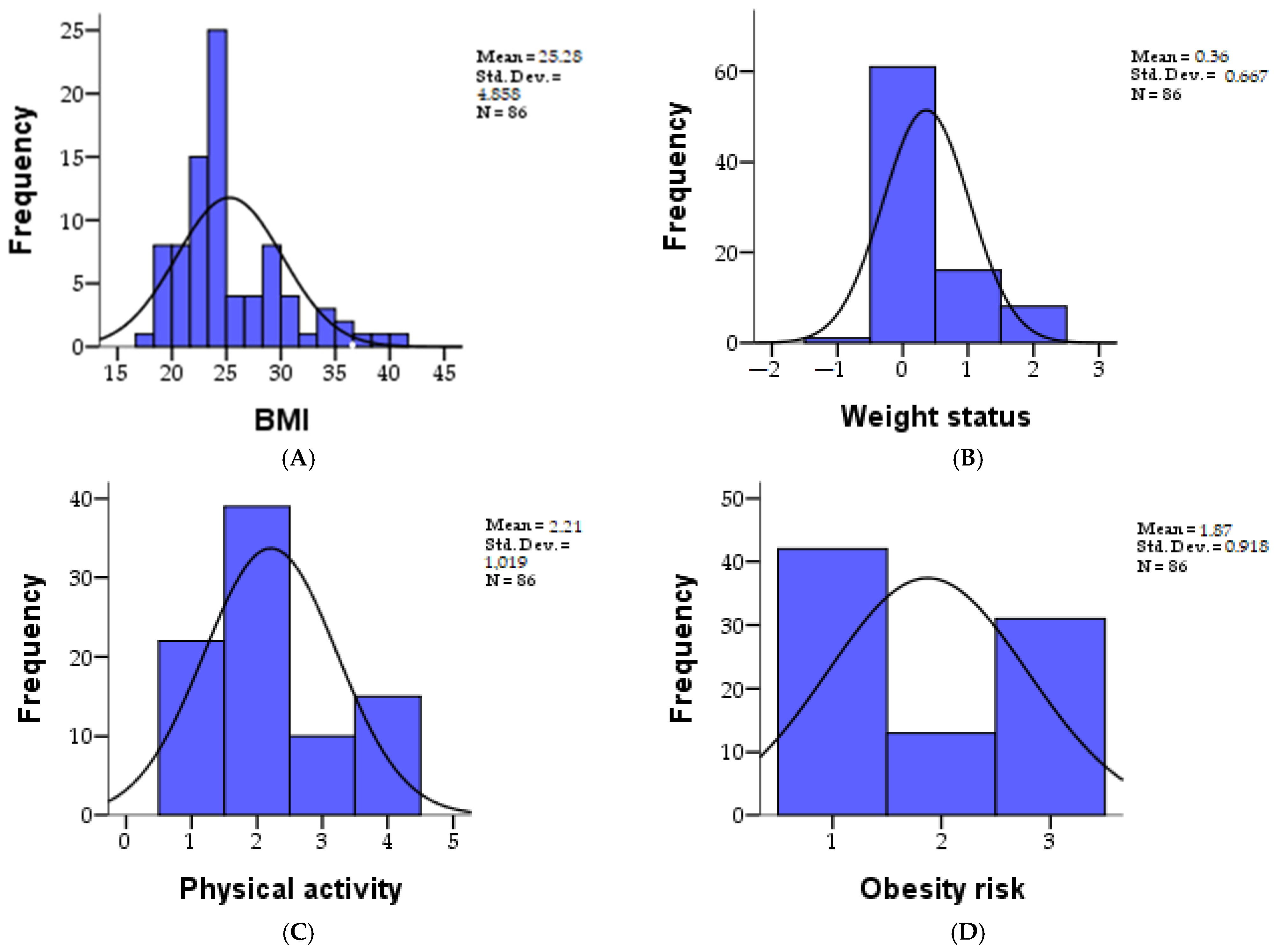
| BMI | 86 | 25.27 | 4.857 | 1.180 | 1.263 | 17.94 | 41.66 | 22.2075 | 24.0900 | 28.0550 |
| Weight status | 86 | 0.36 | 0.667 | 1.382 | 1.031 | −1.00 | 2.00 | 0.0000 | 0.0000 | 1.0000 |
| Physical activity | 86 | 2.20 | 1.018 | 0.590 | −0.704 | 1.00 | 4.00 | 1.0000 | 2.0000 | 3.0000 |
| Obesity risk | 86 | 1.87 | 0.917 | 0.259 | −1.784 | 1.00 | 3.00 | 1.0000 | 2.0000 | 3.0000 |
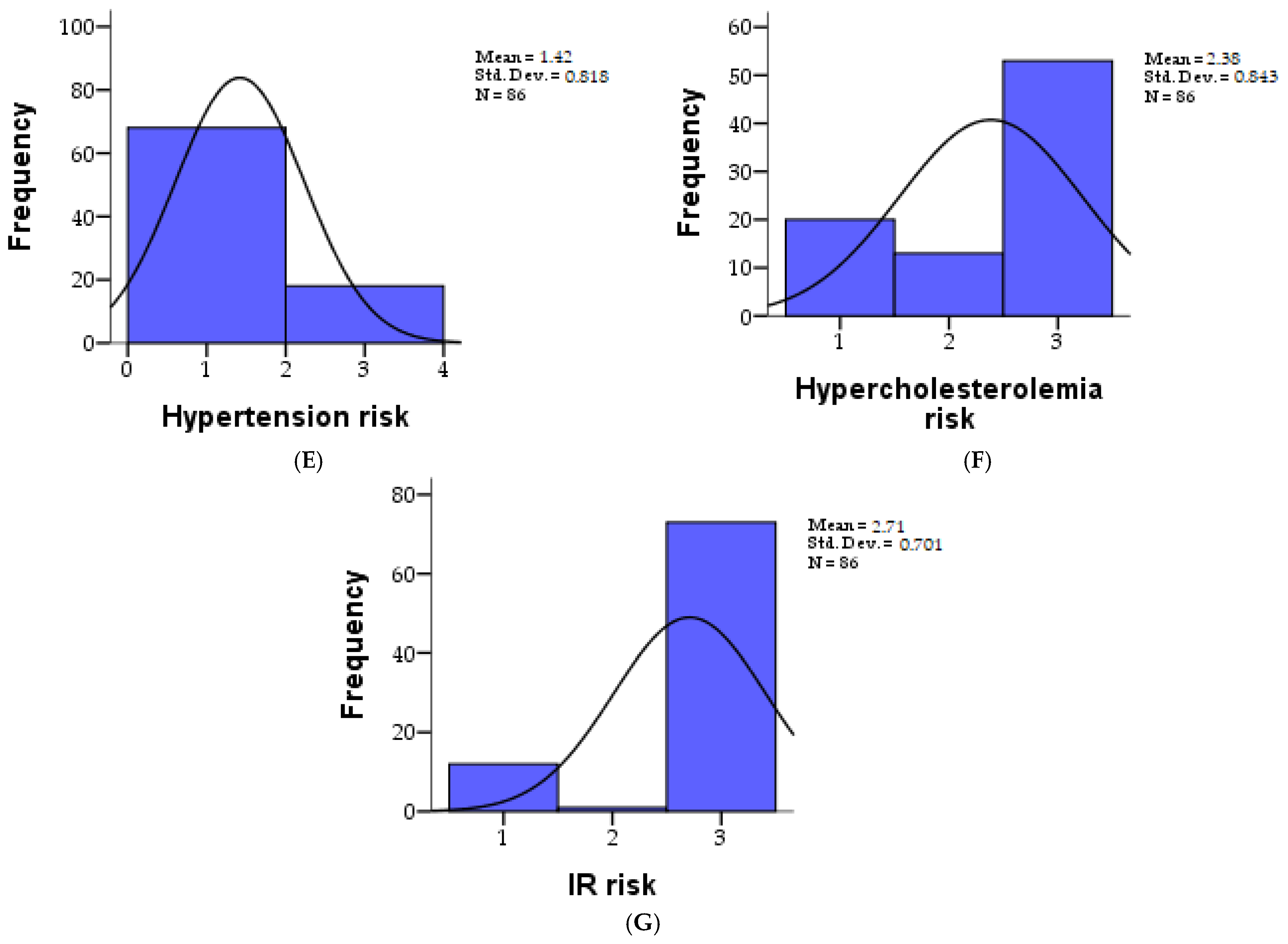
| Hypertension risk | 86 | 1.41 | 0.818 | 1.455 | 0.118 | 1.00 | 3.00 | 1.0000 | 1.0000 | 1.0000 |
| Risk of hypercholesterolemia | 86 | 2.38 | 0.842 | −0.832 | −1.073 | 1.00 | 3.00 | 2.0000 | 3.0000 | 3.0000 |
| IR risk | 86 | 2.70 | 0.700 | −2.048 | 2.297 | 1.00 | 3.00 | 3.0000 | 3.0000 | 3.0000 |
| Parameters | N | Mean | SD | Skewness | Kurtosis | Minim | Maxim | Percentiles | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| Required zinc mg/day | 86 | 12.04 | 3.300 | 0.889 | 0.845 | 8.00 | 22.00 | 10.0000 | 11.0000 | 14.0000 |
| Magnesium required mg/day | 435.46 | 61.675 | 0.066 | −1.025 | 250.00 | 510.00 | 390.0000 | 390.0000 | 510.0000 | |
| Folic acid required day | 1.30 | 0.461 | 0.876 | −1.262 | 1.00 | 2.00 | 1.0000 | 1.0000 | 2.0000 | |
| Vit B2 required mg/day | 2.32 | 0.275 | −1.498 | 4.578 | 1.30 | 2.60 | 2.2000 | 2.2000 | 2.6000 | |
| Vit B3 required mg/day | 21.83 | 2.365 | −1.635 | 3.759 | 14.00 | 24.00 | 21.0000 | 21.0000 | 24.0000 | |
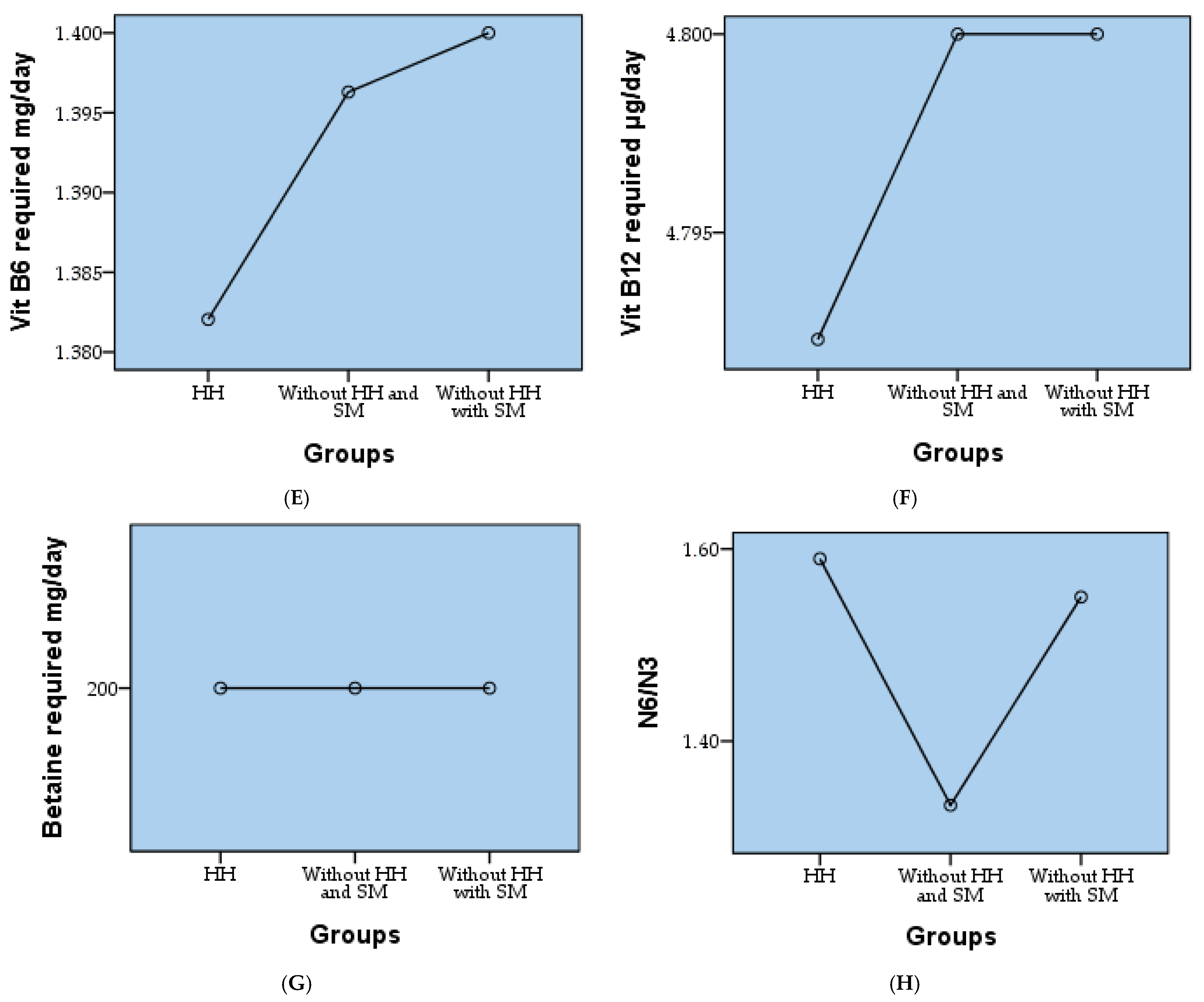
| Vit B6 required mg/day | 1.39 | 0.149 | 1.294 | 0.072 | 1.30 | 1.70 | 1.3000 | 1.3000 | 1.5000 | |
| Vit B12 required µg/day | 4.79 | 0.032 | −9.274 | 86.000 | 4.50 | 4.80 | 4.8000 | 4.8000 | 4.8000 | |
| Betaine required mg/day | 200.00 | 0.000 | - | - | 200.00 | 200.00 | 200.0000 | 200.0000 | 200.0000 | |
| N6/N3 | 1.50 | 0.502 | 0.000 | −2.048 | 1.00 | 2.00 | 1.0000 | 1.5000 | 2.0000 | |
| Parameters | Groups | ||||||
|---|---|---|---|---|---|---|---|
| HH | Without HH and SM | Without HH with SM | |||||
| Count | % | Count | % | Count | % | ||
| BMI (Mean ± SD) | 25.42 ± 4.26 | 23.06 ± 2.69 | 27.99 ± 6.68 | ||||
| Weight status | underweight | 0 | 0.0% | 0 | 0.0% | 1 | 1.2% |
| normal | 26 | 30.2% | 25 | 29.1% | 10 | 11.6% | |
| overweight | 8 | 9.3% | 1 | 1.2% | 7 | 8.1% | |
| obesity I | 5 | 5.8% | 1 | 1.2% | 2 | 2.3% | |
| obesity II | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| obesity III | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Physical activity (Mean ± SD) | 2.21 ± 1.03 | 2.41 ± 0.97 | 1.95 ± 1.05 | ||||
| Obesity risk | low | 12 | 14.0% | 21 | 24.4% | 9 | 10.5% |
| medium | 13 | 15.1% | 0 | 0.0% | 0 | 0.0% | |
| high | 14 | 16.3% | 6 | 7.0% | 11 | 12.8% | |
| Hypertension risk | low | 29 | 33.7% | 27 | 31.4% | 12 | 14.0% |
| medium | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| high | 10 | 11.6% | 0 | 0.0% | 8 | 9.3% | |
| Risk of hypercholesterolemia | low | 2 | 2.3% | 15 | 17.4% | 3 | 3.5% |
| medium | 12 | 14.0% | 0 | 0.0% | 1 | 1.2% | |
| high | 25 | 29.1% | 12 | 14.0% | 16 | 18.6% | |
| IR risk | low | 4 | 4.7% | 8 | 9.3% | 0 | 0.0% |
| medium | 1 | 1.2% | 0 | 0.0% | 0 | 0.0% | |
| high | 34 | 39.5% | 19 | 22.1% | 20 | 23.3% | |
| Parameters | Groups | ||||||
|---|---|---|---|---|---|---|---|
| HH | Without HH and SM | Without HH with SM | |||||
| Count | % | Count | % | Count | % | ||
| Required zinc mg/day (Mean ± SD) | 12.49 ± 3.42 | 11.19 ± 2.40 | 12.35 ± 3.99 | ||||
| Magnesium required mg/day (Mean ± SD) | 429.49 ± 64.11 | 447.41 ± 60.17 | 431.00 ± 59.55 | ||||
| Folic acid required day | 400 UEF-5-MTHF | 30 | 34.9% | 13 | 15.1% | 17 | 19.8% |
| 400 | 9 | 10.5% | 14 | 16.3% | 3 | 3.5% | |
| Vit B2 required mg/day (Mean ± SD) | 2.35 ± 0.20 | 2.31 ± 0.35 | 2.28 ± 0.30 | ||||
| Vit B3 required mg/day (Mean ± SD) | 21.23 ± 3.01 | 22.56 ± 1.53 | 22.05 ± 1.47 | ||||
| Vit B6 required mg/day (Mean ± SD) | 1.38 ± 0.14 | 1.40 ± 0.16 | 1.40 ± 0.15 | ||||
| Vit B12 required µg/day (Mean ± SD) | 4.79 ± 0.05 | 4.80 ± 0.00 | 4.80 ± 0.00 | ||||
| Betaine required mg/day | 200.00 ± 0.00 | 200.00 ± 0.00 | 200.00 ± 0.00 | ||||
| N6/N3 | <4 | 16 | 18.6% | 18 | 20.9% | 9 | 10.5% |
| <10 | 23 | 26.7% | 9 | 10.5% | 11 | 12.8% | |
| Pearson Correlation | Sex | Age | Weight Status | Physical Activity | Obesity Risk | Hypertension Risk | Hypercholesterolemia Risk | N6/N3 | |
|---|---|---|---|---|---|---|---|---|---|
| Required zinc mg/day | r | −0.421 ** | 0.076 | −0.221 * | 0.228 * | −0.282 ** | 0.672 ** | 0.167 | −0.298 ** |
| p | 0.000 | 0.489 | 0.040 | 0.035 | 0.009 | 0.000 | 0.124 | 0.005 | |
| Vit B3 required mg/day | r | −0.681 ** | 0.126 | −0.015 | 0.131 | −0.394 ** | −0.268 * | −0.045 | −0.633 ** |
| p | 0.000 | 0.246 | 0.894 | 0.228 | 0.000 | 0.013 | 0.681 | 0.000 | |
| Magnesium required mg/day | r | −0.938 ** | 0.106 | −0.129 | 0.219 * | −0.624 ** | −0.186 | −0.011 | −0.825 ** |
| p | 0.000 | 0.329 | 0.238 | 0.042 | 0.000 | 0.087 | 0.917 | 0.000 | |
| Folic acid required day | r | −0.073 | −0.234 * | −0.129 | −0.061 | 0.065 | −0.276 ** | −0.302 ** | −0.101 |
| p | 0.504 | 0.030 | 0.237 | 0.577 | 0.555 | 0.010 | 0.005 | 0.354 | |
| Vit B2 required mg/day | r | −0.504 ** | 0.077 | −0.038 | 0.139 | −0.315 ** | −0.021 | 0.146 | −0.447 ** |
| p | 0.000 | 0.479 | 0.732 | 0.203 | 0.003 | 0.850 | 0.180 | 0.000 | |
| Vit B6 required mg/day | r | −0.323 ** | 0.647 ** | 0.046 | −0.064 | −0.138 | −0.083 | 0.038 | −0.235 * |
| p | 0.002 | 0.000 | 0.675 | 0.556 | 0.206 | 0.446 | 0.728 | 0.029 | |
| Vit B12 required µg/day | r | −0.090 | −0.074 | 0.059 | 0.129 | 0.104 | 0.056 | −0.080 | −0.108 |
| p | 0.411 | 0.498 | 0.590 | 0.235 | 0.342 | 0.610 | 0.465 | 0.320 | |
| N | 86 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarcau, B.M.; Negru, A.; Ghitea, T.C.; Marian, E. Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome? Biomedicines 2024, 12, 1135. https://doi.org/10.3390/biomedicines12061135
Tarcau BM, Negru A, Ghitea TC, Marian E. Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome? Biomedicines. 2024; 12(6):1135. https://doi.org/10.3390/biomedicines12061135
Chicago/Turabian StyleTarcau, Bogdan Mihai, Andra Negru, Timea Claudia Ghitea, and Eleonora Marian. 2024. "Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome?" Biomedicines 12, no. 6: 1135. https://doi.org/10.3390/biomedicines12061135
APA StyleTarcau, B. M., Negru, A., Ghitea, T. C., & Marian, E. (2024). Is There a Connection between Hyperhomocysteinemia and the Cardiometabolic Syndrome? Biomedicines, 12(6), 1135. https://doi.org/10.3390/biomedicines12061135








